Abstract
Introduction
Key knowledge gaps remain to improve reproductive health outcomes for millions of women living with female genital mutilation (FGM). We aimed to update previous reviews and quantify more rigorously maternal and perinatal complications related to FGM across different settings.
Methods
In this systematic review and meta-analysis, we searched 15 electronic databases for studies published between 1 August 1995 and 15 March 2020, reporting on maternal and perinatal complications related to FGM. We included studies comparing women with and without FGM while accounting for confounders. Pooled relative risks (RR) were calculated, using fixed-effects and random-effects models, for a range of maternal and perinatal outcomes, adjusting for individual characteristics and according to delivery settings and study design.
Results
We identified 106 unique references, assessed 72 full-text articles and included 11 studies. We found non-significant elevated risks of instrumental delivery, caesarean delivery, episiotomy, postpartum haemorrhage, perineal laceration, low Apgar score and miscarriage/stillbirth related to FGM. Heterogeneity was present for most outcomes when combining all studies but reduced in subgroup analyses. The risk of caesarean delivery was increased among primiparous women (1.79, 95% CI 1.04 to 3.07) such as the risk of episiotomy in European specialised settings for women with FGM (1.88, 1.14 to 3.09). In Africa, subgroup analyses revealed elevated risks of postpartum haemorrhage (2.59, 1.28 to 5.25). The most common reported type was FGM II. However, few studies provided stratified analyses by type of FGM, which did not allow an assessment of the impact of the severity of typology on studied outcomes.
Conclusion
This review suggests maternal and perinatal morbidity related to FGM vary by study design, context and by subgroup of women. Our study also draws attention to the complications that may extend to the postpartum period. This work contributes to shaping a reference framework for future research and clinical guidelines.
Keywords: epidemiology, maternal health, obstetrics, systematic review
Key questions.
What is already known?
A review identified obstetric outcomes and postpartum care related to female genital mutilation (FGM) as research areas with significant evidence gaps.
Studies suggest that obstetric risks are mainly due to the scarring resulting from the procedure of FGM.
Previous reviews suggest that prolonged labour, obstruction, episiotomies, perineal tears, postpartum haemorrhage and instrumental delivery are the most common obstetric complications of FGM.
What are the new findings?
Delivery settings, individual factors, study design and analytical strategies are important when predicting maternal and perinatal morbidity related to FGM.
Our subgroup analyses revealed contrasting risks according to context, with elevated risks of episiotomy in Europe versus elevated risks of postpartum haemorrhage in Africa, and increased risks of caesarean section among primiparous women.
What do the new findings imply?
Prevention of maternal and perinatal complications should include providing healthcare professionals with guidelines regarding the clinical management of FGM complications in order to assist women in making informed decisions.
More research is needed to study the benefits of episiotomies and caesarean delivery for FGM and evaluate postpartum risks.
Studies on postpartum period are warranted to improve the healing of women with FGM from perineal trauma.
Introduction
WHO defines female genital mutilation (FGM) as all procedures involving partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.1 FGM is most prevalent in 30 countries extending from regions of Africa, the Middle East and Asia. According to recent estimates, at least 200 million girls and women living in countries where the practice is prevalent or in diaspora communities (as a result of migration) have undergone FGM.2 Despite numerous efforts accomplished globally to enact policies and establish effective strategies for preventing FGM, key knowledge gaps remain to deliver optimal evidence-based care to improve health outcomes for girls and women with FGM.
Health sequelae following FGM have been broadly investigated in the literature. Short-term health risks include severe pain, haemorrhage, infection and in the worst cases, death, while long-term complications entail the formation of cysts, keloids, sexual dysfunction, chronic pelvic infection and obstetric problems.1 Among the available literature on the clinical care of women with FGM, a study identified obstetric outcomes and postpartum care as research areas with significant evidence gaps.3 Three reviews published between 2000 and 2014 suggested that obstetric complications related to FGM (performed prior to the index pregnancy) were mainly attributed to the scarring resulting from the procedure.4–6 A systematic review of the health complications of FGM identified 422 articles investigating a range of childbirth sequelae related to FGM. The most common complications were prolonged labour, obstruction, episiotomies, perineal tears and postpartum haemorrhage.4 Another descriptive review including 35 studies reported increased risks for perineal tears, fetal distress and general difficulties but no significant associations with other maternal and perinatal complications.5 Lastly, the first meta-analysis published on the matter suggested that women with FGM were at greater risk of prolonged labour, obstetric lacerations, instrumental delivery, obstetric haemorrhage and difficult delivery.6
While these reviews have been informative in highlighting the growing body of evidence on the consequences of FGM on maternal and perinatal health, the quality of these studies aiming to investigate the causality between FGM and these outcomes is questionable. Indeed, these reviews included a vast volume of publications without distinguishing studies of different designs, therefore not of comparable quality. Furthermore, the conclusions are weakened by the fact that potential confounding factors related to maternal and perinatal morbidity are not considered and most studies included in the previous reviews and meta-analysis were conducted in African countries where FGM is prevalent and where maternal and perinatal morbidity rates are higher than in high-income countries. In such cases, it is difficult to discern the effect of FGM from the effects of maternal attributes or delivery setting. Another limitation is the lack of attention on whether exposure and outcomes are defined and measured in a standardised way. We also identified a paucity of evidence on the risks of different types of FGM making it difficult to judge whether the more extensive operations are associated with more severe outcomes.
More studies have since been published on the subject, particularly studies conducted in high-income countries. Considering strengths and weaknesses of earlier reviews, we aimed to conduct a more rigorous systematic review and meta-analysis to update findings and ascertain maternal and perinatal consequences of FGM across different settings, worldwide. Our study focused on the comparison of women with and without FGM while accounting for individual characteristics of women, study design and healthcare settings.
Methods
We conducted a systematic review and meta-analysis according to the MOOSE guidelines for Meta-Analyses of Observational Studies in Epidemiology (online supplemental text 1).7
bmjgh-2020-003307supp001.pdf (49.4KB, pdf)
Search strategy and selection criteria
We searched 15 electronic databases (MEDLINE, POPLINE, Scopus, Cochrane Library, BMC, Web of Science, EBSCO Discovery Service, Science Direct, Global Index Medicus, Google Scholar, ScieLO, LILACS, LiSSA, Pascal Francis and RefDoc) for articles on maternal and perinatal consequences related to FGM. We searched for studies published in any language between 1 August 1995 and 5 July 2019. This search was updated on 15 March 2020. The research time span starting from 1995 was chosen as pertinent to the year WHO developed the first standardised classification of FGM,1 which was used to define FGM in the current study (box 1). Search terms included “female genital mutilation”, “female genital cutting”, “female circumcision”, “clitoridectomy”, “infibulation”, “sunna”, “obstetric*”, “gyn*”, “reproductive health”, “childbirth”, “delivery”, “neonatal” and “pregnancy”, with no language restrictions. We supplemented these database searches with grey literature using OpenGrey, screening online resources maintained by active FGM networks (End FGM European Network, FGM Specialist Network, FGM Education and Networking Project, Orchid Project and Excision Parlons-en), contacting authors when articles were not available and screening reference lists of retrieved articles. FS and AA independently screened titles and abstracts, then full texts of eligible articles.
Box 1. WHO classification of female genital mutilation (FGM).
No FGM: no evidence of any genital mutilation.
FGM I: excision of the prepuce, with or without excision of part or all of the clitoris.
FGM II: excision of the clitoris with partial or total removal of the labia minora.
FGM III: excision of part or all of the external genitalia and stitching, or narrowing of the vaginal opening (infibulation).
FGM IV: all other harmful procedures to the female genitalia.
We included studies providing primary quantitative data on any maternal and perinatal consequences of FGM, matching with the following criteria: (a) studies including women of reproductive age (between 15 and 49 years of age) with or without any type of FGM, (b) studies reporting estimates on complications of pregnancy, childbirth or the immediate postpartum period, including perinatal complications, (c) studies conducted in any country, (d) observational studies. In accordance with the aim of this study, we excluded studies with no comparison group (women without FGM). Furthermore, to minimise potential confounding bias, we excluded studies where comparison groups were not matched or for which analyses did not adjust for potential confounders.8
Data extraction and quality assessment
FS and AA extracted the following data from eligible studies: authors’ names, date of publication, country where the study was conducted, study design, number of participants, mean age of participants, FGM type and method of assessment, outcome measures and method of assessment, matching criteria or list of confounders and level of specialisation of healthcare facilities where women delivered.
FS and AA assessed the quality of each study using the Newcastle-Ottawa Scale recommended by The Cochrane Collaboration for observational studies.9 This scale is a nine-item instrument used to assess the selection of study population, study comparability and ascertainment of exposure (online supplemental text 2).10 We considered a study to be of good quality when it met seven or more of the nine recommended items.
Statistical analysis
Risk ratios (RR) and 95% CIs adjusted for sociodemographic factors, maternal attributes and delivery setting were extracted. When RRs and CIs were not available, RRs were manually calculated based on frequencies in each comparison group. We considered a two-tailed p value of <0.05 for significant associations. According to the level of heterogeneity between studies, we used Mantel-Haenszel fixed-effects models or random-effects models, a robust method used to estimate pooled RR and CIs for dichotomous variables.11 Cochran’s Q statistic and the I² statistic were used to assess statistical heterogeneity.12 A p<0.05 was considered significant for heterogeneity and I² values of 25%, 50% and 75% indicated low, moderate and high heterogeneity, respectively.13 We ran a fixed-effect meta-analytical model when heterogeneity was not significant and used a random-effects model when heterogeneity was significant. Subgroup analyses were performed based on study quality, study design, geographic setting, parity, level of specialisation of healthcare facilities related to FGM and adjustment or matching for confounders.
Among the studies eligible for our meta-analysis, we identified potential confounders known in the literature for their relevancy to FGM status and their influence on maternal and perinatal outcomes. For statistical purpose, we included confounding categories which were accounted in at least two studies. While ethnicity is known as the most influential factor on the practice of FGM and the type of procedure,1 the literature shows that marital status is also associated with FGM status.14 On the other hand, studies show that low socioeconomic status is a risk factor for severe maternal and perinatal outcomes.15 Advanced maternal age and high parity are also known to be linked to maternal and perinatal morbidity.16 Therefore, ethnicity, marital status, socioeconomic status (education or occupation), maternal age and parity were judged a priori to be confounding factors to consider in our subgroup analyses.
Meta-analyses were conducted using the software R, V.3.6.0 (The R foundation, 2019).
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Characteristics of studies found in the literature
We retrieved 300 articles in our initial search (figure 1). After removing duplicates (leaving 106 unique references) and excluding 34 articles based on titles and abstracts, we assessed the 72 full remaining texts. Sixty-one of these articles were excluded because they were descriptive (n=7), did not match nor adjust for potential confounders (n=15), did not include relevant maternal and perinatal health outcomes (n=20), did not use comparison groups based on FGM status (yes/no) (n=15) or did not have the required data available (n=4). Eleven articles met all eligibility criteria for our meta-analysis.
Figure 1.
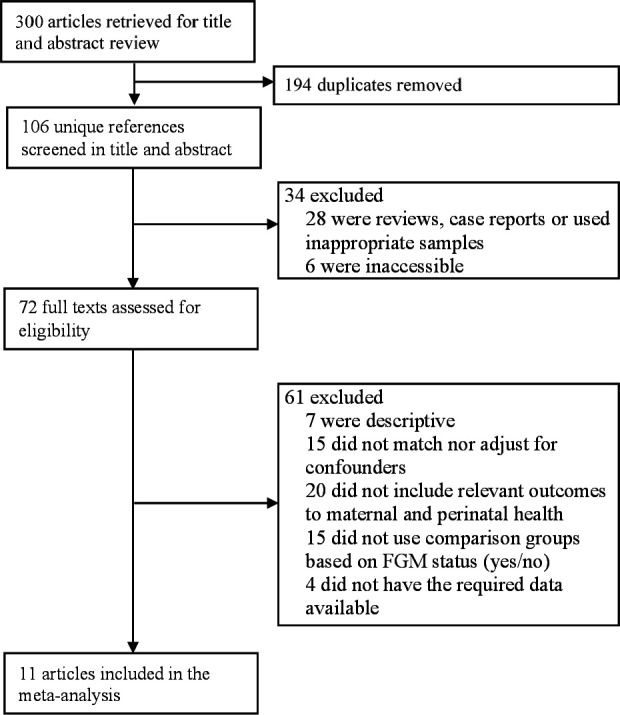
Study selection flow chart. FGM, female genital mutilation.
The characteristics of the 11 studies are shown in table 1.17–27 Eight studies were conducted in Africa19–21 23–27 and three in Europe.17 18 22 Altogether, the studies included 111 558 participants, 61 218 of whom had undergone FGM. Across studies, sample sizes ranged from 232 to 103 095 women. Most studies were facility-based,17–19 21–26 one study was community-based27 and one study consolidated data from 13 Demographic and Health Surveys.20 FGM type was identified in 917–19 22–27 of the 11 studies and the most reported type was FGM II. In seven studies, FGM type was ascertained by medical examination,17–19 22 25–27 in one study it was self-reported21 and in three studies the method of assessment was not specified.20 23 24 Regarding outcomes, there were nearly as many studies relying on women’s self-report19–22 26 27 as there were studies in which outcomes were ascertained clinically.17 18 23–25 Using the Newcastle-Ottawa Scale, the overall quality score of the 11 studies ranged from 5 to 8, indicative of moderate to good quality (online supplemental text 3).
Table 1.
Summary of included studies
| Study | Region | Study design, setting, | FGM/No FGM | Mean age (years) |
Type of FGM (assessment) | Outcome (assessment) | Adjustment/Matching for confounding variables | Quality (score) |
| Andro et al22 | France | Case-control, health centres and hospitals | 678/1076 | 30 | 57% FGM I or II, 2% FGM III, 40% uncertain (self-reported, medical exam) | A, B, C, D, E, G (self-reported) | Age, education, occupation, years since migration, miscarriage/stillbirth, parity, pain at first intercourse | Good (7/9) |
| Balachandran et al17 | UK | Case-control, specialised clinic | 121/121 | 29 (FGM) 30 (no FGM) | 31% FGM I, 45% FGM II, 16% FGM III, 1% FGM IV (medical exam) | A, B, C, D, E (medical records) | Age, ethnicity, parity and gestational age | Good (8/9) |
| Gebremicheal et al25 | Ethiopia | Cohort, hospital | 142/139 | 25 (FGM) 26 (no FGM) | 26% FGM I, 32% FGM II, 42% FGM III (medical exam) | F (medical records) | Parity, age, birth weight, education, antenatal care visit, height, distance travelled and nutritional status | Good (8/9) |
| Kasim et al21 | Egypt | Case-control, maternal clinics | 200/200 | N/A | Type not precised (self-reported) | D (self-reported) | Age, sex, education, occupation and residence | Moderate (5/9) |
| Larsen, Okonofua19 | Nigeria | Cross-sectional, family planning and antenatal services | 827/1009 | N/A | 71% FGM I, 24% FGM II (self-reported, medical exam) | B, C, E, G (self-reported) | Age, education, residence, region, ethnicity, religion, age at first pregnancy, marital status, place at delivery, parity | Good (7/9) |
| Milogo-Traore et al24 | Burkina Faso | Case-control, antenatal services | 227/227 | 25 | 27.7% FGM I, 69.6% FGM II, 2.6% FGM III (assessment N/A) | A, C, E, F, G (medical records) | Age and parity | Good (7/9) |
| Morison et al27 | Gambia | Cross-sectional, rural areas | 668/489 | N/A | 0.4% FGM I, 98% FGM II, 1% FGM III (medical exam) | G (self-reported) | Age, marital status and parity | Good (7/9) |
| Slanger et al26 | Nigeria | Cross-sectional, family planning and antenatal services | 621/486 | 34 | 72% FGM I, 24% FGM II, 4% FGM III or IV (medical exam) | B, C, D, E (self-reported) | Sociodemographic correlates | Moderate (6/9) |
| Thera et al23 | Mali | Case-control, rural health centre | 280/130 | 18 (FGM) 19 (no FGM) | 6% FGM I, 91% FGM II, 3% FGM III (assessment N/A) | A, B, E, G (medical records) | Age, parity and number of fetus | Good (7/9) |
| Wagner20 | African countries* | Cross-sectional, households and family planning | 57 332/45 763 | 31 | N/A | B (self-reported) | Age, education and marriage status | Moderate (5/9) |
| Wuest et al18 | Switzerland | Case-control, specialised hospital | 122/110 | 27 (FGM) 29 (no FGM) | 17% FGM I, 24% FGM II, 47% FGM III, 11% FGM IV (medical exam) | A, B, C, E, F (medical records) | Maternal age | Good (7/9) |
Outcomes: A indicates instrumental delivery, B indicates caesarean delivery, C indicates episiotomy, D indicates postpartum haemorrhage, E indicates perineal laceration, F indicates 5 min Apgar score <7, G indicates miscarriage or stillbirth.
*Benin, Burkina Faso, Cameroon, Chad, Ethiopia, Ghana, Guinea, Kenya, Mali, Nigeria, Niger, Senegal, Sierra Leone.
FGM, female genital mutilation; N/A, not available.
Types of maternal and perinatal health outcomes found in the articles
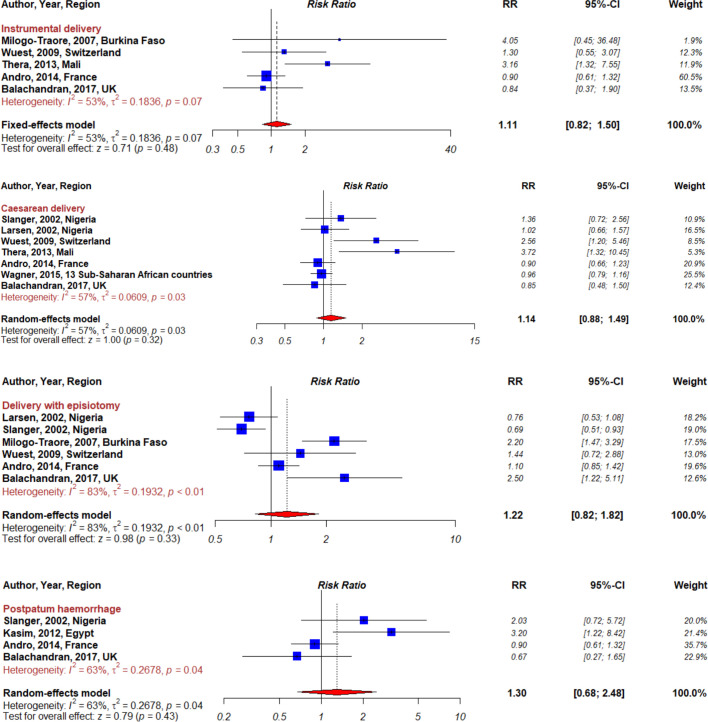
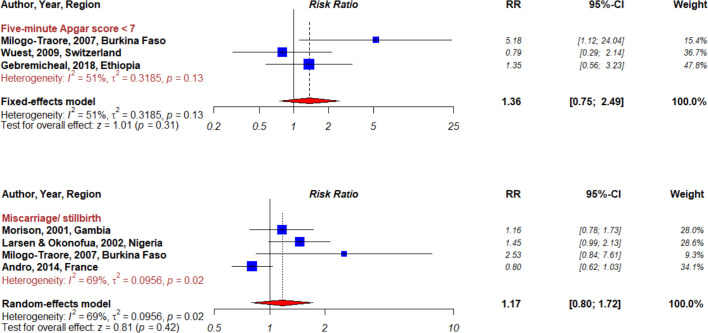
Maternal and perinatal health outcomes found in the articles included instrumental delivery, caesarean delivery, episiotomy, postpartum haemorrhage, perineal laceration, 5 min Apgar score <7 and miscarriage or stillbirth (box 2). Individual study results for these outcomes are presented in figures 2 and 3.
Box 2. Definitions of maternal and perinatal health outcomes.
Instrumental delivery: vaginal delivery assisted by instruments such as forceps or vacuum extraction during the second stage of labour.
Caesarean delivery: delivery of a fetus or infant by a surgical incision through the maternal abdominal wall and uterus.
Episiotomy: an incision into the perineal body to enlarge the outlet area and facilitate delivery.
Postpartum haemorrhage: estimated blood loss of >500 mL for a vaginal delivery or >1000 mL for a caesarean delivery after the delivery of a fetus or infant.
Perineal laceration: an injury characterised by a laceration to the maternal perineum during delivery.
5 min Apgar score <7: score indicating a condition characterised by cardiorespiratory and neurological depression in a newborn at 5 min following birth.
Miscarriage: spontaneous loss of a fetus weighing <500 g or at gestational age <20 weeks.
Stillbirth: customary term of pregnancy loss after 20 weeks of gestation.
Figure 2.
Forest plot summarising meta-analyses for female genital mutilation and maternal outcomes.
Figure 3.
Forest plot summarising meta-analyses for female genital mutilation and perinatal outcomes.
Instrumental delivery
Five studies assessed the risk of instrumental delivery associated with FGM17 18 22–24 and the summary RR was 1.11 (95% CI 0.82 to 1.50, I²=53%, pheterogeneity=0.07; figure 2). Exclusion of specific studies did not change our main result except for subgroup analyses based on geographic location (table 2). Heterogeneity was observed between studies conducted in Africa and Europe (pheterogeneity=0.005) with stronger associations among studies conducted in Africa (n=2, RR=3.27, 95% CI 1.45 to 7.34, I²=0%, pheterogeneity=0.84).
Table 2.
Pooled relative risks for female genital mutilation status and instrumental delivery
| Studies (n) | RR (95% CI) | I2 (%) | Ph* | Ph† | |
| All studies | 5 | 1.11 (0.82 to 1.50) | 53 | 0.07 | |
| Study quality | |||||
| Moderate (4–6) | – | – | – | – | – |
| Good (7–9) | 5 | 1.11 (0.82 to 1.50) | 53 | 0.07 | |
| Study design | |||||
| Case-control | 5 | 1.11 (0.82 to 1.50) | 53 | 0.07 | – |
| Cross-sectional | – | – | – | ||
| Geographic location | |||||
| Africa | 2 | 3.27 (1.45 to 7.34) | 0 | 0.84 | 0.005 |
| Europe | 3 | 0.94 (0.68 to 1.30) | 0 | 0.72 | |
| Parity | |||||
| Primiparous | 1 | 3.16 (1.34 to 7.65) | – | – | 0.01 |
| Primiparous and multiparous | 4 | 0.97 (0.70 to 1.33) | 0 | 0.51 | |
| Specialised delivery setting | |||||
| Yes | 2 | 1.03 (0.57 to 1.87) | 0 | 0.47 | 0.36 |
| No | 3 | 1.83 (0.63 to 5.38) | 75 | 0.02 | |
| Adjustment/Matching for confounders | |||||
| Maternal age | |||||
| Yes | 4 | 1.54 (0.96 to 2.49) | 47 | 0.13 | 0.14 |
| No | 1 | 0.90 (0.60 to 1.30) | – | – | |
| Parity | |||||
| Yes | 4 | 1.39 (0.68 to 2.82) | 64 | 0.04 | 0.91 |
| No | 1 | 1.30 (0.55 to 3.06) | – | – | |
| Education or socioeconomic status | |||||
| Yes | 1 | 0.90 (0.60 to 1.30) | – | – | 0.14 |
| No | 4 | 1.54 (0.96 to 2.49) | 47 | 0.13 | |
| Ethnicity | |||||
| Yes | 1 | 0.84 (0.37 to 1.90) | – | – | 0.26 |
| No | 4 | 1.56 (0.77 to 3.15) | 63 | 0.04 | |
| Marital status | |||||
| Yes | – | – | – | – | – |
| No | 5 | 1.11 (0.82 to 1.50) | 53 | 0.07 |
n denotes the number of studies.
*P for heterogeneity within each subgroup.
†P for heterogeneity between subgroups.
Caesarean delivery
Pooled results of seven studies measuring the risk of caesarean delivery associated with FGM indicated a non-significant summary RR of 1.14 (95% CI 0.88 to 1.49), however, heterogeneity was substantial between studies (I²=57%, pheterogeneity=0.03; figure 2).17 18 22 23 26 In subgroup analyses by parity, the risk of caesarean delivery associated with FGM was significantly elevated among primiparous women (RR=1.79, 95% CI 1.04 to 3.07, I²=62%, pheterogeneity=0.10; table 3) based on the pooled results of two studies reporting on parity.
Table 3.
Pooled relative risks for female genital mutilation status and caesarean delivery
| Studies (n) | RR (95% CI) | I2 (%) | Ph* | Ph† | |
| All studies | 7 | 1.14 (0.88 to 1.49) | 57 | 0.03 | |
| Study quality | |||||
| Moderate (4–6) | 2 | 0.99 (0.82 to 1.19) | 6 | 0.30 | 0.32 |
| Good (7–9) | 5 | 1.28 (0.83 to 1.97) | 68 | 0.01 | |
| Study design | |||||
| Case-control | 4 | 1.45 (0.78 to 2.69) | 76 | 0.006 | 0.25 |
| Cross-sectional | 3 | 0.99 (0.84 to 1.17) | 0 | 0.58 | |
| Geographic location | |||||
| Africa | 4 | 1.03 (0.87 to 1.21) | 58 | 0.07 | 0.91 |
| Europe | 3 | 1.15 (0.66 to 2.01) | 70 | 0.04 | |
| Parity | |||||
| Primiparous | 2 | 1.79 (1.04 to 3.07) | 62 | 0.10 | 0.44 |
| Primiparous and multiparous | 5 | 0.98 (0.85 to 1.13) | 41 | 0.15 | |
| Specialised delivery setting | |||||
| Yes | 2 | 1.43 (0.49 to 4.21) | 81 | 0.02 | 0.60 |
| No | 5 | 1.00 (0.86 to 1.16) | 48 | 0.10 | |
| Adjustment/Matching for confounders | |||||
| Maternal age | |||||
| Yes | 5 | 1.44 (0.91 to 2.29) | 62 | 0.03 | 0.09 |
| No | 2 | 0.94 (0.80 to 1.11) | 67 | 0.73 | |
| Parity | |||||
| Yes | 4 | 1.19 (0.75 to 1.90) | 62 | 0.05 | 0.99 |
| No | 3 | 1.19 (0.78 to 1.81) | 61 | 0.05 | |
| Education or socioeconomic status | |||||
| Yes | 4 | 0.97 (0.84 to 1.13) | 0 | 0.71 | 0.17 |
| No | 3 | 1.88 (0.74 to 4.75) | 77 | 0.01 | |
| Ethnicity | |||||
| Yes | 2 | 0.95 (0.68 to 1.34) | 0 | 0.62 | 0.22 |
| No | 5 | 1.31 (0.90 to 1.91) | 70 | <0.01 | |
| Marital status | |||||
| Yes | 2 | 0.97 (0.81 to 1.16) | 0 | 0.80 | 0.05 |
| No | 5 | 1.39 (0.86 to 2.26) | 69 | 0.01 |
n denotes the number of studies.
*P for heterogeneity within each subgroup.
†P for heterogeneity between subgroups.
Episiotomy
Six studies examined the association between FGM and episiotomy.17–19 22 24 26 Pooled results indicated a non-significant positive association (RR=1.22, 95% CI 0.82 to 1.82), but heterogeneity between the studies was high (I²=83%, pheterogeneity <0.01; figure 2). Subgroup analyses in three studies grouped by geographic location also showed a non-significant positive association between episiotomy and FGM for women delivering in Europe (RR=1.23, 95% CI 0.98 to 1.54, I²=58%, pheterogeneity=0.09; table 4). The association was stronger and significant when restricting the analysis to two European studies in which the delivery settings had clinical guidelines for women delivering with FGM (RR=1.88, 95% CI 1.14 to 3.09, I²=15%, pheterogeneity=0.28). Subgroup analyses by level of specialisation of delivery setting (yes vs no) showed no significant heterogeneity (pheterogeneity=0.10), while heterogeneity was significant according to study design (pheterogeneity=0.001) and adjustment/matching for confounders (pheterogeneity <0.0001). Specifically, we found an inverse association between cross-sectional studies (n=2, RR=0.72, 95% CI 0.57 to 0.90, I²=0%, pheterogeneity=0.68) and case-control studies (n=4, RR=1.65, 95% CI 1.06 to 2.50, I²=72%, pheterogeneity=0.01). In addition, the observed association was stronger in studies that did not adjust/match for education or socioeconomic status (n=3, RR=2.07, 95% CI 1.51 to 2.83, I²=0%, pheterogeneity=0.49).
Table 4.
Pooled relative risks for female genital mutilation status and episiotomy
| Studies (n) | RR (95% CI) | I2 (%) | Ph* | Ph† | |
| All studies | 6 | 1.22 (0.82 to 1.82) | 83 | <0.01 | |
| Study quality | |||||
| Moderate (4–6) | 1 | 0.69 (0.51 to 0.93) | – | – | 0.008 |
| Good (7–9) | 5 | 1.39 (0.91 to 2.13) | 80 | <0.01 | |
| Study design | |||||
| Case-control | 4 | 1.65 (1.06 to 2.50) | 72 | 0.01 | 0.001 |
| Cross-sectional | 2 | 0.72 (0.57 to 0.90) | 0 | 0.68 | |
| Geographic location | |||||
| Africa | 3 | 1.04 (0.53 to 2.05) | 91 | <0.01 | 0.43 |
| Europe | 3 | 1.23 (0.98 to 1.54) | 58 | 0.09 | |
| Parity | |||||
| Primiparous | 1 | 0.69 (0.51 to 0.93) | – | – | 0.008 |
| Primiparous and multiparous | 5 | 1.39 (0.91 to 2.13) | 80 | <0.01 | |
| Specialised delivery setting | |||||
| Yes | 2 | 1.88 (1.14 to 3.09) | 15 | 0.28 | 0.10 |
| No | 4 | 1.05 (0.67 to 1.65) | 87 | <0.01 | |
| Adjustment/Matching for confounders | |||||
| Maternal age | |||||
| Yes | 5 | 1.28 (0.74 to 2.20) | 87 | <0.01 | 0.63 |
| No | 1 | 1.10 (0.85 to 1.42) | – | – | |
| Parity | |||||
| Yes | 4 | 1.36 (0.79 to 2.33) | 88 | <0.01 | 0.42 |
| No | 2 | 0.87 (0.63 to 1.19) | 61 | 0.11 | |
| Education or socioeconomic status | |||||
| Yes | 3 | 0.84 (0.62 to 1.14) | 67 | 0.05 | <0.0001 |
| No | 3 | 2.07 (1.51 to 2.83) | 0 | 0.49 | |
| Ethnicity | |||||
| Yes | 2 | 1.32 (0.41 to 4.23) | 88 | <0.01 | 0.90 |
| No | 4 | 1.21 (0.74 to 1.99) | 86 | <0.01 | |
| Marital status | |||||
| Yes | 1 | 0.76 (0.53 to 1.08) | – | – | 0.05 |
| No | 5 | 1.36 (0.85 to 2.18) | 85 | <0.01 |
n denotes the number of studies.
*P for heterogeneity within each subgroup.
†P for heterogeneity between subgroups.
Postpartum haemorrhage
Four studies reporting on the risk of postpartum haemorrhage showed a non-significant positive overall association with FGM (RR=1.30, 95% CI 0.68 to 2.48, I²=63%, pheterogeneity=0.04; figure 2).17 21 22 26 However, significant associations emerged in subgroup analysis with evidence of between-subgroup heterogeneity (table 5). For instance, the association was stronger in studies of moderate quality compared with studies of good quality. Likewise, we found an increased risk of postpartum haemorrhage in studies conducted in Africa (n=2, RR=2.59, 95% CI 1.28 to 5.25, I²=0%, pheterogeneity=0.53) while the association was negative and not significant in studies conducted in Europe (n=2, RR=0.86, 95% CI 0.60 to 1.23, I²=0%, pheterogeneity=0.55).
Table 5.
Pooled relative risks for female genital mutilation status and postpartum haemorrhage
| Studies (n) | RR (95% CI) | I2 (%) | Ph* | Ph† | |
| All studies | 4 | 1.30 (0.68 to 2.48) | 63 | 0.04 | |
| Study quality | |||||
| Moderate (4–6) | 2 | 2.59 (1.28 to 5.25) | 0 | 0.53 | 0.006 |
| Good (7–9) | 2 | 0.86 (0.60 to 1.23) | 0 | 0.55 | |
| Study design | |||||
| Case-control | 3 | 1.17 (0.54 to 2.54) | 70 | 0.04 | 0.40 |
| Cross-sectional | 1 | 2.03 (0.72 to 5.71) | – | – | |
| Geographic location | |||||
| Africa | 2 | 2.59 (1.28 to 5.25) | 0 | 0.53 | 0.006 |
| Europe | 2 | 0.86 (0.60 to 1.23) | 0 | 0.55 | |
| Parity | |||||
| Primiparous | 1 | 2.03 (0.72 to 5.72) | 0 | 0.53 | 0.40 |
| Primiparous and multiparous | 3 | 1.17 (0.54 to 2.54) | 70 | 0.04 | |
| Specialised delivery setting | |||||
| Yes | 1 | 0.67 (0.27 to 1.63) | – | – | 0.16 |
| No | 3 | 1.63 (0.70 to 3.81) | 71 | 0.03 | |
| Adjustment/Matching for confounders | |||||
| Maternal age | |||||
| Yes | 2 | 1.08 (0.55 to 2.13) | 60 | 0.11 | 0.70 |
| No | 2 | 1.57 (0.46 to 5.37) | 82 | 0.02 | |
| Parity | |||||
| Yes | 3 | 0.94 (0.67 to 1.32) | 26 | 0.26 | 0.03 |
| No | 1 | 3.20 (1.22 to 8.42) | – | – | |
| Education or socioeconomic status | |||||
| Yes | 3 | 1.63 (0.70 to 3.81) | 71 | 0.03 | 0.70 |
| No | 1 | 0.67 (0.27 to 1.65) | – | – | |
| Ethnicity | |||||
| Yes | 1 | 0.67 (0.27 to 1.65) | – | – | |
| No | 3 | 1.63 (0.70 to 3.81) | 71 | 0.03 | 0.16 |
| Marital status | |||||
| Yes | 0 | – | – | – | – |
| No | 4 | 1.30 (0.68 to 2.48) | 63 | 0.04 |
n denotes the number of studies.
*P for heterogeneity within each subgroup.
†P for heterogeneity between subgroups.
Perineal laceration
Seven studies reported on perineal laceration associated with FGM and the summary RR was 1.37 (95% CI 0.67 to 2.78, I²=92%, pheterogeneity <0.01; figure 2).17 19 22–24 26 Studies adjusting/matching for education or socioeconomic status revealed a stronger significant positive association (RR=1.49, 95% CI 1.19 to 1.86, I²=18%, pheterogeneity=0.30; table 6). Stronger associations were also identified among women with FGM delivering in settings not specialised in FGM care (n=5, RR=2.15, 95% CI 1.08 to 4.27), however, heterogeneity persisted across these studies (pheterogeneity=0.02).
Table 6.
Pooled relative risks for female genital mutilation status and perineal laceration
| Studies (n) | RR (95% CI) | I2 (%) | Ph* | Ph† | |
| All studies | 7 | 1.37 (0.67 to 2.78) | 92 | <0.01 | |
| Study quality | |||||
| Moderate (4–6) | 1 | 0.92 (0.48 to 1.75) | – | – | 0.38 |
| Good (7–9) | 6 | 1.47 (0.65 to 3.31) | 93 | <0.01 | |
| Study design | |||||
| Case-control | 5 | 1.46 (0.55 to 3.91) | 94 | <0.01 | 0.71 |
| Cross-sectional | 2 | 1.19 (0.73 to 1.94) | 16 | 0.28 | |
| Geographic location | |||||
| Africa | 4 | 2.38 (0.82 to 6.89) | 89 | <0.01 | 0.11 |
| Europe | 3 | 0.69 (0.23 to 2.05) | 94 | <0.01 | |
| Parity | |||||
| Primiparous | 2 | 3.46 (0.25 to 47.52) | 96 | <0.01 | 0.36 |
| Primiparous and multiparous | 5 | 0.98 (0.49 to 1.94) | 89 | <0.01 | |
| Specialised delivery setting | |||||
| Yes | 2 | 0.47 (0.31 to 0.70) | 88 | <0.01 | 0.02 |
| No | 5 | 2.15 (1.08 to 4.27) | 87 | <0.01 | |
| Adjustment/Matching for confounders | |||||
| Maternal age | |||||
| Yes | 6 | 1.34 (0.51 to 3.54) | 93 | <0.01 | 0.73 |
| No | 1 | 1.60 (1.20 to 2.00) | – | – | |
| Parity | |||||
| Yes | 5 | 1.88 (0.90 to 3.93) | 89 | <0.01 | 0.25 |
| No | 2 | 0.60 (0.10 to 3.68) | 94 | <0.01 | |
| Education or socioeconomic status | |||||
| Yes | 3 | 1.49 (1.19 to 1.86) | 18 | 0.30 | 0.99 |
| No | 4 | 1.45 (0.31 to 6.68) | 96 | <0.01 | |
| Ethnicity | |||||
| Yes | 2 | 1.04 (0.69 to 1.58) | 58 | 0.12 | 0.57 |
| No | 5 | 1.51 (0.55 to 4.14) | 94 | <0.01 | |
| Marital status | |||||
| Yes | 1 | 1.52 (0.81 to 2.86) | – | – | 0.82 |
| No | 6 | 1.35 (0.59 to 3.08) | 93 | <0.01 |
n denotes the number of studies.
*P for heterogeneity within each subgroup.
†P for heterogeneity between subgroups.
Five-minute Apgar score
Three studies assessed 5 min Apgar score comparing children of mothers with and without FGM.18 24 25 The overall risk for a 5 min Apgar score <7 was positive but not significant (RR=1.36, 95% CI 0.75 to 2.49, I²=51%, pheterogeneity=0.13; figure 3).
Miscarriage or stillbirth
The risk of miscarriage or stillbirth associated with FGM, assessed in four studies18 21 23 26 (three studies conducted in Africa and one in Europe) indicated a non-significant positive summary RR of 1.17 (95% CI 0.80 to 1.72, I²=69%, pheterogeneity=0.02; figure 3). Subgroup analysis in African settings showed a significant positive association (RR=1.35, 95% CI 1.04 to 1.77, I²=0%, pheterogeneity=0.38; table 7), but heterogeneity was present between subgroups of geographic location (pheterogeneity=0.005). In addition, subgroup analyses accounting for heterogeneity by study design and adjustment/matching on confounders indicated stronger associations in cross-sectional studies (RR=1.30, 95% CI 0.99 to 1.72, I²=0%, pheterogeneity=0.43), in studies adjusting/matching on maternal age (RR=1.54, 95% CI 1.07 to 2.22, I²=0%, pheterogeneity=0.35) and on marital status (RR=1.30, 95% CI 0.99 to 1.72, I²=0%, pheterogeneity=0.43).
Table 7.
Pooled relative risks for female genital mutilation status and miscarriage or stillbirth
| Studies (n) | RR (95% CI) | I2 (%) | Ph* | Ph† | |
| All studies | 4 | 1.17 (0.80 to 1.72) | 69 | 0.02 | |
| Study quality | |||||
| Moderate (4–6) | – | – | – | – | – |
| Good (7–9) | 4 | 1.17 (0.80 to 1.72) | 69 | 0.02 | |
| Study design | |||||
| Case-control | 2 | 1.25 (0.42 to 3.75) | 75 | 0.05 | 0.94 |
| Cross-sectional | 2 | 1.30 (0.99 to 1.72) | 0 | 0.43 | |
| Geographic location | |||||
| Africa | 3 | 1.35 (1.04 to 1.77) | 0 | 0.38 | 0.005 |
| Europe | 1 | 0.80 (0.62 to 1.03) | – | – | |
| Parity | |||||
| Primiparous | 0 | – | – | – | – |
| Primiparous and multiparous | 4 | 1.17 (0.80 to 1.72) | 69 | 0.02 | |
| Specialised delivery setting | |||||
| Yes | 0 | – | – | – | – |
| No | 4 | 1.17 (0.80 to 1.72) | 69 | 0.02 | |
| Adjustment/Matching for confounders | |||||
| Maternal age | |||||
| Yes | 2 | 1.54 (1.07 to 2.22) | 0 | 0.35 | 0.05 |
| No | 2 | 0.89 (0.72 to 1.11) | 58 | 0.12 | |
| Parity | |||||
| Yes | 3 | 1.23 (0.70 to 2.18) | 78 | 0.01 | 0.87 |
| No | 1 | 1.16 (0.78 to 1.73) | – | – | |
| Education or socioeconomic status | |||||
| Yes | 2 | 1.06 (0.59 to 1.89) | 84 | 0.01 | 0.50 |
| No | 2 | 1.27 (0.87 to 1.85) | 41 | 0.19 | |
| Ethnicity | |||||
| Yes | 1 | 1.45 (0.99 to 2.13) | – | – | 0.32 |
| No | 3 | 0.93 (0.75 to 1.14) | 65 | 0.06 | |
| Marital status | |||||
| Yes | 2 | 1.30 (0.99 to 1.72) | 0 | 0.43 | 0.94 |
| No | 2 | 1.25 (0.42 to 3.75) | 75 | 0.05 |
n denotes the number of studies.
*P for heterogeneity within each subgroup.
†P for heterogeneity between subgroups.
Discussion
The purpose of this systematic review and meta-analysis was to depict the global context in which women with FGM deliver and measure maternal and perinatal complications associated with FGM while accounting for study design, maternal attributes and delivery settings. We also aimed to update previous findings with more rigorous statistical methods. Our meta-analysis included 11 studies comprising 111 558 women attending family planning services and antenatal services in private and public health centres in Africa and Europe. The most common reported type was FGM II but only two studies provided stratified analyses by type of FGM, which did not allow an assessment of the impact of the severity of types of FGM on studied outcomes.
In line with previous reviews,4–6 we found elevated maternal and perinatal complications among women with FGM, in the form of instrumental delivery, caesarean delivery, episiotomy, postpartum haemorrhage, perineal laceration, low Apgar score and miscarriage/stillbirth, although these risks varied by study design, study context and by subgroups of women. Indeed, similar to a previous meta-analysis,6 heterogeneity was present for most outcomes when considering all studies combined. However, our study put in evidence that heterogeneity was reduced in subgroup analyses when selecting more robust adjustment/matching strategies or stratifying by study geography (Africa and Europe) and parity. Specifically, subgroup analyses revealed contrasting risks according to context, with elevated risks of episiotomy in Europe versus elevated risks of postpartum haemorrhage in Africa, and increased risks of caesarean section among primiparous women.
While increased use of episiotomy in Europe draws attention to the potential for excessive medical interventions among women with FGM in high-income settings, the studies conducted in healthcare facilities specialised for management of women with FGM in our study suggest that episiotomies were performed for specific indications by health professionals who underwent extensive training on the appropriate use of episiotomies with the goal of reducing obstetric complications.17 18 Even though episiotomies are minor incisions that can facilitate delivery in certain cases, the literature highlights that these interventions involve substantial risks such as pain, perineal tears and excessive blood loss if performed too early.16 Currently in Europe, there is a lack of appropriate training on FGM in most medical and public health curriculums, and recommendations about clinical management are not well known.28 The majority of existing recommendations for episiotomy practice in this population are based on expert opinion.3 More studies are needed to help providers decide the optimal type of episiotomy and time to perform when a woman with FGM presents in labour.
The higher frequency of obstetric interventions among women with FGM is also reflected in higher caesarean deliveries performed among primiparous women with FGM in our subgroup analyses. Surprisingly, this result contrasted with a borderline negative association found in studies including both primiparous and multiparous participants. However, in this subgroup of studies, there was a proportionally higher number of participants who self-reported the outcome of caesarean delivery. Response bias may explain these different results. When performed for medically indicated reasons, caesarean delivery can be a life-saving intervention for women and neonates. Nevertheless, it remains a major surgery that can lead to significant morbidity and has implications for delivery mode of subsequent pregnancies.29 Indeed, the incidence of postpartum haemorrhage is higher with caesarean delivery than vaginal delivery and 10% of caesarean deliveries are affected by postpartum uterine infection.16 Although the literature is scarce on the association between FGM and caesarean delivery, several mechanisms are evoked. Indeed, caesarean sections may be indicated to address varying amounts of scar tissue resulting from the FGM procedure and restricting the vaginal opening.4 6 Additionally, the literature indicates that many healthcare providers are unfamiliar with the management of FGM, particularly infibulation, limiting their ability to conduct pelvic examination to assess the safety and feasibility of vaginal delivery.4 30
On the other hand, the absence of an association between FGM and these obstetric interventions in the African context may signal a lack of healthcare resources to perform caesarean sections or episiotomies even when indicated. The elevated risks of postpartum haemorrhage among women with FGM delivering in African countries in our study could be a consequence of insufficient obstetric care to remedy obstructed labour caused by FGM. The frequency and effect of postpartum haemorrhage could have more serious complications outside the hospital setting. Indeed, postpartum haemorrhage is the leading cause of maternal mortality in Africa.31
Our study has some limitations which may affect our conclusions. Specifically, the internal validity of our study may be compromised by measurement error, bias, confounding or lack of statistical power. Recall bias and reporting bias should not be ignored, especially in studies where FGM status was self-reported (n=3). It is possible that some women were unaware of their FGM status, particularly if it was performed in early childhood, while others may have chosen not to disclose it, especially if living in a country where FGM has been legally banned. In addition, even though special attention was taken to include studies that adjusted for major confounding variables such as sociodemographic factors, maternal age and parity, there is the potential for unobserved confounding, including chronic disease and quality of prenatal care. As previously discussed, significant heterogeneity in estimates between studies was partly explained by individual characteristics, delivery settings, study quality, study design and adjustment or matching strategies. While reducing heterogeneity and uncovering different associations according to context, our subgroup analyses must be interpreted with caution due to small sample size. In addition, the studies were mostly facility based, omitting home births occurring in the absence of skilled birth attendants which remain common in some low-income countries in Africa where the prevalence of FGM is high.32 33 Thus, the findings of our research may not be generalisable to women who deliver at home.
Despite these limitations, the present study provides several contributions by updating previous reviews, synthetizing reported associations by using meta-analytical techniques and highlighting the importance of individual factors, healthcare context and study design in the interpretation of results. In this review, we expanded the geographical representation of the research by including studies published in French (FGM is prevalent in many Francophone countries). Compared with previous reviews, we examined in more depth sources of heterogeneity by conducting subgroup analyses by region, revealing different implications of FGM for maternal and perinatal health according to healthcare settings and maternal attributes. Finally, our findings rely on moderate and good quality studies, which provides robustness to our results.
The current findings have important public health implications considering the high prevalence of girls and women affected by FGM globally. Prevention should be a matter of condemning the practice and educating families, and it should provide healthcare professionals with guidelines regarding the clinical management of FGM complications in order to assist women in making informed decisions. Our study contributes to shaping a reference framework by suggesting that delivery settings, individual factors, study design and analytical strategies are important when predicting maternal and perinatal morbidity related to FGM. The experience of interdisciplinary specialised clinics is needed to help healthcare providers anticipate any complications arising throughout pregnancy and labour. More data are also needed to study the benefits of episiotomies and caesarean delivery for FGM. These interventions are not without risks and our study draws attention to the complications that may extend to the postpartum period due to perineal trauma that may affect the well-being of women with FGM. Studies on this period are warranted to improve postpartum care of women with FGM. When caring for women with FGM in the context of migration, we should also be conscious of the cultural background and of their personal history, and any other experiences of violence that could impact their mental health and well-being. We encourage practitioners to include women’s perspectives in this process to help us understand what research they need.
Acknowledgments
The authors would like to thank Guillemette Antoni and Emilie Cordina Duverger for their assistance with data analysis. The authors would also like to thank Yahya Mahamat-Saleh for his comments on the meta-analysis.
Footnotes
Handling editor: Sanni Yaya
Contributors: FS, CM and AA designed the study. FS and AA undertook review activities including searches, study selection, data extraction and calculation and quality assessment, with support from CM. FS and CM developed the statistical model and FS conducted the meta-analyses. FS wrote the manuscript with contributions from CM and AA. All authors reviewed the study findings, read and approved the final version before submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Data availability statement: Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. All data come from published journal articles. https://docs.google.com/spreadsheets/d/1tavrGBdmi4DiB1Xz6j98M_QSH5ighckVHtf1evcMOKo/edit?usp=sharing.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.World Health Organization, Fund UNP, Fund (UNICEF) UNC Female genital mutilation: a joint WHO/UNICEF/UNFPA statement, 1997. Available: https://apps.who.int/iris/handle/10665/41903 [Accessed 06 Mar 2020].
- 2.UNICEF Female Genital Mutilation/cutting: a Global Concern. UNICEF’s Data Work on FGM/C. Unicef, 2016. [Google Scholar]
- 3.Abdulcadir J, Rodriguez MI, Say L. Research gaps in the care of women with female genital mutilation: an analysis. BJOG 2015;122:294–303. 10.1111/1471-0528.13217 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization A systematic review of the health complications of female genital mutilation including sequelae in childbirth. WHO, 2000. [Google Scholar]
- 5.Obermeyer CM. The consequences of female circumcision for health and sexuality: an update on the evidence. Cult Health Sex 2005;7:443–61. 10.1080/14789940500181495 [DOI] [PubMed] [Google Scholar]
- 6.Berg RC, Odgaard-Jensen J, Fretheim A, et al. An updated systematic review and meta-analysis of the obstetric consequences of female genital mutilation/cutting. Obstet Gynecol Int 2014;2014:1–8. 10.1155/2014/542859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, et al. Meta-Analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 8.Simunovic N, Sprague S, Bhandari M. Methodological issues in systematic reviews and meta-analyses of observational studies in orthopaedic research. J Bone Joint Surg Am 2009;91:87–94. 10.2106/JBJS.H.01576 [DOI] [PubMed] [Google Scholar]
- 9.Deeks JJ, Higgins J, Altman DG, et al. Cochrane Handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
- 10.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of non randomised studies in meta-analyses, 2001. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed 06 Mar 2020].
- 11.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- 12.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. 10.2307/3001666 [DOI] [Google Scholar]
- 13.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakeah E, Debpuur C, Oduro AR, et al. Prevalence and factors associated with female genital mutilation among women of reproductive age in the Bawku municipality and Pusiga district of northern Ghana. BMC Womens Health 2018;18:150. 10.1186/s12905-018-0643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindquist A, Knight M, Kurinczuk JJ. Variation in severe maternal morbidity according to socioeconomic position: a UK National case-control study. BMJ Open 2013;3:e002742. 10.1136/bmjopen-2013-002742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bienstock JL, Fox HE, Wallach EE, et al. Johns Hopkins manual of gynecology and obstetrics. Lippincott Williams & Wilkins, 2015. [Google Scholar]
- 17.Balachandran AA, Duvalla S, Sultan AH, et al. Are obstetric outcomes affected by female genital mutilation? Int Urogynecol J 2018;29:339–44. 10.1007/s00192-017-3466-5 [DOI] [PubMed] [Google Scholar]
- 18.Wuest S, Raio L, Wyssmueller D, et al. Effects of female genital mutilation on birth outcomes in Switzerland. BJOG 2009;116:1204–9. 10.1111/j.1471-0528.2009.02215.x [DOI] [PubMed] [Google Scholar]
- 19.Larsen U, Okonofua FE. Female circumcision and obstetric complications. Int J Gynaecol Obstet 2002;77:255–65. 10.1016/S0020-7292(02)00028-0 [DOI] [PubMed] [Google Scholar]
- 20.Wagner N, Cutting FG. Female genital cutting and long-term health consequences – nationally representative estimates across 13 countries. J Dev Stud 2015;16:1–21. 10.1080/00220388.2014.976620 [DOI] [Google Scholar]
- 21.Kasim K, Shaaban S, El Sadak AE, et al. Impacts of Female Genital Mutilation on Women’s Reproductive Health. Journal of Community Medicine and Health Education 2012;02:258–78. [Google Scholar]
- 22.Andro A, Cambois E, Lesclingand M. Long-Term consequences of female genital mutilation in a European context: self perceived health of FGM women compared to non-FGM women. Soc Sci Med 2014;106:177–84. 10.1016/j.socscimed.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 23.Théra T, Kouma A, Touré M, et al. [Obstetrical complications of genital mutilation in Malian rural environment]. J Gynecol Obstet Biol Reprod 2015;44:276–9. 10.1016/j.jgyn.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 24.Millogo-Traore F, Kaba STA, Thieba B, et al. Pronostic maternel et fœtal au cours de l’accouchement chez la femme excisée. La Revue Sage-Femme 2007;6:192–7. 10.1016/S1637-4088(07)79643-8 [DOI] [PubMed] [Google Scholar]
- 25.Gebremicheal K, Alemseged F, Ewunetu H, et al. Sequela of female genital mutilation on birth outcomes in Jijiga town, Ethiopian Somali region: a prospective cohort study. BMC Pregnancy Childbirth 2018;18:305. 10.1186/s12884-018-1937-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slanger TE, Snow RC, Okonofua FE. The impact of female genital cutting on first delivery in Southwest Nigeria. Stud Fam Plann 2002;33:173–84. 10.1111/j.1728-4465.2002.00173.x [DOI] [PubMed] [Google Scholar]
- 27.Morison L, Scherf C, Ekpo G, et al. The long-term reproductive health consequences of female genital cutting in rural Gambia: a community-based survey. Trop Med Int Health 2001;6:643–53. 10.1046/j.1365-3156.2001.00749.x [DOI] [PubMed] [Google Scholar]
- 28.Johansen REB, Ziyada MM, Shell-Duncan B, et al. Health sector involvement in the management of female genital mutilation/cutting in 30 countries. BMC Health Serv Res 2018;18:240. 10.1186/s12913-018-3033-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Who recommendations non-clinical interventions to reduce unnecessary caesarean sections, 2018. Available: https://apps.who.int/iris/bitstream/handle/10665/275377/9789241550338-eng.pdf?ua=1 [Accessed 25 Jun 2020]. [PubMed]
- 30.Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol 2007;27:161–4. 10.1080/01443610601124257 [DOI] [PubMed] [Google Scholar]
- 31.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a who systematic analysis. Lancet Glob Health 2014;2:e323–33. 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 32.Prual A, Bouvier-Colle MH, de Bernis L, et al. Severe maternal morbidity from direct obstetric causes in West Africa: incidence and case fatality rates. Bull World Health Organ 2000;78:593–602. [PMC free article] [PubMed] [Google Scholar]
- 33.Ronsmans C, Etard JF, Walraven G, et al. Maternal mortality and access to obstetric services in West Africa. Trop Med Int Health 2003;8:940–8. 10.1046/j.1365-3156.2003.01111.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-003307supp001.pdf (49.4KB, pdf)