Abstract
Introduction
Virtual reality (VR) offers an innovative method to deliver non-pharmacological pain management. Distraction-based VR (VR-D) using immersive games to redirect attention has shown short-term pain reductions in various settings. To create lasting pain reduction, VR-based strategies must go beyond distraction. Guided relaxation-based VR (VR-GR) integrates pain-relieving mind–body based guided relaxation with VR, a novel therapy delivery mechanism. The primary aim of this study is to assess the impact of daily VR-GR, VR-D and 360 video (passive control) on pain intensity. We will also assess the impact of these interventions on pain unpleasantness, anxiety and opioid and benzodiazepine consumption. The secondary aim of this study will assess the impact of psychological factors (anxiety sensitivity and pain catastrophising) on pain following VR.
Methods and analysis
This is a single centre, prospective, randomised, clinical trial. Ninety children/adolescents, aged 8–18 years, presenting for Nuss repair of pectus excavatum will be randomised to 1 of 3 study arms (VR-GR, VR-D and 360 video). Patients will use the Starlight Xperience (Google Daydream) VR suite for 10 min. Patients randomised to VR-GR (n=30) will engage in guided relaxation/mindfulness with the Aurora application. Patients randomised to VR-D (n=30) will play 1 of 3 distraction-based games, and those randomised to the 360 video (n=30) will watch the Aurora application without audio instructions or sound. Primary outcome is pain intensity. Secondary outcomes include pain unpleasantness, anxiety and opioid and benzodiazepine consumption.
Ethics and dissemination
This study follows Standard Protocol Items: Recommendations for Interventional Trials guidelines. The protocol was approved by the Cincinnati Children’s Hospital Medical Center’s institutional review board. Patient recruitment began in July 2020. Written informed consent will be obtained for all participants. All information acquired will be disseminated via scientific meetings and published in peer-reviewed journals.
Trial registration number
Keywords: pain management, paediatric anaesthesia, complementary medicine, paediatric thoracic surgery, clinical trials
Strengths and limitations of this study.
This is a prospective, randomised clinical trial, which provides the best clinical evidence and support for virtual reality (VR) as an intervention.
This is the first study examining the use of VR-based interventions in a postoperative paediatric population.
Due to the nature of the study, it cannot be blinded.
One limitation is the specific patient population being studied: children and adolescents between the ages of 8 years and 18 years undergoing Nuss repair of pectus excavatum. Patient selection may limit generalisability of findings.
A second limitation is the conduction of the study at an academic, tertiary care, paediatric hospital; as such, these results may not be generalisable to patients in other clinical settings.
Introduction
Background and rationale
Children and adolescents with pain are at risk of opioid abuse,1 and many are initially exposed to narcotics prescribed to treat pain.2 More specifically, children and adolescents are at risk of persistent pain and opioid use after surgery, with the surgical period being a significant risk for the initial opioid exposure in children.3–5 Over 25% of patients with chronic pain who are on opioids were first exposed after surgery.6 Even short-term opioid use after surgery places a patient at risk of long-term abuse. Just 5 days of opioid use can increase the risk of persistent use, and use for more than 8 days may increase the risk to as much as 13.5%.7 While this risk is well documented in adults, few studies address this topic in children.8 A recent retrospective study of opioid-naïve surgical patients found persistent opioid use in 4.8% of adolescents versus 0.1% in a matched, non-surgical cohort, equating to a 50-fold increase in risk.3
Pectus excavatum, a depression of the anterior chest wall, is often corrected via the Nuss repair, a minimally invasive procedure in which a bar(s) is inserted beneath the sternum and flipped to elevate the chest.9 Although minimally invasive, this procedure is associated with significant postoperative pain.10 Despite efforts at multimodal therapy, the percentage of patients experiencing severe pain after surgery has not changed over the last 20 years.11 12 Existing paediatric studies have identified an approximately 20% incidence of persistent postsurgical pain beyond what is expected from surgery alone.13 While 80% of these patients recover within about 1 month, 20% maintain a reduced quality of life secondary to persistent pain.13 While the consequences of opioids exposure are significant, poorly controlled postsurgical pain is also problematic. Ineffective postoperative pain management is associated with increased morbidity, poorer physical functioning, longer recover and higher cost.14 15 Multimodal pain management requires the exploration of safe, effective, non-pharmacological strategies that reduce pain and opioid consumption.16 Non-pharmacological methods to treat pain can both improve analgesia after surgery and decrease opioid exposure, a risk factor for future addiction.1
Virtual reality (VR) may offer a safe, innovative, non-pharmacological tool with the potential to decrease pain and medication consumption. VR provides an immersive, multisensory, three-dimensional environment that enables individuals to have modified experiences of reality by creating a sense of ‘presence’, making it an excellent candidate for distraction-based therapy.17 Distraction-based VR (VR-D) is hypothesised to reduce pain through the redirection of attention augmented by the immersion created by VR.18 19 VR-D has been used during painful procedures, the postoperative period and labour to help decrease pain by redirecting attention.20–32 These studies show transient reductions in pain insufficient to treat prolonged acute pain experiences,33 34 including postoperative pain, suggesting that redirection of attention alone is not adequate to help manage pain that is more sustained. Comparatively, non-pharmacological alternatives that use mind–body-based therapies delivered in a traditional format, like relaxation and slow breathing, are able to decrease anxiety and pain in children undergoing surgery.35 Unlike distraction, slow breathing during relaxation results in increased heart rate variability,36 which activates the parasympathetic nervous system, resulting in pain reduction.37 38 However, despite their efficacy, these therapies are fraught with challenges, such as barriers to accessing care, high cost, need for multiple visits and provider shortages.39 VR can increase accessibility to these mind–body therapies and enhance acceptability, motivation and adherence in paediatric patients compared with methods without VR.40 Combining strategies of traditional mind–body therapies, like relaxation and slow breathing, with the immersive nature of VR opens new possibilities for multimodal analgesia in the paediatric population and has the potential to simultaneously minimise acute postoperative pain and opioid consumption. Guided relaxation-based VR (VR-GR) is a promising mechanism to deliver mind–body-based therapy, improve postoperative pain control and avoid challenges common with mind–body therapies delivered in the traditional format.
We have designed a prospective, randomised, clinical trial to assess the efficacy of VR-GR to decrease pain, anxiety and opioid consumption in children and adolescents undergoing Nuss repair of pectus excavatum and hypothesise that VR-GR will be more effective at reducing pain, anxiety and opioid consumption in this population than VR-D or a passive control.
Objectives
The primary objective of this study is to determine the impact of VR-GR on pain intensity in children and adolescents undergoing Nuss repair of pectus excavatum compared with VR-D and 360 video both during the hospitalisation (primary) and up to 1 month following discharge (secondary). We will also assess the impact of VR-GR on pain unpleasantness, anxiety, and opioid and benzodiazepine consumption compared with VR-D and 360 video. The secondary objective of this study is to determine the role of anxiety sensitivity and pain catastrophising on changes in pain and anxiety following VR-GR, VR-D and 360 video both during hospitalisation and 1 month after discharge in this same patient population using standard questionnaires.
Methods and analysis
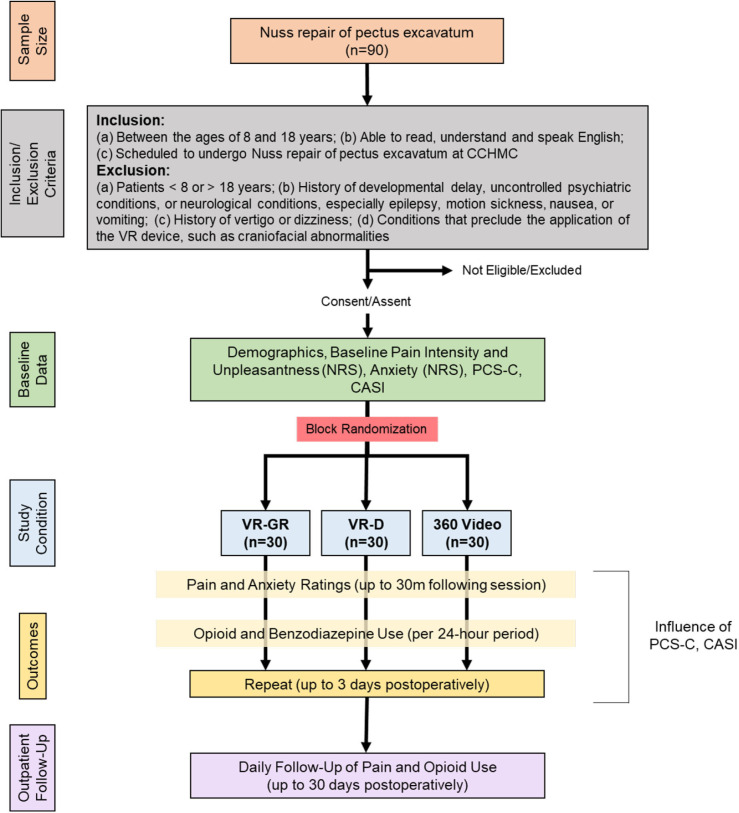
The FOREVR Peds study is a single centre, prospective, unblinded, randomised clinical trial with three groups: VR-GR, VR-D or 360 video. A daily, 10-minute session of these respective interventions is administered to children and adolescents between the age of 8 years and 18 years undergoing Nuss repair of pectus excavatum for up to 3 days after surgery. The primary objective is to determine the impact of VR-GR on pain intensity compared with VR-D and 360 video during hospitalisation. Patient recruitment began in July 2020 and we anticipate a total study duration of 2 years. This study protocol complies with the Standard Protocol Items: Recommendations for Interventional Trials Statement as well as the Consolidated Standards of Reporting Trials Statement (figure 1). The study was registered at ClinicalTrials.gov on 3 April 2020 and all trial registration data can be found on the ClinicalTrials.gov website.
Figure 1.
Study flow chart (CONSORT Diagram). CASI, Child Anxiety Sensitivity Index; CCHMC, Cincinnati Children’s Hospital Medical Center; CONSORT, Consolidated Standards of Reporting Trials; NRS, Numerical Rating Scale; PCS-C, Pain Catastrophizing Scale for Children; VR, virtual reality; VR-D, distraction-based VR; VR-GR, guided relaxation-based VR.
Study setting
Cincinnati Children’s Hospital Medical Center (CCHMC), a tertiary care, academic, paediatric hospital.
Study design
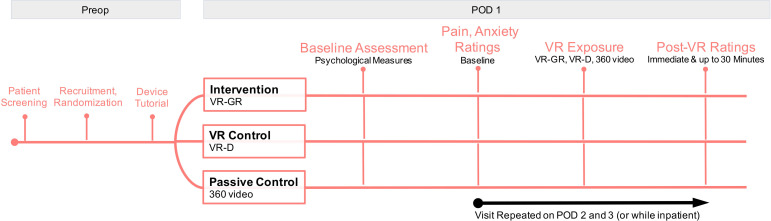
This is a single-centre, prospective, randomised clinical trial of children and adolescents with acute postoperative pain following Nuss repair of pectus excavatum to assess the impact of multiple VR-GR sessions on pain and medication utilisation in relation to patient anxiety and pain catastrophising. Figure 1 summarises the study design. We are assessing the acute and long-term impact of each intervention on changes in pain intensity, pain unpleasantness, anxiety, and opioid and benzodiazepine consumption during hospitalisation and following discharge, where acute impact on pain intensity is the primary focus. Figure 2 summarises this experimental design. All patients are managed postoperatively via the pectus surgery pain management protocol, which standardises all non-controlled medications received by patients. Patients enrolled in this study are managed per this protocol (standard care) and receive the additional intervention of VR or 360 video.
Figure 2.
Experimental design of the study. POD, postoperative day; VR, virtual reality; VR-D, distraction-based VR; VR-GR, guided relaxation-based VR.
Outcome measures
Primary outcome
Our primary outcome is pain intensity following daily VR-GR, VR-D and 360 video in our population during hospitalisation.
Secondary outcomes
Our secondary outcomes are pain unpleasantness, anxiety, and opioid and benzodiazepine consumption following daily VR-GR, VR-D and 360 video in our population during hospitalisation and up to 1 month following discharge. We will also assess the impact of pain catastrophising and anxiety sensitivity on these outcomes.
Participants
We are recruiting 90 adolescents (30 per group) between the age of 8 years and 18 years undergoing Nuss repair of pectus excavatum. Eligibility criteria have been chosen to correspond with our prior work and result in a population with whom our group has substantial experience.
Inclusion criteria
Patients are included based on the following criteria: (a) between the ages of 8 years and 18 years; (b) able to read, understand and speak English; and (c) scheduled to undergo Nuss repair of pectus excavatum at CCHMC.
Exclusion criteria
Patients are excluded for the following reasons: (a) patients <8 years or >18 years of age at the time study enrolment; (b) history of significant developmental delay, underlying psychiatric disease associated with delusions or hallucinations, or significant neurological conditions, including epilepsy, severe motion sickness or active nausea/vomiting; and (c) conditions that preclude application and use of the VR device, including craniofacial abnormalities.
Randomisation
Eligible patients are randomly assigned in a 1:1:1 fashion to the following three study groups: VR-GR, VR-D and 360 video (passive control) following study enrollment based on subject number. The randomisation scheme was generated using an online randomising tool (www.randomizer.org) to assign patient study numbers into 1 of 3 groups. The randomisation scheme is stored in our Research Electronic Data Capture (REDCap) database (https://www.project-redcap.org/), a secure web application for building and maintaining secure databases and surveys. Randomisation has allowed for equal distribution of demographic characteristics among the three groups. Our clinical research coordinator (CRC) is responsible for assigning patients to each study group based on this randomisation scheme.
Interventions
All patients use the VR device and software from the Starlight Children’s Foundation, the Starlight Xperience device (Google Daydream). This VR device is commercially available and is not Food and Drug Administration (FDA) regulated. The Google Daydream is an all-in-one headset, so no additional hardware is required to deliver the VR experience. A set of headphones, included with the headset, is used to deliver audio instructions and sound, creating a fully immersive experience. Patients are visited daily to undergo a single, 10-minute session with the VR headset for up to 3 days after surgery. The 10-minute daily session is based on a standard time duration and frequency for mind–body therapies.38 41 We will work with the care team to standardise the timing of the daily study visit for all patients.
VR-GR (intervention)
Patients randomised to the VR-GR group use the Aurora application to receive relaxation/mindfulness content. This application acts as an escape for patients as well as a tool to teach slow breathing and relaxation techniques. Patients are transported to an alpine meadow with dynamic daytime, and later, night-time scenery. With the help of a 10 min narrative, participants are guided to sync their breathing with their surroundings: the rise and fall of a floating butterfly during the day and the movement of the northern lights in the sky at night.
VR-D (active control)
Patients randomised to the VR-D group choose one of three distraction-based games: Space Pups, Pebbles the Penguin or Wonderglade. Each provides a similar distraction-based experience for the user. (1) Space Pups: user controls an astronaut space puppy and works to collect treats to the beat of the music. (2) Pebbles the Penguin: user controls a penguin sliding down a mountain and works to collect shiny pebbles to unlock new power-ups. (3) Wonderglade: 5 different carnival-themed mini-games like basketball, miniature golf and racing.
360 video (passive control)
Patients view a 360 video of a nature scene like the Aurora application but will not receive a guided tutorial on how to relax and sync their breathing with the application. They also do not receive any audio instructions or sound, decreasing the fully immersive experience.
Patient recruitment
On average, 125–150 Nuss repairs are performed at CCHMC annually. We plan to enrol a total of 90 patients. Patients scheduled to undergo Nuss repair of pectus excavatum are being recruited continuously throughout the course of the study until enrolment targets are met. We are recruiting about two patients per week given our surgical volume. We receive notification of all Nuss repair surgery bookings by the surgery schedulers to identify possible participants, allowing for eligible patients to be identified greater than 1 week prior to surgery. The operating room schedule as well as the surgical patient list is screened for eligible patients based on age criteria. Patients meeting age criteria undergo screening of their available electronic medical record to assess study eligibility. Eligible patients are approached prior to surgery. If patients wish to participate, appropriate consent (and assent for patients >11 years of age) is obtained and eligibility criteria are verified. Patients are randomised (1:1:1 ratio) to VR-GR (intervention), VR-D (active control) and 360 video (passive control). A randomisation scheme was created prior to the start of the study using an online tool (www.randomizer.org) and patients are assigned to a group based on study number. Patients receive a tutorial on the VR device at the time of enrolment. Demographic, health information and medical history are recorded and documented in the REDCap database. Patients are given a small stipend for participation to help increase recruitment and adherence. Our CRC is responsible for enroling patients.
Study visits
Patients are visited daily to undergo a single, 10-minute session. Every effort is made to ensure consistency in timing of the visits for all patients. Prior to surgery, patients complete two validated questionnaires to assess baseline trait measures: the Pain Catastrophizing Scale for Children (PCS-C)42 and the Child Anxiety Sensitivity Index (CASI).43 They also complete a health history questionnaire and a baseline pain intensity, pain unpleasantness and anxiety rating is obtained using the Numerical Rating Scale (NRS).44 45 Pain intensity, pain unpleasantness and anxiety ratings are repeated immediately, 15 min, and 30 min following session completion. Patients typically remain in the hospital for 3–4 days following Nuss repair. During their inpatient stay, participants have daily study visits, repeating the same process as the first session; patients will not repeat the PCS-C or CASI surveys. At the last visit, patients are given a satisfaction survey to gather qualitative feedback about the VR experience.
Data collection
For each eligible participant, data are collected from patient history/interview and the electronic medical record in a standardised case report form in the REDCap system by a CRC or student who maintain Collaborative Institutional Training Initiative (CITI) training in accordance with our local institutional review board (IRB) under the direct supervision of the principal investigator (PI).
Total opioid and benzodiazepine usage are collected from the electronic medical record for 24 hours after each session in mg/kg/day to account for patient weight. All medication consumption is collected for assessment of non-opioid analgesics and to ensure consistency with the pectus pain management protocol. To assess pain intensity and unpleasantness after hospital discharge, patients use a daily log to record pain scores using the NRS for 1 month. We use electronic capture pill dispenser (https://www.informationmediary.com/nfc-smart-packaging-devices/ecap-smart-pill-bottle/) to document medication consumption. Weekly reminders are sent using Twilio, and telephone follow-up is done at 2 weeks and 1 month to help improve patient adherence. Prescription cross-verification is done using controlled substance reporting databases for Ohio, Kentucky and Indiana (Ohio Automated Rx Reporting System (OARRS), Kentucky All Schedule Prescription Electronic Reporting (KASPER) and Indiana's Prescription Drug Monitoring Program (INSPECT), respectively) to verify data collected from patient logs and eCAP.
Measurements
(a) Pain intensity, pain unpleasantness and anxiety ratings are assessed using the NRS.44 45 (b) Pain catastrophising is assessed using PCS-C.42 (c) Anxiety sensitivity is assessed using CASI.43 (d) Total opioid and benzodiazepine usage is collected from EPIC for 24 hours after each session and up to 1-month post-hospital discharge via eCAP. Opioid consumption is converted to morphine equivalents in mg/kg/day. All medication consumption is collected for assessment of non-opioid analgesics and converted to mg/kg/day. Box 1 summarises the measurements used in the study.
Box 1. Scales and questionnaires used in the study.
Numerical Rating Scale (NRS): NRS where children are asked to give a number on a scale of 0–10 of how bad their pain hurts, with 0 being no pain and 10 being the worst pain of their life.
Pain Catastrophizing Scale for Children (PCS-C): children rate 13 items assessing rumination, magnification and helplessness related to thoughts about pain. PCS summary scores can be interpreted as low (0–14), moderate (15–25) and high (≥26). Internal reliability for our distraction-based virtual reality (VR-D) pilot data was 0.94 (Cronbach’s α).
Child Anxiety Sensitivity Index (CASI): 18-item self-report tool designed to measure symptoms of anxiety in children and adolescents, with total scores ranging from 18 to 54. Internal reliability for our VR-D pilot data was 0.84 (Cronbach’s α).
Sample size calculation
Sample size calculation is based on preliminary data assessing the impact of VR-D to affect changes in pain intensity in children and adolescents following surgery (unpublished). Preliminary data showed that the average change in pain intensity across time was −1, with SD 1.2 and correlation between measurement pairs of 0.88. Assuming similar results in the passive control group, sample size of 30 per group will have 80% power to detect differences in mean changes of one between VR-GR and the two control groups. We expect a difference of ≥1 between VR-GR and VR-D to emerge with multiple sessions as proposed with this study. Significance (alpha) is 0.025 to control for two comparisons. We are recruiting 90 patients, 30 per group.
Statistical analysis
Statistical analysis will be done with SAS V.9.4. Descriptive statistics will be calculated and summarised (continuous: mean±SD; categorical: frequency percentage). Prior to analysis, assumption of normality will be assessed for continuous variables and corrected using log transformation when appropriate. All statistical tests will be two-sided. Bonferroni correction will be made as appropriate for comparisons. Change from baseline for primary and secondary outcomes will be tested for normality and deviation from zero using paired tests (t-test or signed-rank, as appropriate) at individual time points after interventions. Change from baseline will be compared between groups using two-sample t-test or Wilcoxon rank-sum test (between two groups, ie, VR-GR vs VR-D and VR-GR vs 360 video) and ANOVA or Kruskal-Wallis test (across three groups) at individual time points after the sessions.
Primary analysis for the primary outcome, pain intensity during hospitalisation, will be conducted on the intent to treat population, which is defined as all patients who were randomised. All patients who were randomised will be included in analysis and analysed according to the group to which they were originally assigned, regardless of the treatment (if any) they received. The primary analysis will be mixed-effects models for repeated measures with baseline value, intervention group, time (0 min, 15 min and 30 min after intervention), and group and time interaction to test the hypothesis that VR-GR reduces pain more than controls. Similar analysis will be run for secondary outcomes, including anxiety, and opioid and benzodiazepine consumption. Potential covariates (such as age and sex) will be tested for association with the outcomes using univariate approaches and included in the mixed-effect models if significant. Pain and opioid use 1 month after discharge will be compared between intervention groups using ANOVA (with adjustment of possible covariates) or Kruskal-Wallis test, as appropriate, based on data distribution.
Anxiety sensitivity (or pain catastrophising) will be dichotomised using the sample median (or tertiles depending on distribution) and its effect on response to intervention (change in pain intensity from baseline) will be tested using the two-sample t-test or Wilcoxon rank-sum test, as appropriate at individual time points (0 min, 15 min and 30 min) after intervention. Mixed-effects models for repeated measures (change in pain intensity from baseline) with high or low anxiety sensitivity (or pain catastrophising) group, time (0 min, 15 min and 30 min after intervention), and group and time interaction will be used to test the hypothesis that patients with greater anxiety sensitivity and pain catastrophising will have a larger reduction in pain versus patients with less anxiety sensitivity and pain catastrophising. Assuming the same SD and correlation between pain intensity measurement pairs from the primary power analysis, sample size of 15 per group (high vs low anxiety or pain catastrophising dichotomised at median for the VR-GR group) will have 80% power to detect differences in mean changes of pain intensity of 1.3 between the two groups, with α=0.05. The same analysis will be repeated for pain unpleasantness and anxiety.
We are making every effort to ensure that at least one daily VR session is completed for each study participant and that all data extraction is complete to avoid missing data. We are assessing missing data for all study variables. Chart review for missing data on demographics, medical history, etc is performed when feasible. Missing outcome data will be statistically imputed using multiple imputation, and a sensitivity analysis will be conducted to compare analysis results with and without imputation. We do not anticipate that age will have an impact on our findings. Although the trial is not powered to detect overall differences between groups by age, we will perform an exploratory analysis in which we will stratify by age (age 8–13 years and 14–18 years) to explore a possible influence of age.
Ethics, safety and dissemination
Ethics
This study is being conducted in accordance with the rules and regulations applicable to the conduct of ethical research and this study protocol has been approved by the IRB at CCHMC (IRB #2019–1090). This protocol includes clear delineation of the protocol version identifier and date on each protocol amendment submitted to the IRB; clear delineation of plans for data entry, coding, security and storage; clear delineation of mechanisms to ensure patient confidentiality, including how personal information will be collected, shared and maintained in order to protect confidentiality before, during and after the trial; statements regarding who has access to data collected during this study; and a model consent form and other related documentation given to participants and/or guardians. We do not anticipate any major protocol modifications during the duration of this study.
Safety
It is anticipated that the risk to participants in this study is minimal. The specific VR device used in this study is a minimal risk device, and because it is considered a relaxation device by the FDA, it is not regulated as a clinical device. Risks specific to VR are minimal, with the greatest risk being motion sickness and/or nausea while the headset is in place.46 There is a theoretical risk of inducing seizures (0.025% in a paediatric data set supplied by a similar Samsung device). We are minimising these risks by excluding patients with a history of significant neurological disorders, including epilepsy and severe motion sickness/nausea. Patients are also explicitly instructed to remove the headset should any side effects or discomfort occur. The PI continually monitors all risks to the participants. Weekly lab meetings are used to address quality assurance and safety concerns with the study. Research personnel are instructed to inform the PI immediately with any safety concerns or adverse events (AEs). The IRB will also be updated when any serious AEs (SAEs) occur or when mild or moderate AEs determined to be a result from study participation occur. SAEs that are unanticipated, serious and possibly related to study participation will be reported to the data safety monitoring committee (DSMC), IRB and any other necessary study regulatory committee. We do not anticipate any SAEs that would require stopping this trial early. Therefore, we do not plan to conduct interim analysis for safety. This consideration will change if SAEs are reported during the study.
Although the risk to patients from this clinical trial is low, a DSMC is being used to monitor safety. The DSMC is composed of three experts (clinical research, pain management and digital technology) who are independent of the protocol. The DSMC will report to the IRB. This protocol is approved by the IRB at CCHMC in compliance with existing regulations and policies for the conduct of clinical research.
Dissemination
Unique data will be obtained from this research and will be widely disseminated through conference presentations at national and international meetings and through publication of manuscripts in peer-reviewed publications. Participants may receive trial results if interested. All authors are eligible to participate in dissemination and we do not plan to use professional writers to disseminate study results.
Patient and public involvement
No patients or members of the public were involved in the design, recruitment or conduct of this study. Consideration of the burden of the intervention and time required to participate in this research was assessed during pilot data collection using VR in the acute postoperative pain population at our institution and information gathered from this pilot study helped guide the development of this clinical trial. Participants may receive information about study results if they wish via a letter describing results to participants. We will share access to the full protocol to requesting individuals/institutions.
Supplementary Material
Footnotes
Correction notice: This article has been corrected since it first published. The provenance and peer review statement has been included.
Contributors: VAO, SEW and CDK contributed to the conception of this idea, the design of the research protocol and study, and the writing of this manuscript. KTO'C, COB, GWM, SMG and KJG provided input regarding the design and implementation of the study protocol and procedures. LD and GY contributed to the design of the research protocol and the statistical analysis plan development. All authors revised and modified this manuscript. They will all approve the final version.
Funding: This study is supported by an internal Cincinnati Children’s Hospital Medical Center's research grant, research innovation/pilot funding programme as well as the Department of Anesthesiology. Award/grant number not applicable.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Hudgins JD, Porter JJ, Monuteaux MC, et al. Trends in opioid prescribing for adolescents and young adults in ambulatory care settings. Pediatrics 2019;143:e20181578–9. 10.1542/peds.2018-1578 [DOI] [PubMed] [Google Scholar]
- 2.Johnston LD, O’Malley PM. Key findings on adolescent drug use. National survey results on drug use 1975-2017, 2017. [Google Scholar]
- 3.Harbaugh CM, Lee JS, Hu HM, et al. Persistent opioid use among pediatric patients after surgery. Pediatrics 2018;141 10.1542/peds.2017-2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 2003;160:1041–52. 10.1176/appi.ajp.160.6.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabbitts JA, Kain Z. Perioperative care for adolescents undergoing major surgery: a biopsychosocial conceptual framework. Anesth Analg 2019;129:1181–4. 10.1213/ANE.0000000000004048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callinan CE, Neuman MD, Lacy KE, et al. The initiation of chronic opioids: a survey of chronic pain patients. J Pain 2017;18:360–5. 10.1016/j.jpain.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 7.Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017;66:265–9. 10.15585/mmwr.mm6610a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortier MA, Chou J, Maurer EL, et al. Acute to chronic postoperative pain in children: preliminary findings. J Pediatr Surg 2011;46:1700–5. 10.1016/j.jpedsurg.2011.03.074 [DOI] [PubMed] [Google Scholar]
- 9.Kelly RE, Shamberger RC, Mellins RB, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg 2007;205:205–16. 10.1016/j.jamcollsurg.2007.03.027 [DOI] [PubMed] [Google Scholar]
- 10.Muhly WT, Beltran RJ, Bielsky A, et al. Perioperative management and in-hospital outcomes after minimally invasive repair of pectus excavatum: a multicenter registry report from the society for pediatric anesthesia improvement network. Anesth Analg 2019;128:315–27. 10.1213/ANE.0000000000003829 [DOI] [PubMed] [Google Scholar]
- 11.Groenewald CB, Rabbitts JA, Schroeder DR, et al. Prevalence of moderate-severe pain in hospitalized children. Paediatr Anaesth 2012;22:661–8. 10.1111/j.1460-9592.2012.03807.x [DOI] [PubMed] [Google Scholar]
- 12.Kozlowski LJ, Kost-Byerly S, Colantuoni E, et al. Pain prevalence, intensity, assessment and management in a hospitalized pediatric population. Pain Manag Nurs 2014;15:22–35. 10.1016/j.pmn.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 13.Rabbitts JA, Zhou C, Groenewald CB, et al. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain 2015;156:2383–9. 10.1097/j.pain.0000000000000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res 2017;10:2287–98. 10.2147/JPR.S144066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters ML, Sommer M, de Rijke JM, et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg 2007;245:487–94. 10.1097/01.sla.0000245495.79781.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tick H, Nielsen A, Pelletier KR, et al. Evidence-based nonpharmacologic strategies for comprehensive pain care: the Consortium pain task force white paper. Explore 2018;14:177–211. 10.1016/j.explore.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 17.Carrougher GJ, Hoffman HG, Nakamura D, et al. The effect of virtual reality on pain and range of motion in adults with burn injuries. J Burn Care Res 2009;30:785–91. 10.1097/BCR.0b013e3181b485d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tashjian VC, Mosadeghi S, Howard AR, et al. Virtual reality for management of pain in hospitalized patients: results of a controlled trial. JMIR Ment Health 2017;4:e9. 10.2196/mental.7387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegel B, Fuller G, Lopez M, et al. Virtual reality for management of pain in hospitalized patients: a randomized comparative effectiveness trial. PLoS One 2019;14:e0219115. 10.1371/journal.pone.0219115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris LD, Louw QA, Grimmer-Somers K. The effectiveness of virtual reality on reducing pain and anxiety in burn injury patients: a systematic review. Clin J Pain 2009;25:815–26. 10.1097/AJP.0b013e3181aaa909 [DOI] [PubMed] [Google Scholar]
- 21.Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psychol Rev 2010;30:1011–8. 10.1016/j.cpr.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Hoffman HG, Doctor JN, Patterson DR, et al. Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain 2000;85:305–9. 10.1016/S0304-3959(99)00275-4 [DOI] [PubMed] [Google Scholar]
- 23.Li A, Montaño Z, Chen VJ, et al. Virtual reality and pain management: current trends and future directions. Pain Manag 2011;1:147–57. 10.2217/pmt.10.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrett B, Taverner T, Masinde W, et al. A rapid evidence assessment of immersive virtual reality as an adjunct therapy in acute pain management in clinical practice. Clin J Pain 2014;30:1089–98. 10.1097/AJP.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 25.Gold JI, Kim SH, Kant AJ, et al. Effectiveness of virtual reality for pediatric pain distraction during i.v. placement. Cyberpsychol Behav 2006;9:207–12. 10.1089/cpb.2006.9.207 [DOI] [PubMed] [Google Scholar]
- 26.Furman E, Jasinevicius TR, Bissada NF, et al. Virtual reality distraction for pain control during periodontal scaling and root planing procedures. J Am Dent Assoc 2009;140:1508–16. 10.14219/jada.archive.2009.0102 [DOI] [PubMed] [Google Scholar]
- 27.Gold JI, Mahrer NE. Is virtual reality ready for prime time in the medical space? A randomized control trial of pediatric virtual reality for acute procedural pain management. J Pediatr Psychol 2018;43:266–75. 10.1093/jpepsy/jsx129 [DOI] [PubMed] [Google Scholar]
- 28.Indovina P, Barone D, Gallo L, et al. Virtual reality as a distraction intervention to relieve pain and distress during medical procedures: a comprehensive literature review. Clin J Pain 2018;34:858–77. 10.1097/AJP.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 29.Cacau LdeAP, Oliveira GU, Maynard LG, et al. The use of the virtual reality as intervention tool in the postoperative of cardiac surgery. Rev Bras Cir Cardiovasc 2013;28:281–9. 10.5935/1678-9741.20130039 [DOI] [PubMed] [Google Scholar]
- 30.Mosso-Vázquez JL, Gao K, Wiederhold BK, et al. Virtual reality for pain management in cardiac surgery. Cyberpsychol Behav Soc Netw 2014;17:371–8. 10.1089/cyber.2014.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frey DP, Bauer ME, Bell CL, et al. Virtual reality analgesia in labor: the VRAIL pilot Study-A preliminary randomized controlled trial suggesting benefit of immersive virtual reality analgesia in unmedicated laboring women. Anesth Analg 2019;128:e93–6. 10.1213/ANE.0000000000003649 [DOI] [PubMed] [Google Scholar]
- 32.JahaniShoorab N, Ebrahimzadeh Zagami S, Nahvi A, et al. The effect of virtual reality on pain in Primiparity women during episiotomy repair: a randomize clinical trial. Iran J Med Sci 2015;40:219–24. [PMC free article] [PubMed] [Google Scholar]
- 33.Van Ryckeghem DM, Van Damme S, Eccleston C, et al. The efficacy of attentional distraction and sensory monitoring in chronic pain patients: a meta-analysis. Clin Psychol Rev 2018;59:16–29. 10.1016/j.cpr.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 34.Gupta A, Scott K, Dukewich M. Innovative technology using virtual reality in the treatment of pain: does it reduce pain via distraction, or is there more to it? Pain Med 2018;19:151–9. 10.1093/pm/pnx109 [DOI] [PubMed] [Google Scholar]
- 35.Vagnoli L, Bettini A, Amore E, et al. Relaxation-guided imagery reduces perioperative anxiety and pain in children: a randomized study. Eur J Pediatr 2019;178:913–21. 10.1007/s00431-019-03376-x [DOI] [PubMed] [Google Scholar]
- 36.Zaccaro A, Piarulli A, Laurino M, et al. How breath-control can change your life: a systematic review on Psycho-physiological correlates of slow breathing. Front Hum Neurosci 2018;12:353. 10.3389/fnhum.2018.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehrer PM, Gevirtz R. Heart rate variability biofeedback: how and why does it work? Front Psychol 2014;5:756. 10.3389/fpsyg.2014.00756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowder E, Gevirtz R, Shapiro W, et al. Restoration of vagal tone: a possible mechanism for functional abdominal pain. Appl Psychophysiol Biofeedback 2010;35:199–206. 10.1007/s10484-010-9128-8 [DOI] [PubMed] [Google Scholar]
- 39.Peng P, Stinson JN, Choiniere M, et al. Dedicated multidisciplinary pain management centres for children in Canada: the current status. Can J Anaesth 2007;54:985–91. 10.1007/BF03016632 [DOI] [PubMed] [Google Scholar]
- 40.Harris K, Reid D. The influence of virtual reality play on children's motivation. Can J Occup Ther 2005;72:21–9. 10.1177/000841740507200107 [DOI] [PubMed] [Google Scholar]
- 41.Prinsloo GE, Derman WE, Lambert MI, et al. The effect of a single session of short duration biofeedback-induced deep breathing on measures of heart rate variability during laboratory-induced cognitive stress: a pilot study. Appl Psychophysiol Biofeedback 2013;38:81–90. 10.1007/s10484-013-9210-0 [DOI] [PubMed] [Google Scholar]
- 42.Crombez G, Bijttebier P, Eccleston C, et al. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain 2003;104:639–46. 10.1016/S0304-3959(03)00121-0 [DOI] [PubMed] [Google Scholar]
- 43.Silverman WK, Goedhart AW, Barrett P, et al. The facets of anxiety sensitivity represented in the childhood anxiety sensitivity index: confirmatory analyses of factor models from past studies. J Abnorm Psychol 2003;112:364–74. 10.1037/0021-843X.112.3.364 [DOI] [PubMed] [Google Scholar]
- 44.Tsze DS, von Baeyer CL, Pahalyants V, et al. Validity and Reliability of the Verbal Numerical Rating Scale for Children Aged 4 to 17 Years With Acute Pain. Ann Emerg Med 2018;71:691–702. 10.1016/j.annemergmed.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miró J, Castarlenas E, Huguet A. Evidence for the use of a numerical rating scale to assess the intensity of pediatric pain. Eur J Pain 2009;13:1089–95. 10.1016/j.ejpain.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 46.Nichols S, Patel H. Health and safety implications of virtual reality: a review of empirical evidence. Appl Ergon 2002;33:251–71. 10.1016/S0003-6870(02)00020-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.