Abstract
Haemophagocytic lymphohistiocytosis (HLH) is an aggressive syndrome which has characteristic symptoms and laboratory findings. Infection is a common trigger of HLH. We report a 2700 g male infant with persistent fever, massive hepatosplenomegaly and severe thrombocytopaenia. Laboratory evidence of primary dengue infection was detected. Investigations revealed hypertriglyceridaemia, hypofibrinogenaemia, hyperferritinaemia and elevated soluble CD25. Bone marrow examination revealed haemophagocytes. The diagnostic criteria for HLH were fulfilled. A diagnosis of secondary HLH triggered by primary dengue infection was considered. Dexamethasone was initiated and continued for 8 weeks. He responded clinically with regression of hepatosplenomegaly, was afebrile and platelet counts normalised. Dengue‐associated HLH is often missed clinically as treating physicians focus more on the underlying infection and its treatment. In neonates, HLH should be considered as differential diagnosis of sepsis and other viral infections, particularly in situations of inappropriate response to standard management.
Keywords: haematology (incl blood transfusion), tropical medicine (infectious disease), neonatal intensive care, malignant and benign haematology, infant health
Background
Haemophagocytic lymphohistiocytosis (HLH) is a syndrome, which has characteristic symptoms and laboratory findings. Infection is a common trigger of HLH.1 2 Transmission of the dengue virus from mother is responsible for the pathogenesis of most of the cases of dengue in neonates. Primary dengue infection has also been described in neonates. We report a case of secondary HLH triggered by primary dengue infection in a neonate.
Case presentation
A 2700 g appropriate-for-age male infant presented to the emergency department on day 9 of life with high-grade fever (38.90C) for 4 days and poor physical activity. He was born at 37+4 weeks’ gestation by spontaneous vaginal delivery to a primigravidae. Apgar score was 9 at 5 min of life. There was no perinatal depression. He was born out of a non-consanguineous marriage without any significant antenatal history. The mother’s laboratory tests revealed that HIV was negative, rapid plasma reagin for syphilis was non-reactive, and rubella titres showed immunity. She did not have fever or viral prodrome before delivery. Mother was not diagnosed with any autoimmune disorder previously. Family history was unremarkable.
On physical examination, he had significant hepatosplenomegaly (liver span 11 cm, spleen palpable 6 cm below left costal margin). There was no pallor, icterus, petechiae or purpura. No dysmorphic features were evident. There were no features of capillary leak, hypotension, seizures or refusal to feed. No other systemic manifestations were detected. There was no clinical bleed throughout the hospital stay.
Investigations
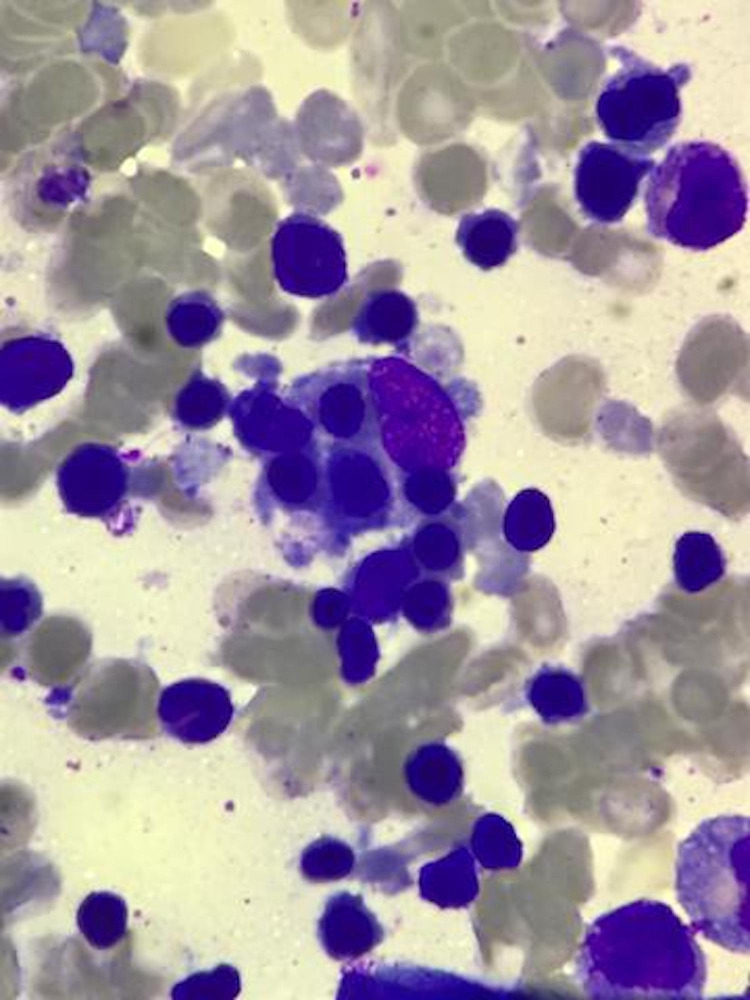
Initial laboratory tests revealed anaemia (90 g/L) with severe thrombocytopaenia (15×109/L). Bacterial cultures were sterile. Cerebrospinal fluid examination was not suggestive of meningitis. Cranial ultrasonography was normal. Coagulation parameters were normal. Investigations for malaria, HIV, chikungunya, rubella, herpes and cytomegalovirus were negative. Serum dengue non-structural protein 1 antigen was positive (detected by enzyme-linked immunosorbent assay). Serum IgM for dengue infection was positive, IgG was negative (mother’s serum IgG, IgM for dengue infection were negative) (all serologies were detected by enzyme-linked immunosorbent assay). Antinuclear antibody was negative. In view of persistent fever and severe thrombocytopaenia, further investigations were carried out which revealed hypertriglyceridaemia (361 mg/dL), hypofibrinogenaemia (121.7 mg/dL) with markedly elevated ferritin levels (10 233 ng/mL) (table 1). Additional test for HLH revealed soluble CD25 (soluble IL-2 receptor alpha) was elevated (4010 units/mL, upper limit of normal <2400 units/mL) (table 1). Bone marrow examination revealed haemophagocytes (figure 1). The diagnostic criteria for HLH were fulfilled.3 To rule out familial HLH, a genetic examination was carried out which did not reveal common mutations specific for HLH (gene encoding perforin PRF1, UNC13D or STX11).
Table 1.
Laboratory investigations
| Investigation | Baseline | Follow-up (3 months of age) |
Reference range |
| Packed cell volume (%) | 26.8 | 27.6 | 40–60 |
| Platelet count (x109/L) | 15 | 258 | 150–450 |
| Triglyceride (mg/dL) | 361 | 86 | <265 |
| Fibrinogen (mg/dL) | 121.7 | 124 | >150 |
| Ferritin (ng/mL) | 10 233 | 39 | <500 |
| Soluble CD25 (U/mL) | 4010 | 1790 | <2400 |
Figure 1.
Bone marrow aspirate showing haemophagocytes (Leishman stain 40×10).
On the basis of the above findings, a diagnosis of secondary HLH triggered by primary dengue infection was considered.
Treatment
Broad-spectrum antimicrobial agents were administered empirically. In view of severe thrombocytopaenia, multiple platelet transfusions were administered. The diagnostic criteria for HLH were fulfilled. In view of persistent fever, significant hepatosplenomegaly and severe thrombocytopaenia, HLH-specific therapy was initiated (HLH-94 protocol).4 In view of inadequate evidence of use of etoposide, ciclosporin and intrathecal methotrexate in neonates and concern for additional immunosuppression, only dexamethasone was initiated and continued for 8 weeks.
Outcome and follow-up
He responded clinically with regression of hepatosplenomegaly, was afebrile and platelet counts normalised. Weight gain was appropriate. There were no adverse effects of dexamethasone (no hyperglycaemia, hypertension, dyselectrolytaemia or evidence of sepsis). Serum triglyceride levels (86 mg/dL) and serum ferritin levels (39 ng/mL) decreased and soluble CD25 (soluble IL-2 receptor alpha) levels were <2400 units/mL (table 1).
Discussion
Vertical transmission of the dengue virus is responsible for the pathogenesis of most of the cases of dengue infections in neonates, although primary dengue has also been described in neonates. HLH is an aggressive and fatal syndrome of excessive immunological activity. HLH can be a familial (genetic) or acquired (secondary) disorder and any event that disrupt immunological balance can trigger HLH. Our patient had secondary HLH triggered by primary dengue infection.
In 2004, Henter et al established the diagnostic criteria for HLH. A molecular diagnosis or altogether five of the eight following criteria must be fulfilled: (1) persistent fever; (2) splenomegaly; (3) cytopenias affecting at least two of three lineages in the peripheral blood (haemoglobin <100 g/L, platelets <100×109/L, neutrophils <1000/mm3); (4) hypertriglyceridaemia (≥265 mg/dL) and/or hypofibrinogenaemia (≤150 mg/dL); (5) haemophagocytosis in the bone marrow, spleen or lymph nodes; (6) low or absent natural killer cell activity; (7) hyperferritinaemia (≥500 ng/mL) and (8) high levels of soluble CD25 (≥2400 U/mL).3 Seven out of the eight criteria were present in the index case. Neonatal HLH is rare and accounts for only 4% of all HLH cases.5 6
A genetic HLH was unlikely in our patient since genetic analysis did not reveal the most common mutations associated with HLH (gene encoding perforin PRF1, UNC13D or STX11).7 8 Infection is a common trigger for secondary HLH in the tropics (44%).6 9 Viruses were identified in 56% of paediatric HLH, out of which dengue constituted 26%.6 9 Giang et al did a systematic review of cases of dengue haemophagocytic syndrome across all ages. They reported 122 such cases out of which more than half were in the paediatric age group. Only two cases were neonates.10 A case of neonatal HLH triggered by dengue infection was first reported by Krithika et al.6 Ours is the third such case report of dengue virus triggered secondary HLH in a neonate.
Serum ferritin levels of >10 000 ng/mL is pathognomonic of HLH.11 The presence of hepatosplenomegaly, hyperferritinaemia and cytopenias warrants a bone marrow examination. The presence of haemophagocytes in bone marrow is one of the diagnostic criteria of HLH but is not a gold-standard test.3 10 In cases of infection triggered HLH, the immediate treatment of the underlying disease is indicated. Our patient clearly had HLH secondary to dengue infection. This is a clinical dilemma since treatment of HLH with steroids and other chemotherapeutic agents pose risk of immunosuppression in neonates with systemic viral infections such as dengue infections. Therapeutic options of HLH include corticosteroids, cyclosporine A, etoposide and intrathecal methotrexate.3 4 There is inadequate evidence regarding usage of such immunosuppressive drugs in neonates. Dexamethasone was the lone drug used in the index case and there was good clinical and biochemical response.
Dengue‐associated HLH is often missed clinically as treating physicians focus more on the underlying infection and its treatment. This disorder is potentially fatal and hence, an early suspicion and diagnosis is required to start life-saving therapy in time. This is of particular importance because such cases might respond to immunomodulation. In neonates, HLH should be considered as differential diagnosis of sepsis and other viral infections, particularly in situations of inappropriate response to standard management.
Learning points.
Haemophagocytic lymphohistiocytosis (HLH) is a fatal syndrome with highly characteristic symptoms and laboratory findings. Infection is a common trigger of HLH.
In infancy, HLH should be considered as differential diagnosis of sepsis and other viral infections, particularly in situations of inappropriate response to standard management.
The presence of hepatosplenomegaly, hyperferritinaemia and cytopenias warrants a bone marrow examination.
Early suspicion and diagnosis might be life-saving as such cases respond to immunomodulation.
Footnotes
Contributors: GA: conceived and wrote the manuscript. SW and AS: critically reviewed and finalised the manuscript. GA, SW, AS and SK were involved in clinical care of the infant, read and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. The who Committee on Histiocytic/Reticulum cell proliferations. reclassification Working group of the histiocyte Society. Med Pediatr Oncol 1997;29:157–66. [DOI] [PubMed] [Google Scholar]
- 2.Janka GE. Hemophagocytic syndromes. Blood Rev 2007;21:245–53. 10.1016/j.blre.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 3.Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. 10.1002/pbc.21039 [DOI] [PubMed] [Google Scholar]
- 4.Henter J-I, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 2002;100:2367–73. 10.1182/blood-2002-01-0172 [DOI] [PubMed] [Google Scholar]
- 5.Isaacs H. Fetal and neonatal histiocytoses. Pediatr Blood Cancer 2006;47:123–9. 10.1002/pbc.20725 [DOI] [PubMed] [Google Scholar]
- 6.Krithika MV, Amboiram P, Latha SM, et al. Neonate with haemophagocytic lymphohistiocytosis secondary to dengue infection: a case report. Trop Doct 2017;47:1–3. 10.1177/0049475516644102 [DOI] [PubMed] [Google Scholar]
- 7.Göransdotter Ericson K, Fadeel B, Nilsson-Ardnor S, et al. Spectrum of perforin gene mutations in familial hemophagocytic lymphohistiocytosis. Am J Hum Genet 2001;68:590–7. 10.1086/318796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmann J, Callebaut I, Raposo G, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 2003;115:461–73. 10.1016/S0092-8674(03)00855-9 [DOI] [PubMed] [Google Scholar]
- 9.Rajagopala S, Singh N. Diagnosing and treating hemophagocytic lymphohistiocytosis in the tropics: systematic review from the Indian subcontinent. Acta Med Acad 2012;41:161–74. 10.5644/ama2006-124.49 [DOI] [PubMed] [Google Scholar]
- 10.Giang HTN, Banno K, Minh LHN, et al. Dengue hemophagocytic syndrome: a systematic review and meta-analysis on epidemiology, clinical signs, outcomes, and risk factors. Rev Med Virol 2018;28:e2005. 10.1002/rmv.2005 [DOI] [PubMed] [Google Scholar]
- 11.Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program 2013;2013:605–11. 10.1182/asheducation-2013.1.605 [DOI] [PubMed] [Google Scholar]