Abstract
Background
Spontaneous coronary artery dissection (SCAD) is a frequently underdiagnosed entity that carries a significant risk of morbidity and mortality. Spontaneous coronary artery dissection is increasingly recognized as an important cause of acute coronary syndrome (ACS) and, the majority of SCAD patients are young healthy women.
Case summary
A 23-year-old female G5P4 presented to the emergency room for severe sub-sternal chest pain, associated with shortness of breath. Past medical history was significant for pre-eclampsia. Initial electrocardiogram was remarkable for ST depressions in V5–V6 with inverted T waves to V1–V2. Troponin I was elevated to 1.13 ng/mL. Two-dimensional echo showed reduced left ventricular function with an ejection fraction of 40%. Cardiac catheterization showed triple vessel dissection involving the left main trunk extending into mid-left anterior descending and dissection extending from ostium of left circumflex artery into large first obtuse marginal branch. She was started on aspirin and heparin. After 48 h she was loaded with clopidogrel. Computed tomography angiography of head, neck, abdomen, and pelvis showed findings compatible with fibromuscular dysplasia. She was haemodynamically stable and symptom free and did not want surgery. She was recommended to continue dual antiplatelet therapy for 12 months and subsequently aspirin and beta blocker only lifelong.
Discussion
Spontaneous coronary artery dissection is a rare condition which is underdiagnosed. A thorough history and high degree of suspicion is required to diagnose in a timely manner and it should be high on differential in a postpartum female presenting with signs and symptoms of ACS.
Keywords: SCAD, ACS, Dissection, Angina, Case report
Introduction
Spontaneous coronary artery dissection (SCAD) poses a dilemma in making an accurate diagnosis and to manage affected patients due to the limited literature. Current pregnancy and history of connective tissue disorders have demonstrated strong associations with SCAD, placing such patients at higher risk.1 Spontaneous coronary artery dissection is a significant contributor to acute coronary syndrome (ACS), especially among young females without traditional risk factors of the atherosclerotic process. A majority of cases demonstrate single artery involvement, most commonly the left anterior descending (LAD).2 Integration of multi-modality imaging assists in making the accurate diagnosis of SCAD, utilizing coronary angiography (CAG) in conjunction with multidetector computed tomography (MDCT). Management strategy is dictated by the severity of disease and consists of either conservative treatment or aggressive management with percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG). We report a rare case of spontaneous triple vessel dissection in a peripartum patient.
Learning points
Spontaneous coronary artery dissection (SCAD) is underdiagnosed but is fairly common in young active females without prior history of cardiovascular diseases who present with chest pain.
Patients with SCAD and suspected connective tissue disorder need a surveillance computed tomography angiography to evaluate the underlying cause and to rule out peripheral dissections/vascular anomalies.
Spontaneous coronary artery dissection has a high association with pregnancy and connective tissue disorders.
Management of SCAD should be individualized based on severity and patient characteristics.
Timeline
| Day 0 | Young female with chest pain, acute coronary syndrome symptoms, postpartum Day 14, lab markers concerning for ischaemia, echo showing reduced left ventricular function |
| Day 1 | Cardiac catheterization confirming dissection of three coronary vessels. Started on aspirin and heparin drip |
| Day 3 | Loaded with clopidogrel after decision made to manage conservatively as patient refused coronary artery bypass graft |
| Day 5 | Found to have fibromuscular dysplasia on screening computed tomography of chest, head, abdomen, and pelvis |
| Day 7 | Discharged home on aspirin and clopidogrel for 12 months and subsequently aspirin only |
| 6 months | Doing well with no recurrence |
Case presentation
We present a 23-year-old African-American female who was gravida 5 para 4, postpartum Day 14, who presented to the emergency room for severe sub-sternal chest pressure without radiation. The patient also complained of associated shortness of breath which was aggravated by exertion and partially relieved by rest. Upon arrival, the patient was haemodynamically stable. Examination of vitals revealed blood pressure was 127/83 mmHg, heart rate was 72 b.p.m., respiratory rate was 16 breaths per minute, and oxygen saturation was 98% on room air. Cardiac auscultation revealed normal heart sounds. The patient had a past medical history significant for pre-eclampsia in a previous pregnancy, asthma, and bipolar disorder. Differential diagnoses included ACS (which could be secondary to acute myocardial infarction), aortic dissection, costochondritis, and gastroesophageal reflux disease.
Initial electrocardiogram (ECG) revealed ST depressions in inferior leads and lateral leads V5–V6 with inverted T waves in leads V1–V2. Initial troponin was normal but serial troponin I was elevated to 1.13 ng/mL (reference range 0.000–0.029 ng/mL). Two-dimensional (2D) echocardiography demonstrated globally reduced left ventricular function with an ejection fraction of 40% (Table 1). In the setting of alarm symptoms with signs of acute myocardial injury, cardiac catheterization was urgently performed. Catheterization displayed triple vessel SCAD involving the left main trunk extending into mid-LAD, dissection in the ostium of the left circumflex artery into the large first obtuse marginal branch and dissection involving distal right coronary artery (RCA) (Figures 1 and 2 and Videos 1 and 2).
Table 1.
Most common treatment modalities used for spontaneous coronary artery dissection
| Treatment modalities | First line | Advantages | Disadvantages |
|---|---|---|---|
| Aspirin | Most commonly used for acute and long term SCAD treatment | Low side effect profile and bleeding risks and clear cut benefits in patients with ACS and secondary prevention of CAD | None |
| Aspirin + clopidogrel | Used in patients after PCI due to SCAD and sometimes in combination with aspirin even in patients without stents | Since SCAD involves intimal tear which is prothrombotic, dual antiplatelet therapy would be empirically beneficial to reduce false lumen thrombus burden and theoretically reduce true lumen compression | Higher risk of bleeding |
| Anticoagulation | Controversial, no clear cut guidelines | Initially administered on patients presenting with ACS | Risk of extension of dissection and extension of intramural haematoma |
| Beta blockers | Indicated | Reduce arterial wall stress | Should be avoided in severe asthma and COPD patients |
| ACE-inhibitors | Not first line | Only indicated in patients with significant LV dysfunction after MI (EF < 40%) | |
| Statin | Not used | No previous studies showing benefit in patients with non-atherosclerotic SCAD | Should only be used in patients with pre-existing dyslipidaemia |
| PCI | Not routinely performed | Indicated in patients with ongoing or recurrent chest pain, haemodynamic or electrical instability or cardiogenic shock and or patients involving LM SCAD3 | Potential risk of further dissection or inability to find true lumen |
| CABG | Not routinely performed | Indicated in high risk patients not amenable to PCI and patients with LM SCAD3 | Higher risk of bleeding complications and inability to find true lumen for bypass anastomosis |
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; LM, left main; LV, left ventricular; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection.
Figure 1.
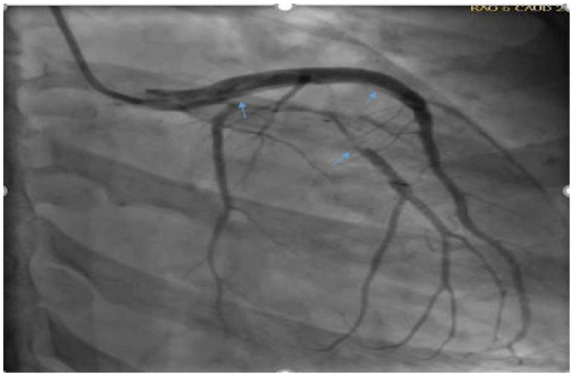
Right anterior oblique cardiac catheterization view showing dissection involving distal left main trunk to proximal left anterior descending artery and left obtuse marginal first branch.
Figure 2.
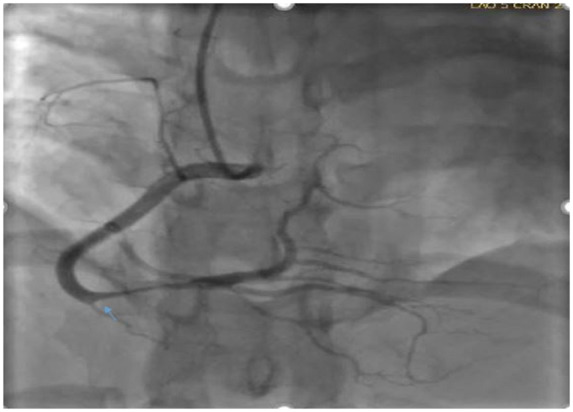
Left anterior oblique cardiac catheterization view showing dissection involving distal right coronary artery.
The patient remained haemodynamically stable with improved Thrombolysis in Myocardial Infarction III flow and resolution of her symptoms, which dictated medical management. The patient was started on aspirin, heparin drip, blood pressure management, and heart rate control and moved to the coronary care unit. After 48 h, clopidogrel loading was initiated. Computed tomography angiography (CTA) of coronary arteries was then performed confirming dissection (Figures 3 and 4).
Figure 3.
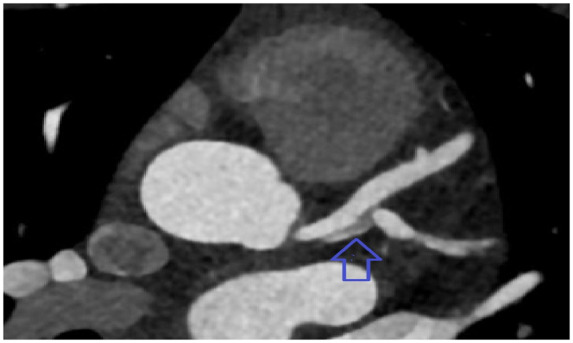
Computed tomography angiography of coronary arteries showing dissection in left main trunk extending to the ostium of left anterior descending artery and obtuse marginal branch.
Figure 4.
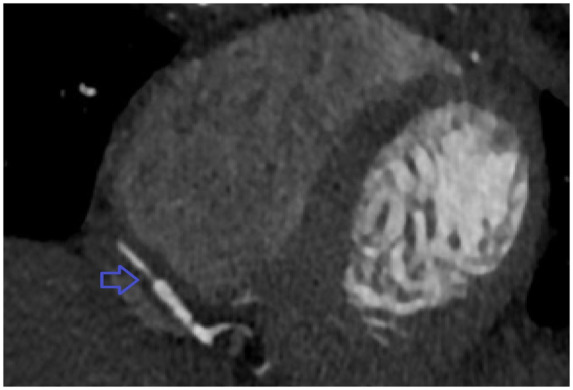
Computed tomography angiography of coronary arteries showing Type 2 spontaneous coronary artery dissection involving distal right coronary artery.
For screening purpose, CTA of head, neck, abdomen, and pelvis was done which showed a constellation of findings compatible with fibromuscular dysplasia (FMD) including irregular calibre and wall thickening of bilateral common iliac arteries, focal intimal defect and ectasia of the very distal infrarenal abdominal aorta and vague abnormality of the left renal artery probably representing wall thickening.
Revascularization was considered but since she became haemodynamically stable with complete regression of her symptoms, conservative management was indicated and preferred by the patient as well and she was managed with dual antiplatelet therapy for 12 months and lifelong aspirin 81 mg. She was also initiated on a beta blocker therapy on discharge as well since beta blockers reduce shear arterial stress and have been presumably beneficial in reducing coronary arterial wall stress.3 The rationale behind initiating dual antiplatelet therapy for this patient included potential help with inflammation since SCAD is a prothrombotic state. The addition of clopidogrel in SCAD patients not treated with stents is of unclear benefit. The role of anticoagulation for SCAD is also controversial with potential risk of dissection extension but also has a benefit of resolution of overlying thrombus and improving the lumen patency.3 In our patient, heparin drip was briefly started at outside hospital but was stopped after clopidogrel initiation at our facility. Prior to discharge she was extensively counselled by the primary team and obstetrics and gynaecology team that in the setting of a SCAD, another pregnancy could be life-threatening. She was advised that were she to desire another pregnancy it should be after lengthy consultation with her cardiology team and with maternal foetal medicine team. She was recommended for long-term birth control and opted for nexplanon implant. The patient is alive at 6 months follow-up with her rheumatologist but she has not followed up with cardiology team. There has been no documentation of repeat echo or CTA at 6 months follow-up.
Discussion
The term dissection refers to the separation of the arterial wall layers, creating an additional channel for blood to flow, which is called a false lumen. This false lumen can collapse upon the true lumen causing occlusion.
Spontaneous coronary artery dissection is a rare but life-threatening manifestation predominantly in postpartum female patients and has been previously documented in case reports.1 Despite its predominance in females, men can also be affected by SCAD with a study showing 8.7% males among all SCAD patients.4,5 The exact pathogenesis of SCAD in pregnancy remains widely unknown. Studies postulate that the physiological cardiac demands, altered hormone levels, and the vascular strains of labour and delivery challenge the integrity of patient vasculature.6 These factors lead to the development of intimal wall tears and degeneration within coronary artery walls. The incidence of SCAD has been identified as 1–4% on CAG for all ACSs.2,7 Dissection most frequently involves the LAD coronary artery followed by the RCA and the left circumflex coronary artery.2 Spontaneous coronary artery dissection can be characterized into three distinct angiographic appearances and patterns.3 Type 1 SCAD is the pathognomonic appearance of SCAD with contrast dye staining of the arterial wall with multiple radiolucent lumens. Type 2 involves diffuse stenosis of varying severity and can be often missed or misdiagnosed on angiography. There is often subtle abrupt change in arterial calibre, with demarcation from normal diameter to diffuse narrowing.3 Type 3 SCAD is the most challenging to differentiate from atherosclerosis and angiographers need to have a high index of suspicion and employ intracoronary imaging for such cases.3
Spontaneous coronary artery dissection has a strong association with pregnancy and those suffering from connective tissue disorders including Ehlers-Danlos syndrome, Marfan syndrome, and FMD. A single-centre retrospective descriptive analysis was done on 116 patients with SCAD who had undergone medical genetics evaluation. Fifty nine out of 116 patients underwent genetic testing and 3 patients received a diagnosis of connective tissue diseases. The connective tissue diseases diagnosed among the cases were vascular Ehlers-Danlos syndrome, Marfan syndrome, and FMD. Twelve of the 59 patients had variants of unknown significance because those mutations were not known to cause disease.8
The variable clinical presentation of SCAD correlates to the degree in which coronary flow is impeded by the dissection. Patients may present asymptomatically, or unstable with angina, ACS, ventricular arrhythmia, or sudden cardiac death.9,10 Subsequent workup may demonstrate ischaemic changes on ECG or elevated cardiac biomarkers. A majority of cases will exhibit ST-segment changes in the left coronary circulation. Diagnosis can be confirmed with CAG but it can be missed on coronary angiogram.7 Coronary angiogram is only a 2D luminogram so even though it is excellent in assessing luminal narrowing but is poor in assessing the arterial wall where key abnormalities associated with SCAD typically occur.3 Multidetector computed tomography is another alternative for early detection and diagnosis of SCAD. In a study comparing MDCT with CAG, MDCT performed significantly better than CAG for coronary artery dissection detection (DCA). The sensitivity (98.6%), specificity (89.7%), and negative predictive value (98.4%) of MDCT for DCA were higher than those of CAG (77.8%, 79.4%, and 77.1%, respectively).11 Intravascular ultrasound (IVUS) and optical computed tomography (OCT) are both helpful tools in assessing and visualizing the arterial wall structure and composition. Both IVUS and OCT have benefits and downfalls associated with detection of arterial wall abnormalities.3 In studies evaluating the IVUS and OCT modalities, OCT was found to be more sensitive for visualizing and detection of characteristic features associated with SCAD as compared to IVUS especially in identifying tears and flaps.12,13
Current management of SCAD remains controversial without clear guidelines, especially regarding the use of dual antiplatelet therapy in patients not undergoing percutaneous coronary artery revascularization. The cornerstone of therapy for SCAD lies in controlling both heart rate and blood pressure to reduce the progression of the current dissection and recurrence. Despite the management above, the recurrence rate for SCAD is estimated to be 30%.14 However, a majority of patients have favourable outcomes with solely conservative therapy, without invasive measures of CABG or PCI. Patients with severe dissection or continual haemodynamic stability may require more aggressive interventions such as temporizing extracorporeal membrane oxygenation, PCI, or CABG.15 Pregnancy after SCAD should be managed with a multidisciplinary team.15
This case presents a novel manifestation of SCAD, implicating three major coronary arteries. Based on our review of the literature, the dissection of multiple coronary arteries is extremely rare.
Conclusion
Spontaneous coronary artery dissection is a rare condition that is well underdiagnosed. A thorough history and a high degree of suspicion are required for timely diagnosis. Spontaneous coronary artery dissection should be highly considered in patients with symptoms of ACS without traditional atherosclerotic risk factors, particularly in young postpartum females. Management for revascularization should be determined on a case by case manner depending on disease severity and presentation.
Lead author biography

Hassan Lak is a resident in Internal Medicine at the Cleveland Clinic. He aspire to be an interventional cardiologist.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
Joshua Parkera, MD, Bakhtawer Sirajb, MD, Connor Kerndtc, BS, Raunak Naird, MD, and Taha Ahmedd, MD
aRobert and Suzanne Tomsich Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH 44195, USA; bKing Edward Medical University, Lahore, Pakistan; cMichigan State University College of Osteopathic Medicine, East Lansing, MI, USA; and dDepartment of Medicine, Cleveland Clinic, Cleveland, OH, USA.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Contributor Information
Hassan Lak, Department of Medicine, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Karim Abdul Rehman, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Mail Code JB-1, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Wael A Jaber, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Mail Code JB-1, 9500 Euclid Avenue, Cleveland, OH 44195, USA; Section of Cardiovascular imaging, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Leslie Cho, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Mail Code JB-1, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
References
- 1. Moussa H, Movahedian M, Leon M, Sibai B.. Acute myocardial infarction due to coronary artery dissection in the postpartum period. AJP Rep 2015;5:e093–e096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pulivarthi S, Lawal T, Li D, Gurram M.. Spontaneous coronary artery dissection and acute myocardial infarction after cesarean section in a postpartum woman with untreated dyslipidemia. Heart Views 2014;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chandrasekhar J, Thakkar J, Starovoytov A, Mayo J, Saw J.. Spontaneous coronary artery dissection—a review. Cardiovasc Diagn Ther 2020;10:636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fahmy P, Prakash R, Starovoytov A, Boone R, Saw J.. Pre-disposing and precipitating factors in men with spontaneous coronary artery dissection. JACC Cardiovasc Interv 2016;9:866–868. [DOI] [PubMed] [Google Scholar]
- 5. Tan NY, Tweet MS.. Spontaneous coronary artery dissection: etiology and recurrence. Exp Rev Cardiovasc Ther 2019;17:497–510. [DOI] [PubMed] [Google Scholar]
- 6. Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A. et al. Pregnancy-associated acute myocardial infarction. Circulation 2014;129:1695–1702. [DOI] [PubMed] [Google Scholar]
- 7. Gilhofer TS, Saw J.. Spontaneous coronary artery dissection. Curr Opin Cardiol 2019;34:594–602. [DOI] [PubMed] [Google Scholar]
- 8. Henkin S, Negrotto SM, Tweet MS, Kirmani S, Deyle DR, Gulati R. et al. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart 2016;102:876–881. [DOI] [PubMed] [Google Scholar]
- 9. Luong C, Starovoytov A, Heydari M, Sedlak T, Aymong E, Saw J.. Clinical presentation of patients with spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2017;89:1149–1154. [DOI] [PubMed] [Google Scholar]
- 10. Krittanawong C, Kumar A, Virk HUH, Yue B, Wang Z, Bhatt DL.. Trends in incidence, characteristics, and in-hospital outcomes of patients presenting with spontaneous coronary artery dissection (from a national population-based cohort study between 2004 and 2015). Am J Cardiol 2018;122:1617–1623. [DOI] [PubMed] [Google Scholar]
- 11. Sun Y, Mao D, Lu F, Chen Y, Shi K, Qi L. et al. Diagnosis of dissection of the coronary artery dissection by multidetector computed tomography. J Comput Assist Tomogr 2015;39:572–577. [DOI] [PubMed] [Google Scholar]
- 12. Paulo M, Sandoval J, Lennie V, Dutary J, Medina M, Gonzalo N. et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging 2013;6:830–832. [DOI] [PubMed] [Google Scholar]
- 13. Poon K, Bell B, Christopher Raffel O, Walters DL, Jang I-K.. Spontaneous coronary artery dissection: utility of intravascular ultrasound and optical coherence tomography during percutaneous coronary intervention. Circ Cardiovasc Interv 2011;4:e5–e7. [DOI] [PubMed] [Google Scholar]
- 14. Krittanawong C, Kumar A, Virk HUH, Wang Z, Johnson KW, Yue B. et al. Recurrent spontaneous coronary artery dissection in the United States. Int J Cardiol 2020;301:34–37. [DOI] [PubMed] [Google Scholar]
- 15. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE. et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


