Abstract
Aims
Acute coronary syndromes with intact fibrous cap (IFC-ACS), i.e. caused by coronary plaque erosion, account for approximately one-third of ACS. However, the underlying pathophysiological mechanisms as compared with ACS caused by plaque rupture (RFC-ACS) remain largely undefined. The prospective translational OPTICO-ACS study programme investigates for the first time the microenvironment of ACS-causing culprit lesions (CL) with intact fibrous cap by molecular high-resolution intracoronary imaging and simultaneous local immunological phenotyping.
Methods and results
The CL of 170 consecutive ACS patients were investigated by optical coherence tomography (OCT) and simultaneous immunophenotyping by flow cytometric analysis as well as by effector molecule concentration measurements across the culprit lesion gradient (ratio local/systemic levels). Within the study cohort, IFC caused 24.6% of ACS while RFC-ACS caused 75.4% as determined and validated by two independent OCT core laboratories. The IFC-CL were characterized by lower lipid content, less calcification, a thicker overlying fibrous cap, and largely localized near a coronary bifurcation as compared with RFC-CL. The microenvironment of IFC-ACS lesions demonstrated selective enrichment in both CD4+ and CD8+ T-lymphocytes (+8.1% and +11.2%, respectively, both P < 0.05) as compared with RFC-ACS lesions. T-cell-associated extracellular circulating microvesicles (MV) were more pronounced in IFC-ACS lesions and a significantly higher amount of CD8+ T-lymphocytes was detectable in thrombi aspirated from IFC-culprit sites. Furthermore, IFC-ACS lesions showed increased levels of the T-cell effector molecules granzyme A (+22.4%), perforin (+58.8%), and granulysin (+75.4%) as compared with RFC plaques (P < 0.005). Endothelial cells subjected to culture in disturbed laminar flow conditions, i.e. to simulate coronary flow near a bifurcation, demonstrated an enhanced adhesion of CD8+T cells. Finally, both CD8+T cells and their cytotoxic effector molecules caused endothelial cell death, a key potential pathophysiological mechanism in IFC-ACS.
Conclusions
The OPTICO-ACS study emphasizes a novel mechanism in the pathogenesis of IFC-ACS, favouring participation of the adaptive immune system, particularly CD4+ and CD8+ T-cells and their effector molecules. The different immune signatures identified in this study advance the understanding of coronary plaque progression and may provide a basis for future development of personalized therapeutic approaches to ACS with IFC.
Trial registration
The study was registered at clinicalTrials.gov (NCT03129503).
Keywords: Acute coronary syndrome, Plaque rupture, Optical coherence tomography, CD8+, T cells, Shear stress, Endothelial erosion
Graphical Abstract
 Listen to the audio abstract of this contribution See page 3561 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa721)
Listen to the audio abstract of this contribution See page 3561 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa721)
Introduction
Acute coronary syndromes (ACS) remain the most devastating clinical manifestation of coronary artery disease (CAD).1 Autopsy studies indicated that ACS frequently arise from rupture of the coronary fibrous cap (RFC = ruptured fibrous cap), whereby the highly thrombogenic necrotic core is exposed to the circulating blood, triggering thrombus formation, and threatening myocardial perfusion.1–4 The interplay between inflammation and coagulation at sites of vulnerable atherosclerotic plaques prone to rupture has undergone extensive investigation and the concept of plaque rupture causing ACS has gained wide acceptance.3,5,6 However, autopsy studies have suggested another mechanism of ACS, i.e. coronary plaque erosion, characterized by thrombus formation on a plaque with intact fibrous cap (IFC = intact fibrous cap) and endothelial injury on the luminal surface of the plaque.2,5,7–9
Contemporary intravascular imaging studies implicate IFC in up to one-third of ACS.10–12 The underlying pathophysiological mechanisms of IFC-ACS remain incompletely understood. As effective lipid lowering and other current interventions alter atherosclerotic plaque composition in a manner that may render them less susceptible to rupture, plaque erosion may rise as a mechanism of ACS and importantly contribute to residual atherothrombotic risk.5,13,14 A better understanding of pathophysiological pathways leading to IFC-ACS may provide the basis for personalized treatment strategies in ACS patients, as the recent EROSION trial started to illustrate.15 Optical coherence tomography (OCT), a high-resolution intravascular imaging modality, permits in vivo visualization of the vessel wall and a detailed characterization of the mechanisms of ACS.16,17 The present study therefore sought to define immunological patterns of IFC- vs. RFC-culprit lesions by combining for the first time in vivo culprit plaque analysis by OCT and a detailed analysis of immunological and biochemical processes directly at the culprit coronary site in a cohort of consecutive ACS patients.
Methods
Study design and patients
The prospective, multicentre OPTICO-ACS (OPTIcal-COherence Tomography in Acute Coronary Syndrome) study was co-ordinated at the Charité—University Medicine Berlin and the Berlin Institute of Health (BIH), Germany. A total of 170 patients presenting with ACS [Non-ST segment elevation myocardial infarction (NSTE-ACS)18 or ST segment elevation myocardial infarction (STE-ACS19] undergoing percutaneous coronary intervention (PCI) of a clearly determinable culprit lesion in the absence of active inflammatory or malignant diseases were consecutively enrolled (Supplementary material online, S1A). Ethical approval was obtained from the local ethics committee (No. EA1/270/16) and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participating subjects and the study was registered at ClinicalTrials.gov (NCT03129503).
Optical coherence tomography image acquisition and analysis
All OCTs were analysed for the underlying ACS-causing culprit pathophysiology by two independent core labs and according to the predefined study-specific standard operating procedure using the Medis QIvus 3.0 (Medis Medical Imaging Service, Leiden, The Netherlands; see Supplementary material online, S1B and C). The rate of absolute agreement/inter-observer variability for the diagnosis RFC-ACS was 94.3% (ĸ = 0.82) and for IFC-ACS 95.4% (ĸ = 0.86), and the rate of absolute agreement/intra-observer variability for RFC-ACS 98.5% (ĸ = 0.96) and 99.2% (ĸ = 0.98) for IFC-ACS, respectively. Cases with disagreement on the type of the ACS-causing culprit lesion were reassessed to reach agreement with respect to all published data and definitions20 (Supplementary material online, S1B and C and S2). Cases without consensus between the two core labs (n = 5; 3.6% of all OCT scans) were excluded from the analysis.
Biosample collection, analysis, flow cytometry-based immunophenotyping, plasma cytokine profiling, thrombus analysis, and plasma microvesicle quantification
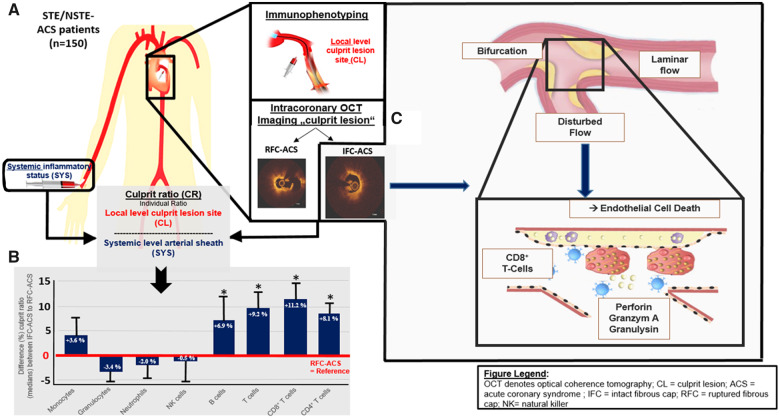
After diagnostic angiography and before PCI, systemic blood samples (SYS) were obtained by aspiration of 30 mL blood from the arterial sheath (Take home figure). Coronary thrombi and blood samples were obtained directly from the ACS-causing culprit lesion (LOC) by aspiration of 30 mL blood using the Export Advance™ aspiration catheter system (Medtronic, Minneapolis, MN, USA, Take home figure). After filtering thrombotic components from the LOC samples, flow cytometry-based immunophenotyping was immediately performed. To determine culprit lesion inflammatory activation, a ‘culprit ratio’ (CR) was calculated by normalizing each individual inflammatory level of the culprit lesion (LOC) to those measured systemically at the level of arterial sheath (SYS; Take home figure, Supplementary material online, S1A). Aspirated culprit thrombi underwent immunofluorescence analysis.21,22Supplementary material online, S1Aprovides more detailed information.
Take home figure.
In total, 150 patients were included into the prospective translational OPTICO-ACS study (A) and its culprit lesions were characterized by OCT as well as by local and systematic immunophenotyping: Culprit lesion ratios revealed differential immunological signature with an enrichment in T-lymphocytes, both CD4+and CD8+ T-cell subpopulations (B) as well as increased T-cell effector molecules at the culprit site distinguishing acute coronary syndromes with intact fibrous cap from ruptured fibrous cap-acute coronary syndrome. Since acute coronary syndromes with intact fibrous cap-lesion were often located at bifurcations, endothelial cells were subjected to culture in disturbed laminar flow conditions (C), i.e. to simulate coronary flow near a bifurcation, demonstrated an enhanced adhesion of CD8+T cells. Finally, both CD8+T cells and their cytotoxic effector molecules caused endothelial cell death, a key pathophysiological mechanism in acute coronary syndromes with intact fibrous cap.
Analysis of endothelial cell death
1 × 105 flow-sorted CD8+ or CD14+ leucocytes were pre-activated by human TransAct (MACS Miltenyl Biotech; Bergisch Gladbach, Germany) and added to preconditioned human aortic endothelial cells (HAECs, Lonza, Basel, Switzerland) for a duration of 24, 48, and 72 h, before cells were harvested and single-cell suspensions were labelled. Similarly, the effect of granulysin, perforin, and granzyme A (Enzo Life Sciences, Loerrach, Germany) in different combinations and at a concentration of 2 ng/mL—as detected in human plasma samples—on HAEC cell death after 72 h was assessed (for details see Supplementary material online, S1A).
Adhesion to flow-primed endothelium
To investigate adhesion to flow-primed endothelium, HAECs were grown to confluence in double-y-shaped microchannel slides (Ibidi, Martinsried, Germany) exposed to 12 dyn/cm2 flow for 48 h prior to the experiment. Flow-sorted CD8+T cells and CD14+monocytes were labelled with DiD and DiO (both Invitrogen, Carlsbad, USA), respectively, and 1 × 106 labelled cells were added to the flow reservoir for 30 min. After flow-termination, non-adherent cells were washed out, cells were fixed with 4% paraformaldehyde and the number of cells adhering in the nine laminar-flowed fields was assessed by fluorescence phase-contrast microscope (for details see Supplementary material online, S1A).
Statistical analysis
Details are provided in Supplementary material online, S1A. In brief, median regression analysis was used to compare the local and peripheral levels as well as the CR for immune cells and cytokines among the three groups. Pairwise group comparisons were conducted by a post hoc test after fitting the median regression model. Multivariable median regression models were fitted to analyse the association between the CR of T cells and cytokines within the three groups and other clinical variables of interest. Panel analysis was conducted by using the LASSO (least absolute shrinkage and selection operator) method for variable selection. Statistical analyses were performed using STATA 12.1 (StataCorp, TX, USA).
Results
Baseline characteristics
Out of 170 ACS patients undergoing OCT imaging, 130 patients were included in the final analysis. Patients were excluded due to suboptimal image quality or massive thrombus burden (n = 28), angiographic non-detectable in-stent thrombosis (n = 2), spontaneous coronary artery dissection (n = 2), calcified nodules (n = 3), and inconclusive findings on OCT imaging across the two independent OCT core labs (n = 5, Supplementary material online, S2). Plaque erosion (IFC-ACS) and plaque rupture (RFC-ACS) were identified in 32 (24.6%) and 98 (75.4%) patients, respectively.
A total of 87 (66.9%) patients presented with STE-ACS. Table 1 summarizes the baseline clinical and laboratory data. Patients were predominantly men (n = 103; 79.2%) and mostly (n = 124; 95.4%) presented with the first CAD event. The prevalence of cardiovascular risk factors was high with hypertension and type 2 diabetes observed in 95 (73.1%) and 25 (19.2%) patients, respectively. The occurrence of cardiovascular risk factors, the type of index ACS events, the localization of the culprit lesion, TIMI flow rate, laboratory data including systemic inflammatory markers and maximum creatinine kinase (CK) levels, as well as left ventricular systolic function did not differ among patients with RFC-ACS and IFC-ACS, except for dyslipidaemia (75% vs. 57%; P = 0.05), respectively.
Table 1.
Baseline characteristics of the study population (n = 130)
| RFC-ACS (n = 98) | IFC-ACS (n = 32) | P-value | |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 64.4 ± 12 | 63.0 ± 10 | 0.44 |
| Male, n (%) | 76 (78%) | 27 (84%) | 0.41 |
| Family history for CAD, n (%) | 39 (45%) | 14 (48%) | 0.79 |
| Smoking, n (%) | 21 (23%) | 7 (25%) | 0.50 |
| Diabetes mellitus, n (%) | 18 (18%) | 7 (22%) | 0.66 |
| Arterial hypertension, n (%) | 72 (74%) | 23 (72%) | 0.86 |
| Dyslipidaemia, n (%) | 73 (75%) | 17 (57%) | 0.05 |
| BMI, mean ± SD | 27.2 ± 4 | 27.5 ± 4 | 0.69 |
| Previous history of PCI, n (%) | 4 (4.1%) | 2 (6.3%) | 0.61 |
| Laboratory data | |||
| Total cholesterol, mg/dL | 187 ± 43 | 172 ± 42 | 0.15 |
| LDL cholesterol, mg/dL | 122 ± 36 | 112 ± 40 | 0.25 |
| Serum creatinine, mg/dL | 0.9 ± 0.2 | 1.00 ± 0.2 | 0.12 |
| Hemoglobin, g/dL | 14.5 ± 1.4 | 14.4 ± 2.0 | 0.92 |
| Leucocytes, per nL | 11.4 ± 3.7 | 11.2 ± 4.1 | 0.78 |
| Lymphocytes, per nL | 2.9 ± 3.7 | 2.3 ± 0.7 | 0.72 |
| hs-CRP, mg/L | 5.6 ± 15.4 | 6.4 ± 20.3 | 0.80 |
| ACS characteristics | |||
| Presentation as STE-ACS, n (%) | 64 (65%) | 23 (72%) | 0.49 |
| CK peak (U/I), mean ± SD | 1498 ± 1444 | 1259 ± 1441 | 0.42 |
| LV-EF at discharge (%), mean ± SD | 56 ± 10 | 54 ± 11 | 0.35 |
| TIMI flow, n (%) | |||
| 0 | 52 (72%) | 12 (71%) | 0.66 |
| 1 | 8 (11%) | 3 (18%) | |
| 2 | 10 (14%) | 1 (6%) | |
| 3 | 2 (3%) | 1 (6%) | |
| Culprit Segment TIMI flow <1, n (%) | 42 (43%) | 11 (34%) | 0.40 |
| Culprit vessel, n (%) | |||
| LM | 0 (0%) | 0 (0%) | 0.69 |
| LAD | 48 (49%) | 18 (56%) | |
| LCX | 14 (14%) | 3 (9%) | |
| RCA | 36 (37%) | 11 (34%) | |
| Involved culprit segment, n (%) | |||
| Proximal | 40 (41%) | 14 (44%) | 0.77 |
| Middle | 44 (45%) | 15 (47%) | |
| Distal | 14 (14%) | 3 (9%) | |
| Number of diseased vessels, n (%) | |||
| 1 | 46 (47%) | 18 (56%) | 0.45 |
| 2 | 33 (34%) | 7 (22%) | |
| 3 | 19 (19%) | 7 (22%) | |
| Medical therapy before admission | |||
| Aspirin 250 mg, n (%) | 96 (98%) | 31 (97%) | 0.41 |
| (LMW-)Heparin, n (%) | 97 (99%) | 30 (94%) | 0.09 |
|
Antiplatelet therapy, n (%) (Loading dose Clopidogrel/Prasugrel/Ticagrelor) |
15 (15%) | 2 (7%) | 0.18 |
| PCI characteristics | |||
| Total stented length (mm) | 34.0 ± 16.4 | 29.6 ± 15.2 | 0.18 |
| Largest stent diameter (mm) | 3.3 ± 0.5 | 3.2 ± 0.5 | 0.38 |
BMI, body mass index; CK, creatinine kinase; CRP, C-reactive protein; HCT, hematocrit; IFC-ACS, intact fibrous cap-acute coronary syndrome; LAD, left anterior descending artery; LCX, left circumflex artery; LDL, low-density lipoprotein; LM, left main; LMW, low-molecular-weight; LVEF, left ventricular ejection fraction; n, number; PCI, percutaneous coronary intervention; RCA, right coronary artery; RFC-ACS, ruptured fibrous cap-acute coronary syndrome; SD, standard deviation; STE-ACS, ST-elevation myocardial infarction; TIMI, thrombolysis in myocardial Infarction.
Optical coherence tomography findings
Lumen reduction as assessed by minimum lumen area23 did not differ between RFC-ACS and IFC-ACS (1.82 ± 0.6 mm2 vs. 1.78 ± 0.8 mm2, P = 0.74, Table 2). Compared with RFC-ACS, IFC-ACS culprit plaques were less often lipid-rich (91% vs. 100%; P = 0.01), had less calcification (mean calcium arc 32 ± 33° vs. 48 ± 39°; P = 0.01), and overlying thrombus (thrombus score23 85 ± 74 vs. 126 ± 87; P = 0.02), and fewer were thin-cap fibroatheroma (TCFA: 50% vs. 98%; P < 0.01). The IFC-ACS lesions were more frequently located near coronary bifurcations (61.3% vs. 29.6%; P = 0.01), yielding a shorter lesion-to-bifurcation distance (3.9 ± 3.9 mm vs. 6.3 ± 4.6 mm; P = 0.01, Table 2).
Table 2.
Optical coherence tomography characteristics of the ACS-causing culprit lesion within the study cohort (n = 130)
| RFC-ACS (n = 98) | IFC-ACS (n = 32) | P-value | |
|---|---|---|---|
| OCT-culprit lesion characteristics | |||
| MLA (mm²), mean ± SD | 1.82 ± 0.6 | 1.78 ± 0.8 | 0.74 |
| Thrombus characteristics | |||
| CL-associated thrombus formation, n (%) | 97 (100%) | 32 (100%) | 1.0 |
| Thrombus score, mean ± SD | 126 ± 87 | 85 ± 74 | 0.02 |
| Thrombus type, n (%) | |||
| Red | 2 (2%) | 0 (0%) | < 0.001 |
| White | 26 (27%) | 22 (69%) | |
| Mixed | 70 (71%) | 10 (31%) | |
| Culprit plaque characteristics | |||
| Fibroatheroma, n (%) | 97 (99%) | 29 (94%) | 0.08 |
| Lipid-rich plaque, n (%) | 98 (100%) | 29 (91%) | 0.01 |
| Thin-attenuated plaque, n (%) | 96 (98%) | 16 (50%) | <0.001 |
| Mean FC thickness (µm), mean ± SD | 55.1 ± 7 | 80.1 ± 55 | <0.001 |
| Lipid index, mean ± SD | 3080.8 ± 1318.9 | 2007.0 ± 1377.8 | <0.001 |
| Calcification | |||
| Mean calcium arc, mean ± SD) | 48 ± 39 | 32 ± 33 | 0.04 |
| Mean calcified length, mean ± SD | 3.85 ± 4.3 | 1.08 ± 6.6 | 0.01 |
| Relation to bifurcation | |||
| Distance to nearest branch, mean ± SD | 6.27 ± 4.6 | 3.92 ± 3.9 | 0.01 |
| Branch within 3 mm, n (%) | 29 (29.6%) | 19 (61.3%) | 0.01 |
| Details of culprit lesions within/near bifurcations | |||
| Bifurcation: LAD/Diagonal branch, n (%) | 15 (51.7%) | 11 (57.9%) | 0.47 |
| Bifurcation: LCX/Marginal-branch, n (%) | 5 (17.2%) | 1 (5.3%) | |
| Bifurcation: Other location, n (%) | 9 (31.1%) | 7 (36.8%) | |
| Bifurcation: Medina 1-1-0, n (%) | 6 (20.7%) | 1 (5.3%) | 0.14 |
| Bifurcation: Medina 1-1-1, n (%) | 6 (20.7%) | 2 (10.5%) | 0.36 |
| Bifurcation: Medina 0-1-0/1-0-0, n (%) | 12 (51.7%) | 9 (47.4%) | 0.77 |
| Bifurcation: Medina 0-0-1, n (%) | 5 (17.2%) | 7 (36.8%) | 0.13 |
FC, fibrous cap; IFC-ACS, intact fibrous cap-acute coronary syndrome; MLA, minimum lumen area; n, number; RFC-ACS, ruptured fibrous cap-acute coronary syndrome; SD, standard deviation; TCFA, thin-cap fibroatheroma.
Concentration of T-lymphocytes and related cytokines
A higher CR of T-lymphocytes (P = 0.02) was observed in patients with plaque erosions (IFC-ACS), including both CD4+ T-cells (1.05 vs. 0.98; P = 0.02) and CD8+ T-cells (1.11 vs. 1.00; P = 0.01, Table 3; Supplementary material online, S3). The observed relations remained significant after adjusting for systemic inflammatory markers, TIMI flow grade, and type of culprit vessel (Supplementary material online, S4). Neutrophils, granulocytes, and natural killer cells only showed trends towards lower levels in patients with RFC-ACS (Table 3 and Supplementary material online, S3). In multivariable linear regression analysis, both plaque erosion (β = 0.256; P < 0.01) and thicker fibrous caps (β = −0.002; P = 0.01) emerged as independent predictors of an increased coronary-to-peripheral CD8+ T-lymphocyte ratio (Figure 1 andTable 4). Thrombotic material harvested at sites of IFC-ACS displayed a higher percentage of CD8+ T-lymphocytes than that from sites of RFC-ACS (Supplementary material online, S5A). Patients with plaque erosion (IFC-ACS) had a higher coronary-to-peripheral ratio of T-lymphocyte-associated extracellular circulating MV and CD8+ T-lymphocytes-associated MV; Supplementary material online, S5B).
Table 3.
Culprit ratio of different immune cells
| RFC-ACS (n = 98) | IFC-ACS (n = 32) | P-value | % IFC-ACS vs. RFC-ACS | |
|---|---|---|---|---|
| Innate immunity | ||||
| Monocytes | 0.99 (0.9) | 1.02 (0.9) | 0.19 | 103.6 |
| Granulocytes | 0.99 (0.9) | 0.96 (0.9) | 0.32 | 96.6 |
| Neutrophils | 0.99 (0.9) | 0.97 (0.9) | 0.44 | 98.0 |
| NK cells | 1.00 (0.7) | 1.00 (0.9) | 0.24 | 99.5 |
| Adaptive immunity | ||||
| B cells | 0.96 (0.8) | 1.03 (0.9) | 0.07 | 106.9 |
| T cells | 0.98 (0.9) | 1.07 (1.0) | 0.02 | 109.2 |
| CD8+ T cells | 1.00 (0.8) | 1.11 (1.0) | 0.01 | 111.2 |
| CD4+ T cells | 0.98 (0.9) | 1.05 (1.0) | 0.02 | 108.1 |
All values are expressed as median (25th percentile); % change based on median.
IFC-ACS, intact fibrous cap-acute coronary syndrome; NK cells, natural killer cells; RFC-ACS, ruptured fibrous cap-acute coronary syndrome; SD, standard deviation.
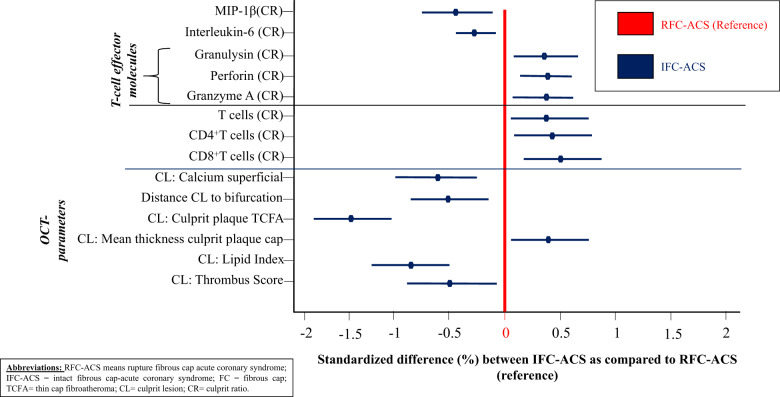
Figure 1.
Hierarchical clustering of Pearson’s correlations including parameters of immunophenotyping and OCT-parameters reveals significant differences among patients with acute coronary syndromes with intact fibrous cap (blue lines depicting standard differences) and ruptured fibrous cap-acute coronary syndrome (reference: red line).
Table 4.
Multivariate regression analysis of different factors influencing culprit ratio of CD8+T-cells
| Unstandardized estimates |
Standardized estimates |
||||
|---|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | P-value | |
| RFC-ACS vs. IFC-ACS | 0.26 | 0.13; 0.39 | 0.26 | 0.06; 0.53 | <0.001 |
| MLA, mm2 | −0.03 | −0.10; 0.05 | −0.10 | −0.33; 0.13 | 0.45 |
| Thrombus score | 0.26 | −0.22; 0.73 | 0.27 | −0.02; 0.52 | 0.29 |
| Mean fibrous cap thickness, µm | −0.002 | −0.004; −0.001 | −0.18 | −0.42; −0.05 | 0.01 |
| Presence of TCFA | 0.001 | −0.008; 0.009 | 0.02 | −0.22; 0.25 | 0.92 |
| Age, years | 0.001 | −0.003; 0.005 | 0.11 | −0.12; 0.33 | 0.61 |
| Time from beginning of symptoms to PCI, min | −0.001 | −0.002; 0.001 | −0.07 | −0.31; 0.17 | 0.68 |
| LVEF, % | −0.002 | −0.036; 0.032 | −0.03 | −0.26; 0.21 | 0.92 |
| Leucocytes, nL | 0.006 | −0.008; 0.020 | 0.19 | −0.06; 0.44 | 0.37 |
| hs-CRP, mg/L | −0.001 | −0.003; 0.004 | −0.06 | −0.31; 0.20 | 0.84 |
| CK Max, U/L | −0.001 | −0.001; 0.001 | −0.07 | −0.32; 0.17 | 0.79 |
| Distance to nearest site-branch, mm | 0.001 | −0.015; 0.016 | 0.04 | −0.31; 0.38 | 0.93 |
| Site-branch within 3 mm to CL | −0.02 | −0.16; 0.12 | −0.12 | −0.44; 0.20 | 0.79 |
CI, confidence interval; CK, creatinine kinase; CL, culprit lesion; hs-CRP, high-sensitive C-reactive protein; IFC-ACS, intact fibrous cap-acute coronary syndrome; LVEF, left ventricular ejection fraction; MLA, minimum lumen area; n, number; RFC-ACS, ruptured fibrous cap-acute coronary syndrome; TCFA, thin-cap fibroatheroma.
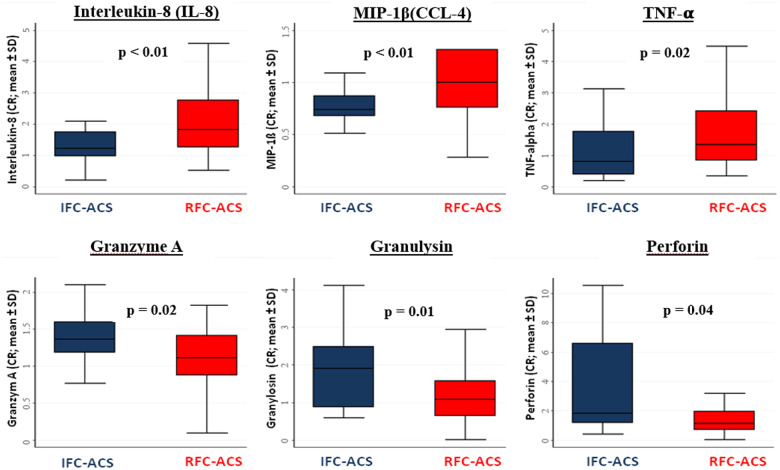
Investigating a panel of cytokines reflecting different immunomodulatory pathways (Supplementary material online, S6) plaque rupture was associated with an increased culprit lesion gradient of IL-8 (P = 0.01), TNF-α (P = 0.02), MIP-1ß (P < 0.01) (Figure 2), while IFC-ACS displayed higher ratios for cytotoxic effector molecules, including granzyme A (P = 0.02), granulysin (P = 0.01), and perforin (P = 0.04) (Figure 2). Differences among groups remained significant after adjustment for systemic inflammatory markers including hs-CRP and leucocytes and TIMI flow grade (Supplementary material online, S4).
Figure 2.
Box plots depicting significant differences in culprit ratio of effector molecules of the innate immune response (Interleukin-8; MIP-1ß, TNF-alpha) and effector molecules involved in the adaptive immune response (Granzyme A; Granulysin, Perforin) between ruptured fibrous cap-acute coronary syndrome and acute coronary syndromes with intact fibrous cap.
Distinct patterns of acute coronary syndromes with intact fibrous cap vs. ruptured fibrous cap-acute coronary syndrome
To discern a distinct clinical pattern for both IFC-ACS and RFC-ACS, hierarchical clustering was performed including clinical characteristics, OCT data and immunological findings. Based on hierarchical clustering, patients with IFC-ACS localized closer to bifurcations, had fewer features of advanced atherosclerotic plaques, lower thrombus burden, increased CD4+ and CD8+ T-lymphocyte and Granzyme A, granulysin, and perforin CR (Figure 1 and Supplementary material online, S6).
CD8+ T-lymphocytes and associated cytokine promote endothelial cell death in vitro
In ex vivo co-culture experiments, we explored whether CD8+ T-lymphocytes or their effector molecules granulysin, granzyme A, and perforin mediate endothelial cell injury, known to be principally involved in the development of plaque erosion. The rate of HAECs positive for the DNA dye Sytox Orange, an indication of compromised cell membrane integrity, significantly increased in co-culture with CD8+ T-lymphocytes as compared with monocytes from the same donor (P < 0.05, Supplementary material online, S7A). In line with this, stimulation of HAECs with a combination of granulysin, granzyme A, and perforin in the concentration range measured in plasma samples increased endothelial cell death, while each molecule alone did not exert any effect (P < 0.01, Supplementary material online, S7B and S8).
In vitro adhesion of CD8+ T-lymphocytes in a model of coronary shear stress
The adhesion patterns of CD8+ T-lymphocytes on flow-preconditioned endothelium were determined in a flow chamber, mimicking the hydrodynamic conditions at sites of coronary bifurcations and curvatures (Supplementary material online, S9 and S10). The percentage of adherent CD8+ T-lymphocytes, but not monocytes, increased significantly (P < 0.01) under conditions of disturbed as compared with ordinary laminar flow (Supplementary material online, S10A–C). Incubation with anti-integrin-β2 or an anti-integrin-α4 blocking antibody reversed this effect (Supplementary material online, S11B and C), highlighting the direct interaction between flow-conditioned endothelium and homing of T-lymphocytes. This effect was not seen under laminar flow conditions or when monocyte adhesion was investigated (Supplementary material online, S11).
Discussion
The prospective, translational OPTICO-ACS study represents an integrated immune and high-resolution intracoronary imaging characterization of distinct ACS entities in a large cohort of ACS patients (Take home figure). In particular, this study demonstrated that IFC caused 24.6% and RFC-ACS 75.4% of ACS events, with IFC-CL being characterized by lower lipid content, less calcification, a thicker overlying fibrous cap, and a co-localization with bifurcation lesions. Second, the microenvironment of IFC-ACS lesions demonstrated a particular enrichment in CD4+ T-lymphocytes, CD8+ T-lymphocytes, and T-cell-associated MV and effector molecules. Consistently, a significant higher amount of CD8+ T-lymphocytes was detectable in thrombi aspirated from IFC-ACS culprit sites. Furthermore, endothelial cells subjected to culture in disturbed laminar flow conditions demonstrated an enhanced adhesion of CD8+ T-lymphocytes.
Hence, this study emphasizes a novel mechanism in the pathogenesis of IFC-ACS, favouring participation of the adaptive immune system that can promote endothelial cell death (Take home figure). CD8+ T-lymphocytes congregated particularly near IFC-ACS lesions, which typically localized near coronary bifurcations, sites of disturbed flow, and low endothelial shear stress that can also promote atherosclerotic plaque formation.24–27 Enhanced integrin-dependent endothelial adhesion of CD8+T-lymphocytes occurred in vitro under conditions of disturbed laminar flow, emphasizing the importance of wall shear stress in the progression of atherosclerotic plaque formation.28
Together these data indicate a novel plausible immunologically driven pathophysiological mechanism for ACS caused by culprit plaques with intact fibrous cap (IFC-ACS), accountable for about one-third of ACS cases.10,11,16
Anatomical characteristics of acute coronary syndromes with intact fibrous cap
The OPTICO-ACS study allowed simultaneous characterization of IFC-ACS as compared with RFC-ACS at both the morphological and inflammatory level. In this study of consecutive cases, plaque erosion (IFC-ACS) caused ACS in about one-fourth of patients. The lower prevalence of IFC-ACS observed in OPTICO-ACS than in other recent series may be due to the stringent use of established OCT diagnostic criteria and external validation by a second OCT core lab. Indeed, most ACS-studies diagnosed IFC-ACS by exclusion.11,29 In contrast to other studies such as EROSION and post-mortem investigations,11,30,31 the baseline clinical risk and type of ACS event at presentation did not differ between the two types of culprit lesions, besides an increased prevalence of dyslipidaemia in RFC-ACS patients.7 The lack of statistically significant differences between clinical characteristics of patients with IFC- and RFC-ACS may be due to the study design, which aimed at a detailed morphological and immunological characterization of IFC-ACS rather than being adequately powered to detect differences in clinical characteristics.
The lesions that provoked IFC-ACS had a lower lipid content and less superficial calcium than those that caused RFC-ACS. In accordance with current concepts,32 TCFA predominated in RFC-ACS, but not IFC-ACS. The lower prevalence of TCFA in IFC-ACS, along with33 the predominance of IFC-ACS lesions near coronary bifurcations6,11 support the hypothesis that these two substrates for coronary artery thrombosis involve distinct pathophysiological mechanisms and implicate the local haemodynamic environment as a determinant.
Distinct immunological characteristics of intact fibrous cap vs. ruptured fibrous cap-acute coronary syndrome
The OPTICO-ACS study tested the hypothesis that IFC-ACS and RFC-ACS involve distinct immunological mechanisms. Our novel results identify a specific immunological signature, characterized by accumulation of T-lymphocytes, especially CD8+ T-lymphocytes and related effector molecules at sites of IFC-ACS.34,35 This finding remained significant after adjustment for potential confounders and received support from analyses of thrombus material retrieved from the culprit artery. The in vitro results demonstrated that CD8+ T-lymphocytes and T-cell-related cytotoxic effector molecules, such as granulysin, perforin, and granzyme A can contribute directly to endothelial cell death. These findings agree with prior experimental and post-mortem studies, which identified distinct inflammatory processes in IFC-ACS lesions.32 Quillard et al.36 demonstrated neutrophil accumulation after endothelial cell denudation in areas of superficial plaques as well as Toll-like receptor-2 (TLR-2)-dependent endothelial cell death. Those findings highlighted the role of leucocyte-endothelial cell interactions during plaque progression in IFC-ACS, particularly the polymorphonuclear granulocyte, an innate immune effector cell.2,7,8,36,37
The novel findings presented here implicate that the adaptive immune system, notably CD8+ T-lymphocytes, may operate in tandem with innate immune pathways in IFC-ACS. Earlier autopsy studies concluded that IFC-ACS did not involve inflammation, focused mostly on macrophages.3 However, our recent findings suggest a role for CD8+T-cells in IFC-ACS, immune pathways distinct from macrophages, a cell type with established functions in RFC-ACS.5,38 There are rather few previous studies that investigated potential immunological differences between RFC-ACS and IFC-ACS, which were mainly focused on cytokine levels, had a limited sample size and measured largely systemic levels.29,39–41
As demonstrated in OPTICO-ACS as well as other studies,29 local immunological alterations do not necessarily correspond with differences in cytokine concentrations measured in the systemic circulation. Future studies are needed to explore intracellular mechanisms induced by T-cell effector molecules in the setting of ACS.
Endothelial flow alterations and acute coronary syndromes with intact fibrous cap
Chronic hydrodynamic disturbances experienced by the endothelium can modulate adhesion molecule expression, plaque formation, progression, and destabilization.28,42,43 Plaque size, composition and geometric changes, along with adverse vessel remodelling alter both endothelial shear stress, thus impinging upon the plaque itself44 and influencing the biomechanical plaque stability.45 The proximity of IFC-ACS lesions to coronary bifurcations—also—provides further evidence linking shear stress alterations to the occurrence of IFC-ACS,24–27,42,46,47 a concept further supported by enhanced adhesion of CD8+ T-lymphocytes in response to disturbed laminar flow shown by the present in vitro experiments. Although the role of CD8+ T-lymphocytes in the pathogenesis of atherosclerotic plaque formation has undergone extensive investigation,48,49 few studies have examined the impact of differential flow conditions on the enhancement of inflammation and plaque activation resulting in ACS. Animal and histopathologic studies interrogating the innate immune system implicated a TLR-2-dependent mechanism regulated by shear stress in the pathophysiology of IFC-ACS.5,50–52 Shear stress-dependent mechanisms could mediate transmigration of T-lymphocytes.53 Its contributions, however, have not received enough attention in the context of different culprit lesion morphologies yet. This study suggests that shear stress-depended accumulation and activation of CD8+ T-lymphocytes and subsequent T-lymphocyte-mediated endothelial cell damage may participate pivotally in the pathogenesis of IFC-ACS in humans. As IFC-ACS lesions do not only occur near coronary bifurcations, mechanisms in addition to flow alterations may influence IFC-ACS events. Of note, a recent study has reported an increased gene expression of hyaluronidase 2 in mononuclear cells from patients with IFC-ACS54 and a potential role of hyaluronan for T-cell biology.55
Limitations of the study
Despite predefined criteria excluding patients with active inflammatory, autoimmune, or malignant disease, as well as patients under anti-inflammatory therapy, we cannot rule out that patients with inapparent co-morbidities may have influenced the results. Second, as thrombus aspiration was performed in the culprit vessel, the aspirated material may not represent exclusively the culprit lesion microenvironment. Furthermore, pre-dilatation, needed to restore coronary blood flow in some patients, may have affected the integrity of the culprit plaque as well as the local inflammatory microenvironment. Third, since OCT imaging was not possible in left main disease, coronary bypass graft disease, at distal locations, or when identification of the culprit lesion was unclear, the findings of this study may not extend to these patient populations. Fourth, as the definition of IFC-ACS and RFC-ACS required agreement between two independent OCT core labs, the exclusion of unclear cases may have introduced selection bias, and patients with particularly high thrombus burden may have thus not entered this study. Finally, this study presents CR as an intra-individual normalization of the local culprit microenvironment for systemic inflammatory effects, thereby accounting for the heterogeneity of immune responses in the study population and minimizing potential systemic confounding. Other methods of normalization, e.g. the aspiration within a non-culprit coronary milieu, may also represent valuable options to minimize bias, but may be difficult to ensure in the setting of ACS.
Conclusions
The OPTICO-ACS study provides novel evidence for a principal role of the adaptive immune system, particularly CD8+ T-lymphocytes and their cytotoxic effector molecules, in the pathogenesis of IFC-ACS, which accounts for approximately one-third of ACS. This investigation revealed distinct immunological patterns in IFC-ACS as compared with RFC-ACS patients, by characterizing the microenvironment of the culprit lesion. The different immune signatures identified in this study advance our understanding of coronary plaque progression and provide the basis for future personalized therapeutic approaches to ACS.
Supplementary Material
Acknowledgements
We greatly appreciate the enthusiastic support of the staff of the catheterization laboratories at Charité University Medicine Berlin. We thank Lisa Steinbeck, MD, Andrea Heuberger, MD, Georg Girke, MD, Nadija Güc, MD and Joachim Weber, MD, Sabine Knüppel, Adelheid Kratzer, PhD, and Hector Giral-Arnal, PhD for their great support as well as the staff of the Berlin Institute of Health (BIH) especially Anne Gössinger (study co-ordinator), Katrin Haug, and Kristin Simon (study nurses).
Funding
This study was supported by a CRU grant form the Berlin Institute of Health (BIH) and the German Centre for Cardiovascular Research (DZHK; FKZ: 81Z2100202). Furthermore an educational grant from Abbott Vascular (formerly: St. Jude Medical) provided OCT imaging. N.K. was supported by the Deutsche Stiftung für Herzforschung (Project: F/39/17); P.L. received funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), and the RRM Charitable Fund.
Conflict of interest: PL is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion, Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Merck, Novartis, Pfizer, Sanofi-Regeneron. PL is also a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, XBiotech, Inc. PL laboratory has received research funding in the last 2 years from Novartis. PL is on the Board of Directors of XBiotech, Inc. PL has a financial interest in Xbiotech, a company developing therapeutic human antibodies. PL interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1. van der Sijde JN, Karanasos A, Villiger M, Bouma BE, Regar E.. First-in-man assessment of plaque rupture by polarization-sensitive optical frequency domain imaging in vivo. Eur Heart J 2016;37:1932–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM.. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262–1275. [DOI] [PubMed] [Google Scholar]
- 3. Bentzon JF, Otsuka F, Virmani R, Falk E.. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- 4. Fujii K, Kawasaki D, Masutani M, Okumura T, Akagami T, Sakoda T, Tsujino T, Ohyanagi M, Masuyama T.. OCT assessment of thin-cap fibroatheroma distribution in native coronary arteries. JACC Cardiovasc Imaging 2010;3:168–175. [DOI] [PubMed] [Google Scholar]
- 5. Libby P, Pasterkamp G, Crea F, Jang IK.. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ Res 2019; 124:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;368:2004–2013. [DOI] [PubMed] [Google Scholar]
- 7. van der Wal AC, Becker AE, van der Loos CM, Das PK.. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89:36–44. [DOI] [PubMed] [Google Scholar]
- 8. Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, Virmani R.. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996;93:1354–1363. [DOI] [PubMed] [Google Scholar]
- 9. Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, Virmani R.. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999;82:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, Aurigemma C, Prati F, Crea F.. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J 2015;36:1377–1384. [DOI] [PubMed] [Google Scholar]
- 11. Dai J, Xing L, Jia H, Zhu Y, Zhang S, Hu S, Lin L, Ma L, Liu H, Xu M, Ren X, Yu H, Li L, Zou Y, Zhang S, Mintz GS, Hou J, Yu B.. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J 2018;39:2077–2085. [DOI] [PubMed] [Google Scholar]
- 12. Kajander OA, Pinilla-Echeverri N, Jolly SS, Bhindi R, Huhtala H, Niemela K, Fung A, Vijayaraghavan R, Alexopoulos D, Sheth T.. Culprit plaque morphology in STEMI—an optical coherence tomography study: insights from the TOTAL-OCT substudy. EuroIntervention 2016;12:716–723. [DOI] [PubMed] [Google Scholar]
- 13. Quillard T, Franck G, Mawson T, Folco E, Libby P.. Mechanisms of erosion of atherosclerotic plaques. Curr Opin Lipidol 2017;28:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Libby P, Pasterkamp G.. Requiem for the ‘vulnerable plaque’. Eur Heart J 2015;36:2984–2987. [DOI] [PubMed] [Google Scholar]
- 15. Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, Xu M, Yao Y, Hu S, Yamamoto E, Lee H, Zhang S, Yu B, Jang IK.. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur Heart J 2017;38:792–800. [DOI] [PubMed] [Google Scholar]
- 16. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK.. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol 2013;62:1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, Vergallo R, Minami Y, Ong DS, Lee H, Okumura K, Jang IK.. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with st-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv 2015;8:1166–1176. [DOI] [PubMed] [Google Scholar]
- 18. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Group E.. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 19. Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D, Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 20. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho J-M, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudeck D, Falk E, Feldman MD, Fitzgerald P, Garcia H, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CCS, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel M-A, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Räber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PWJC, Shimada K, Shinke T, Shite J, Siegel E, Sonada S, Suter M, Takarada S, Tanaka A, Terashima M, Troels T, Uemura S, Ughi GJ, van Beusekom HMM, van der Steen AFW, van Es G-A, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G, International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012;59:1058–1072. [DOI] [PubMed] [Google Scholar]
- 21. Riegger J, Byrne RA, Joner M, Chandraratne S, Gershlick AH, Ten Berg JM, Adriaenssens T, Guagliumi G, Godschalk TC, Neumann FJ, Trenk D, Feldman LJ, Steg PG, Desmet W, Alfonso F, Goodall AH, Wojdyla R, Dudek D, Philippi V, Opinaldo S, Titova A, Malik N, Cotton J, Jhagroe DA, Heestermans AA, Sinnaeve P, Vermeersch P, Valina C, Schulz C, Kastrati A, Massberg S, Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort Investigators. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J 2016;37:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Novotny J, Chandraratne S, Weinberger T, Philippi V, Stark K, Ehrlich A, Pircher J, Konrad I, Oberdieck P, Titova A, Hoti Q, Schubert I, Legate KR, Urtz N, Lorenz M, Pelisek J, Massberg S, von Bruhl ML, Schulz C.. Histological comparison of arterial thrombi in mice and men and the influence of Cl-amidine on thrombus formation. PLoS One 2018;13:e0190728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW, Expert's OCTRD. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401–415. [DOI] [PubMed] [Google Scholar]
- 24. Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL.. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation 2003;108:438–444. [DOI] [PubMed] [Google Scholar]
- 25. Wentzel JJ, Janssen E, Vos J, Schuurbiers JC, Krams R, Serruys PW, de Feyter PJ, Slager CJ.. Extension of increased atherosclerotic wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation 2003;108:17–23. [DOI] [PubMed] [Google Scholar]
- 26. Carlier SG, van Damme LC, Blommerde CP, Wentzel JJ, van Langehove G, Verheye S, Kockx MM, Knaapen MW, Cheng C, Gijsen F, Duncker DJ, Stergiopulos N, Slager CJ, Serruys PW, Krams R.. Augmentation of wall shear stress inhibits neointimal hyperplasia after stent implantation: inhibition through reduction of inflammation? Circulation 2003;107:2741–2746. [DOI] [PubMed] [Google Scholar]
- 27. Stone PH, Coskun AU, Kinlay S, Popma JJ, Sonka M, Wahle A, Yeghiazarians Y, Maynard C, Kuntz RE, Feldman CL.. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: an in vivo serial study. Eur Heart J 2007;28:705–710. [DOI] [PubMed] [Google Scholar]
- 28. Samady H, Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Maynard C, Timmins LH, Quyyumi AA, Giddens DP.. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 2011;124:779–788. [DOI] [PubMed] [Google Scholar]
- 29. Chandran S, Watkins J, Abdul-Aziz A, Shafat M, Calvert PA, Bowles KM, Flather MD, Rushworth SA, Ryding AD.. Inflammatory differences in plaque erosion and rupture in patients with ST-segment elevation myocardial infarction. J Am Heart Assoc 2017;6:e005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R.. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276–1282. [DOI] [PubMed] [Google Scholar]
- 31. Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R.. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation 1998;97:2110–2116. [DOI] [PubMed] [Google Scholar]
- 32. Partida RA, Libby P, Crea F, Jang IK.. Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur Heart J 2018;39:2070–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gijsen FJ, Wentzel JJ, Thury A, Lamers B, Schuurbiers JC, Serruys PW, van der Steen AF.. A new imaging technique to study 3-D plaque and shear stress distribution in human coronary artery bifurcations in vivo. J Biomech 2007;40:2349–2357. [DOI] [PubMed] [Google Scholar]
- 34. Dotiwala F, Mulik S, Polidoro RB, Ansara JA, Burleigh BA, Walch M, Gazzinelli RT, Lieberman J.. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nat Med 2016;22:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walch M, Dotiwala F, Mulik S, Thiery J, Kirchhausen T, Clayberger C, Krensky AM, Martinvalet D, Lieberman J.. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell 2015;161:1229. [DOI] [PubMed] [Google Scholar]
- 36. Quillard T, Araujo HA, Franck G, Shvartz E, Sukhova G, Libby P.. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J 2015;36:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White SJ, Newby AC, Johnson TW.. Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb Haemost 2016;115:509–519. [DOI] [PubMed] [Google Scholar]
- 38. Crea F, Libby P.. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation 2017;136:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrante G, Nakano M, Prati F, Niccoli G, Mallus MT, Ramazzotti V, Montone RA, Kolodgie FD, Virmani R, Crea F.. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation 2010;122:2505–2513. [DOI] [PubMed] [Google Scholar]
- 40. Saia F, Komukai K, Capodanno D, Sirbu V, Musumeci G, Boccuzzi G, Tarantini G, Fineschi M, Tumminello G, Bernelli C, Niccoli G, Coccato M, Bordoni B, Bezerra H, Biondi-Zoccai G, Virmani R, Guagliumi G, OCTAVIA Investigators. Eroded versus ruptured plaques at the culprit site of STEMI: in vivo pathophysiological features and response to primary PCI. JACC Cardiovasc Imaging 2015;8:566–575. [DOI] [PubMed] [Google Scholar]
- 41. Niccoli G, Montone RA, Cataneo L, Cosentino N, Gramegna M, Refaat H, Porto I, Burzotta F, Trani C, Leone AM, Severino A, Crea F.. Morphological-biohumoral correlations in acute coronary syndromes: pathogenetic implications. Int J Cardiol 2014;171:463–466. [DOI] [PubMed] [Google Scholar]
- 42. Brown AJ, Teng Z, Calvert PA, Rajani NK, Hennessy O, Nerlekar N, Obaid DR, Costopoulos C, Huang Y, Hoole SP, Goddard M, West NE, Gillard JH, Bennett MR.. Plaque structural stress estimations improve prediction of future major adverse cardiovascular events after intracoronary imaging. Circ Cardiovasc Imaging 2016;9:e004172. [DOI] [PubMed] [Google Scholar]
- 43. Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Timmins LH, Binongo JN, Golub LJ, Corban MT, Finn AV, Oshinski JN, Quyyumi AA, Giddens DP, Samady H.. Association of coronary wall shear stress with atherosclerotic plaque burden, composition, and distribution in patients with coronary artery disease. J Am Heart Assoc 2012;1:e002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Costopoulos C, Huang Y, Brown AJ, Calvert PA, Hoole SP, West NEJ, Gillard JH, Teng Z, Bennett MR.. Plaque rupture in coronary atherosclerosis is associated with increased plaque structural stress. JACC Cardiovasc Imaging 2017;10:1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Costopoulos C, Timmins LH, Huang Y, Hung OY, Molony DS, Brown AJ, Davis EL, Teng Z, Gillard JH, Samady H, Bennett MR.. Impact of combined plaque structural stress and wall shear stress on coronary plaque progression, regression, and changes in composition. Eur Heart J 2019;40:1411-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thondapu V, Bourantas CV, Foin N, Jang IK, Serruys PW, Barlis P.. Biomechanical stress in coronary atherosclerosis: emerging insights from computational modelling. Eur Heart J 2017;38:81–92. [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto E, Thondapu V, Poon E, Sugiyama T, Fracassi F, Dijkstra J, Lee H, Ooi A, Barlis P, Jang IK.. Endothelial shear stress and plaque erosion: a computational fluid dynamics and optical coherence tomography study. JACC Cardiovasc Imaging 2019;12:374–375. [DOI] [PubMed] [Google Scholar]
- 48. Cochain C, Zernecke A.. Protective and pathogenic roles of CD8(+) T cells in atherosclerosis. Basic Res Cardiol 2016;111:71. [DOI] [PubMed] [Google Scholar]
- 49. Grivel JC, Ivanova O, Pinegina N, Blank PS, Shpektor A, Margolis LB, Vasilieva E.. Activation of T lymphocytes in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2011;31:2929–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mullick AE, Tobias PS, Curtiss LK.. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest 2005;115:3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mullick AE, Soldau K, Kiosses WB, Bell TA 3rd, Tobias PS, Curtiss LK.. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med 2008;205:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, Shvartz E, Sukhova GK, Swirski FK, Nahrendorf M, Aikawa E, Croce KJ, Libby P.. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ Res 2017;121:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schreiber TH, Shinder V, Cain DW, Alon R, Sackstein R.. Shear flow-dependent integration of apical and subendothelial chemokines in T-cell transmigration: implications for locomotion and the multistep paradigm. Blood 2007;109:1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pedicino D, Vinci R, Giglio AF, Pisano E, Porto I, Vergallo R, Russo G, Ruggio A, D’Aiello A, Flego D, Annibali G, Trotta F, Piacentini R, Niccoli G, Liuzzo G, Crea F.. Alterations of hyaluronan metabolism in acute coronary syndrome: implications for plaque erosion. J Am Coll Cardiol 2018;72:1490–1503. [DOI] [PubMed] [Google Scholar]
- 55. Mahaffey CL, Mummert ME.. Hyaluronan synthesis is required for IL-2-mediated T cell proliferation. J Immunol 2007;179:8191–8199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.