Abstract
Background:
The incidence of and risk factors for Hirschsprung’s-associated enterocolitis (HAEC) following pull-through have been limited to single institutions studies. We characterized the incidence of, risk factors for, and consequences of post-operative HAEC.
Methods:
We identified children with Hirschsprung’s Disease (HD) at US Children’s Hospitals from 2007 to 2017 with and an associated pull-through operation at less than 1 year of age. HAEC readmissions were identified using ICD9/10 Diagnosis Codes and antibiotic administration. Hierarchical logistic regression models were developed for the risk factors for HAEC after pull-through and effects of recurrent HAEC on HD-related reoperations.
Results:
We identified 2030 children with HD, and 138 (7%) who had two or more readmissions related to HAEC. The frequency of recurrent HAEC by hospital ranged from 0 to 33%. Pre-operative HAEC, history of central nervous system infection, and congenital neurologic anomalies were associated with increased risk of recurrent HAEC. Recurrent HAEC was associated with HD-specific re-operation (OR 5.2, CI 3.3–8.1, p < 0.001); however, it was not associated with risk of in-hospital mortality (OR 3.3, CI 0.88–12.1, p = 0.08).
Conclusions:
HAEC following pull-through occurs in a large proportion of infants with HD and predicts reoperation. Multicenter studies are needed to develop prediction models and treatment protocols for HAEC.
Level of Evidence:
II
Type of Study:
Retrospective cohort study.
Keywords: Hirschsprung disease, Enterocolitis, Outcome
For children with Hirschsprung disease (HD), Hirschsprung-associated enterocolitis (HAEC) remains one of the most significant causes of morbidity and mortality [1–3]. HAEC can occur both before and after definitive pull-through operation for HD. Studies estimating the rates of pre- and post-operative HAEC have largely been single-center studies and have ranged widely: for pre-operative HAEC 15%–50% and for post-operative HAEC from 2%–33% [2,4–6]. Similarly, studies to identify risk factors for HAEC have also largely relied on single-center data. Previously identified risk factors for HAEC include delay in diagnosis, the length of intestine involved in HD, prior episodes of HAEC, female gender, trisomy 21, and presence of other congenital anomalies including cardiac and neurologic anomalies [2, 4, 7–10].
Few large database or multi-center studies of HD and HAEC exist. One reason for the absence of these studies has been a lack of a clear clinical or coding definition of HAEC. Pastor et al. surveyed experts in the field using a Delphi analysis to develop a consensus definition to attempt to standardize HAEC as an outcome that could be measured and compared across centers and studies [11]. This analysis was used to develop a standardized HAEC score. Since that time multiple studies have been done to validate this score with patient data from multiple hospitals to assess the reliability and applicability of this definition and scoring system [12–14]. Nevertheless, there are no standardized International Classification of Diseases (ICD) codes for HAEC, which is a significant limitation in the ability to study HAEC on a national level using administrative databases [15]. The goal of this study was to use a national database to better understand the incidence of HAEC at US children’s hospitals and to identify risk factors for recurrent post-operative HAEC and its effects on condition-specific re-operation, in-hospital mortality, and hospital costs.
1. Methods
1.1. Data source and study population
We conducted a retrospective cohort study using the Pediatric Health Information System Database (PHIS). PHIS is an administrative database that contains inpatient, emergency department, ambulatory surgery and observation encounter-level data from over 49 not-for-profit, tertiary care pediatric hospitals in the United States. These hospitals are affiliated with the Children’s Hospital Association (Lenexa, KS). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. Portions of the data submission and data quality processes for the PHIS database are managed by Truven Health Analytics (Ann Arbor, MI). For the purposes of external benchmarking, participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. Nearly all of these hospitals also submit resource utilization data (e.g. pharmaceuticals, imaging, and laboratory) into PHIS. Data are de-identified at the time of data submission, and data are subjected to a number of reliability and validity checks before being included in the database. The study was approved by the University of Utah institutional review board.
1.2. Patient selection
Patients were included if they had an ICD 9/10 diagnosis code of Hirschsprung disease and ICD 9/10 Procedure code for a pull-through procedure from January 1st 2006 until December 31st 2017. A list of ICD 9/10 diagnosis and procedure codes are provided in the supplementary appendix. Children were excluded if they were admitted for their initial pull-through procedure after 1 year of life.
1.3. Primary exposure and outcomes
All encounters in PHIS associated with the cohort of patients with HD and a pull-through that had HD as a key diagnosis code were selected (Supplementary Fig. 1). We undertook a review of patients at our institution who were known to have had one or more episodes of HAEC and identified all diagnosis codes associated with those admissions that were consistent with HAEC (See Supplementary Table 2). Using this code list, all related ICD 9 and 10 diagnosis codes were reviewed to generate a complete list of codes associated with HAEC-type symptoms. All encounters associated with our HD cohort were then filtered for the presence of one of these diagnosis codes (Supplementary Fig. 1). As a final filter, all encounters with these diagnoses underwent an additional filter for the administration of antibiotics with gram negative coverage using the clinical transaction classification (CTC) for medication administration (Supplementary Fig. 1). As a sensitivity analysis to determine if a similar analysis could be conducted in other databases our analysis was also run with all patients with diagnosis codes consistent with HAEC with and without antibiotic administration.
The primary outcome of interest was identification of risk factors for recurrent (two or more episodes during the duration of follow up) HAEC following pull-through. Our secondary outcomes of interest were in-hospital mortality, condition-specific re-operation in the first year following pull-through, and cost of recurrent HAEC (total billed hospital charges in the first year of life). A table of procedure specific re-operation codes is provided in the supplementary appendix.
1.4. Covariates
The PHIS database provides data regarding patient demographics, including race, ethnicity, payer sources, and birthweight, and median household income. Patient gestational age was determined using a combination of the specific gestational age data provided in PHIS and ICD-9 diagnosis codes representing gestational age. We used the Agency for Healthcare Research and Quality Clinical Classification Software (CCS) to model patient comorbidities and other surgical procedures based on encounter ICD-9 diagnosis and procedure codes (See Supplementary Table 4). The following covariates were included: congenital cardiac anomalies, neurologic anomalies, chromosomal abnormalities, history of central nervous system infection, cleft lip and palate, congenital diaphragmatic hernia, previous cardiac arrest or hypoxic episode, and ostomy placement prior to definitive pull-through procedure. Using hospital and surgeon identifiers in PHIS, annual pull-through volume was calculated for both hospitals and individual surgeons and averaged over the 11-year period. Hospitals and surgeons were then divided into tertiles based on volume and were incorporated as patient-level covariates.
1.5. Statistical analysis
All statistical analysis was performed using R version 3.5.2. All statistical tests were two-tailed and a p < 0.05 was considered significant. We first performed a univariate analysis to assess the relationship between recurrent HAEC and patient characteristics and the secondary outcomes. Chi squared tests were used for categorical variables and two-sample t-test with unpooled variances for continuous variables. To assess the effect of hospital volume on our primary and secondary outcomes we developed mixed-effect logistic regression models to adjust for salient patient-level covariates while clustering within hospital as a random effect [16]. For each model forward stepwise selection was performed and patient characteristics with a p < 0.05 were selected to remain in the model. Following stepwise selection, patient risk factors for HAEC that had been identified in prior studies including chromosomal abnormalities and cardiac anomalies were also included. We then included these patient-level characteristics along with hospital and surgeon volume tertile. Discrimination was assessed using the Area Under the Receiver Operating Curve and calibration was assessed using the Brier Score [17,18]. Absolute differences in billed hospital charges were calculated using the least-squares means.
2. Results
2.1. Patient demographics
From 2007 to 2017 we identified 2030 patients who underwent a pull-through for Hirschsprung disease. The mortality rate in this cohort was 0.3% and the condition-specific re-operation rate was 8%. Baseline patient characteristics were compared by between patients who had recurrent HAEC and those who did not (Table 1). The majority of patients were male and had private insurance. The most common racial/ethnic group was non-Hispanic White. There were no significant differences in race/ethnicity, gender, insurance type, birthweight, gestational age, presence of congenital cardiac anomalies or presence of chromosome abnormalities between the two groups. Children with recurrent HAEC were more likely to have congenital neurologic anomalies (3.6% vs 0.9%, p = 0.01). With regards to operative characteristics children with recurrent HAEC were more likely to have had an ostomy placed prior to their index pull-through operation (31.9% vs 22.1%, p = 0.01), to have a longer length of stay during their admission at the time of pull-through operation (mean 24.7 vs 13.5 days, p < 0.001), and to have had an episode of HAEC pre-operatively (21.0% vs 6.9%, p < 0.001). There were no differences seen in age at pull-through, open versus laparoscopic operative technique for pull-through, or disposition following pull-through. Surgeon annual pull-through volume and hospital annual pull-through volume were not statistically different between the two groups.
Table 1.
Patient characteristics.
| Without recurrent HAEC (n = 1892) | With recurrent HAEC (n = 138) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Sex | 1 | ||
| Female | 440 (23.3%) | 32 (23.2%) | |
| Male | 1452 (76.7%) | 106 (76.8%) | |
| Race | 0.9 | ||
| Non-Hispanic White | 818 (43.2%) | 56 (40.6%) | |
| Non-Hispanic Black | 251 (13.3%) | 21 (15.2%) | |
| Hispanic or Latino | 209 (11.0%) | 16 (11.6%) | |
| Other | 614 (32.5%) | 45 (32.6%) | |
| Insurance Type | 0.6 | ||
| Private | 763 (40.3%) | 60 (43.5%) | |
| Public | 983 (52.0%) | 71 (51.4%) | |
| Self | 42 (2.2%) | 1 (0.7%) | |
| Other | 82 (4.3%) | 6 (4.3%) | |
| Missing | 22 (1.2%) | 0 (0%) | |
| Birthweight (kg) | 0.3 | ||
| Mean (SD) | 3.27 (0.54) | 3.22 (0.51) | |
| Gestational age (weeks) | 0.9 | ||
| Mean (SD) | 38.0 (5.20) | 37.9 (4.74) | |
| Congenital cardiac anomalies | 68 (3.6%) | 7 (5.1%) | 0.5 |
| Congenital neurologic anomalies | 17 (0.9%) | 5 (3.6%) | 0.01 |
| Chromosome abnormalities | 17 (0.9%) | 2 (1.4%) | 0.8 |
| Congenital diaphragmatic hernia | 1 (0.1%) | 0 (0%) | 1 |
| Characteristics of Index Pull-through | |||
| Age at pull-through (days) | 0.2 | ||
| Mean (SD) | 85.0 (93.5) | 97.6 (99.2) | |
| Ostomy placement before pull-through | 419 (22.1%) | 44(31.9%) | 0.01 |
| Operative technique of pull-through | 0.6 | ||
| Open | 1457 (77%) | 103 (74.6%) | |
| Laparoscopic | 435 (23.0%) | 35 (25.4%) | |
| Length of stay during index admission (days) | <0.001 | ||
| Mean (SD) | 13.5 (22.5) | 24.7 (34.0) | |
| Disposition | 0.1 | ||
| Home | 1741 (92.0%) | 119 (86.2%) | |
| Home with Home Health | 106 (5.6%) | 10 (7.2%) | |
| Transfer to Another | 11 (0.6%) | 2 (1.4%) | |
| Hospital | |||
| Other | 13 (0.7%) | 3 (2.2%) | |
| Other Patient and Hospital Factors | |||
| Repeat pull-through | 61 (3.2%) | 9 (6.5%) | 0.07 |
| Ostomy placement following pull-through | 72 (3.8%) | 31 (22.5%) | <0.001 |
| Pre-operative HAEC | 131 (6.9%) | 29 (21.0%) | <0.001 |
| Surgeon annual volume (cases per year) | 0.2 | ||
| Mean (SD) | 1.09 (0.907) | 0.994(0.861) | |
| Hospital annual volume (cases per year) | 0.2 | ||
| Mean (SD) | 4.98 (2.02) | 4.77 (1.95) | |
2.2. Epidemiology of HAEC
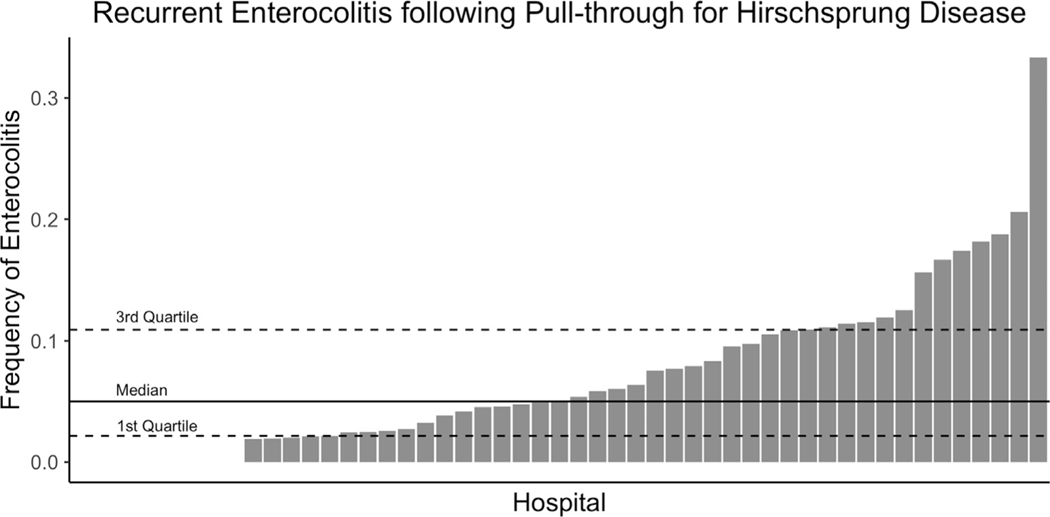
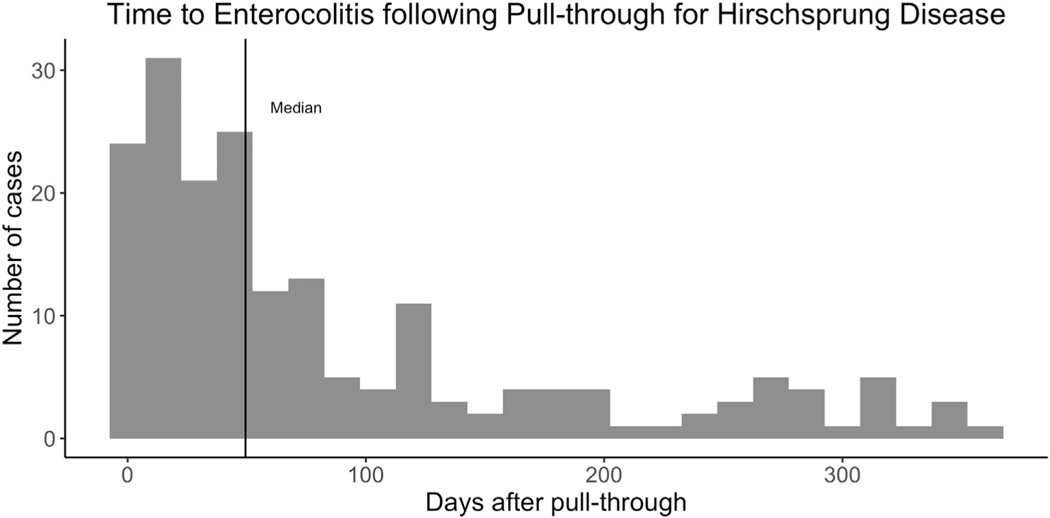
We identified 265 (13%) of children who had at least one episode of post-operative HAEC and 138 (7%) children who had recurrent (two or more episodes) HAEC following pull-through. Hospital rates of recurrent HAEC ranged from 0% to 33% with a median of 5% (Fig. 1). Increasing annual volume of pull-throughs was weakly correlated with lower rates of recurrent enterocolitis (R2=- = 0.13, p < 0.001). In the first year following pull-through the median time from pull-through to initial episode of HAEC was 50 days (Fig. 2). Following pull-through the median time from pull-through to second episode of HAEC was 144 days.
Fig. 1.
Rates of recurrent enterocolitis following pull-through. The median, 1st and 3rd quartiles are shown.
Fig. 2.
Days from pull-through to enterocolitis episode in the first year following pull-through. The median is shown with a solid black line.
2.3. Risk factors for recurrent HAEC
After adjusting for patient characteristics (Supplementary Table 4), neither hospital volume (medium compared to low volume hospitals (adjusted Odd Ratio: [aOR] 1.6, CI 0.75–3.2, p-value 0.24; high compared to low volume hospitals ([aOR] 1.7, CI 0.80–3.5, p-value 0.17)) nor surgeon volume (medium compared to low volume surgeons ([aOR] 0.84, CI 0.48–1.4, p-value 0.52; high compared to low volume surgeons ([aOR] 0.69, CI 0.40–1.2, p-value 0.17)) was associated with an increased odds of recurrent HAEC (Table 2). A ten percentage point increase in hospital recurrent HAEC rate was associated with a 3.5-fold increase in the odds a child developing recurrent HAEC ([aOR] 3.5, CI 2.4–4.9, p < 0.001). Presence of congenital neurologic anomalies ([aOR] 4.5, CI 1.4–14.4, p-value 0.01), history of central nervous system infection ([aOR] 2.5, CI 1.0–6.0, p-value 0.05) and history of pre-operative HAEC ([aOR] 3.6, CI 2.2–5.8, p < 0.001) were all associated with a significant increase in the odds of recurrent post-operative HAEC (Table 2). Placement of an ostomy prior to pull-though ([aOR] CI 0.96–2.3, p-value 0.08), presence of congenital cardiac anomalies ([aOR] 1.3, CI 0.48–3.6, p-value 0.59), and presence of chromosomal abnormalities ([aOR] 0.75, CI 0.14–4.1, p-value 0.74) were not associated with a significant increase in the odds of recurrent post-operative HAEC (Table 2). The same analysis was run using all patients with HAEC symptoms regardless of antibiotic administration and there was no change in the risk factors identified (Supplementary Table 5).
Table 2.
Risk factors for recurrent HAEC *
| Risk factor | Odds ratio | Confidence interval | p-Value |
|---|---|---|---|
| Hospital and surgeon factors | |||
| Medium to low volume hospitals | 1.6 | 0.75–3.2 | 0.24 |
| High to low volume hospitals | 1.7 | 0.80–3.5 | 0.17 |
| Medium to low volume surgeons | 0.84 | 0.48–1.4 | 0.52 |
| High to low volume surgeons | 0.69 | 0.40–1.2 | 0.17 |
| Hospital recurrent HAEC rate (in 10% increments) | 3.5 | 2.4–4.9 | <0.001 |
| Patient factors | |||
| Pre-operative HAEC | 3.6 | 2.2–5.8 | <0.001 |
| Ostomy placement prior to pull-through | 1.5 | 0.96–2.3 | 0.08 |
| History of central nervous system infection | 2.5 | 1.0–6.0 | 0.05 |
| Congenital neurologic anomalies | 4.5 | 1.4–14.4 | 0.01 |
| Congenital cardiac anomalies | 1.3 | 0.48–3.6 | 0.59 |
| Chromosomal abnormality | 0.75 | 0.14–4.1 | 0.74 |
AUC 0.88, Brier Score 0.07.
2.4. Consequences of recurrent HAEC
On univariate analysis, no significant increase in mortality was seen in recurrent HAEC patients (unadjusted Odd ratio [OR]: 2.8, CI 0.6–8.6, p = 0.1, Table 3). On univariate analysis, a significant increase in re-operation was seen in recurrent HAEC patients (unadjusted Odd ratio [OR]: 5.0, CI3.3–7.6, p < 0.001, Table 3). On univariate analysis, a significant increase in absolute billed hospital charges was seen in recurrent HAEC patients (absolute increase: $794,444, CI $794,444–794,448, p < 0.001, Table 3).
Table 3.
Unadjusted odds ratios for mortality and re-operation and unadjusted absolute difference in billed hospital charges.
| Outcome | Odds Ratio | Confidence Interval | p-Value |
|---|---|---|---|
| Mortality | 2.8 | 0.6–8.6 | 0.1 |
| Re-operation | 5.0 | 3.3–7.6 | <0.001 |
| Absolute difference | Confidence interval | p-value | |
| Billed hospital charges | $794,446 | $794,444–794,448 | <0.001 |
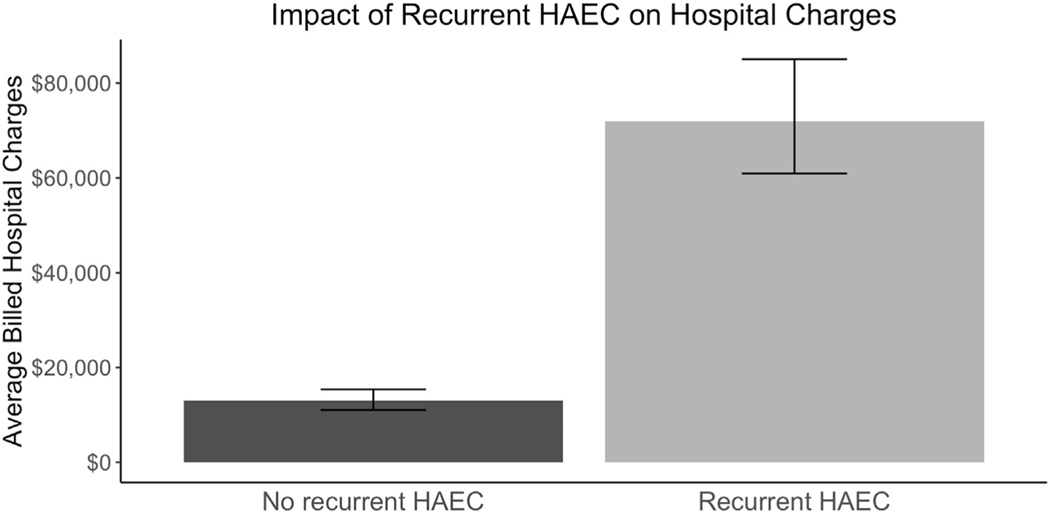
After adjusting for patient co-variates (Supplementary Table 6), recurrent post-operative HAEC was not associated with an increased odds of one-year in-hospital mortality (adjusted Odd Ratio: [aOR] 3.3, CI 0.88–12.1, p-value 0.08) (Table 4). On univariate analysis, children who developed recurrent HAEC were more likely to undergo ostomy placement following their index pull-through procedure (22.5% vs 3.8%, p < 0.001) and had an increased rate of repeat pull-through that approached statistical significance (6.5% vs 3.2%, p = 0.07). After adjusting for patient co-variates (Supplementary Table 6), recurrent post-operative HAEC was associated with a significant increase in the odds of repeat pull-through or placement of an ostomy after a pull-through (adjusted Odd Ratio: [aOR] 5.2, CI 3.3–8.1, p < 0.001) (Table 5). After adjusting for salient patient characteristics (Supplementary Table 6), recurrent HAEC was associated with an absolute increase in total hospital charges in the first year of life of $58,964 (95%CI: $58,962–58,966, p < 0.001, Fig. 3).
Table 4.
Factors predicting in-hospital mortality in multi-variable model*
| Risk factor | Odds ratio | Confidence Interval | p-Value |
|---|---|---|---|
| Recurrent HAEC | 3.3 | 0.88–12.1 | 0.08 |
| Birth weight (kg) | 0.29 | 0.15–0.57 | <0.001 |
AUC 0.83, Brier Score 0.008.
Table 5.
Factors predicting condition-specific re-operation in multi-variable model*
| Risk factor | Odds ratio | Confidence interval | p-Value |
|---|---|---|---|
| Recurrent HAEC | 5.2 | 3.3–8.1 | <0.001 |
| History of central nervous system infection | 2.3 | 1.0–5.0 | 0.04 |
AUC 0.73, Brier Score 0.07.
Fig. 3.
Average billed hospitals charges by recurrent enterocolitis status. Error bars represent the 95% confidence interval.
3. Discussion
3.1. Summary of results
In a large cohort of children with HD treated at U.S. children’s hospitals we identified a 7% rate of recurrent post-operative enterocolitis. Rates varied from 0 to 33% by hospital. Risk factors for recurrent post-operative HAEC included pre-operative HAEC, history of central nervous system infection, and congenital chromosome abnormalities, but did not include hospital or surgeon volume, placement of an ostomy prior to pull-through, congenital cardiac anomalies or chromosome abnormalities. Recurrent post-operative HAEC did not have an impact on inpatient mortality, but it did increase odds of condition-specific re-operation and billed hospital charges.
3.2. Comparison to previous literature
There are few previous large database studies of post-operative HAEC to which we can compare our identified rate of HAEC. Nevertheless, our post-operative HAEC rate of 13% with a range from 0% to 33% is consistent with previous literature which has reported a range from 2% to 33% in single center studies [2, 4–6]. Most previous studies do not break down post-operative HAEC into single episodes versus recurrent HAEC, however Teitelbaum at al reported a rate of recurrent HAEC of 4%, which is slightly lower than our recurrent rate of 7% [4]. We chose to study recurrent HAEC due to the limitations of using an administrative database to define HAEC. A single episode of HAEC may be related to miscoding, however, it is significantly less likely that two or more episodes could be related to miscoding.
While previous studies had identified trisomy 21 as a risk factors for HAEC [4, 10], this was not supported in our data, as presence of any chromosomal abnormality was not a significant risk factor for HAEC. This is consistent with a more recent single center retrospective study by Kwendakwema et al., which showed that there was no increase in rates of HAEC in trisomy 21 patients in their series [19]. Previous studies have identified the presence of additional congenital anomalies including neurologic anomalies and cardiac anomalies as risk factors for HAEC, however, in these studies these anomalies are specifically mentioned in the context of trisomy 21 and were not analyzed independently [2,8]. In our study, while trisomy 21 and congenital cardiac anomalies were not found to be a risk factor for post-operative HAEC, congenital neurologic anomalies and history of central nervous system infection were significant risk factors. Previous studies have been conflicting about whether or not pre-operative HAEC infers a greater risk of post-operative HAEC [2, 20, 21]. Our study found that patients who had a previous episode of HAEC were more likely to develop subsequent episodes.
In our univariate analysis, children who developed recurrent HAEC were more likely to have to had an ostomy placed prior to their initial pull-through procedure. This in contrast to the multi-center study by Teitelbaum et al., which found that patients undergoing a single-stage pull-through were more likely to develop post-operative HAEC [22]. One possible explanation for this finding is that ostomy placement (a two-stage procedure) is actually a surrogate marker for other patient characteristics that are not easily measured in an administrative database such as patient condition at the time of the operation, patient size, length of involved intestine or family comfort with performing rectal irrigations that would allow the possibility of a delayed single-stage repair. Additionally, individual surgeon and local practice patterns may influence the likelihood of choosing a two-stage repair.
Recurrent post-operative HAEC had a strong association with condition-specific re-operation, either placement of an ostomy after pull-through or repeat pull-through. This is consistent with Menezes et al. as well as Thakkar et al., whose studies of long-term outcomes of children in children with HAEC often require additional procedures following pull-through [6, 23]. A previous study by Teitelbaum et al. have shown increased costs of care for patients with HAEC [4]. Similarly, we identified a nearly $60,000 increase in billed hospital charges in patients with recurrent HAEC.
3.3. Implications
This study reveals the wide variation in recurrent HAEC rates across the country, which may be due in part to the challenge of developing a standard definition of HAEC. This challenge is compounded by the difficulty identifying specific codes which represent HAEC in a large administrative dataset. The importance of better understanding this variation is underscored by the finding that a ten percentage point increase in a hospital’s recurrent HAEC rate is associated with a 3.5-fold increase in the odds of a child developing recurrent HAEC at that facility. Additional in-depth studies are needed to better understand the pathophysiology underlying this significant variation between hospitals including existing treatment protocols and possible differences in the patient intestinal microbiome. Identification of risk factors for recurrent HAEC allows us to identify high-risk patients and adapt family education and treatment protocols to better address this condition.
3.4. Limitations
One of the major limitations of our study is the lack of a validated protocol for identifying HAEC using administrative codes as a comparison for our data. Previously reported rates of HAEC are so variable that it is difficult to assess whether or not we are identifying the majority of cases and if all cases that we have identified are actually HAEC. Additionally, cases of HAEC that are treated on an outpatient basis would not be captured in our cohort. The PHIS database is limited to U.S. freestanding children’s hospitals, therefore this data also fails to capture the experience of patients with HD that are treated at other types of hospitals across the United States.
4. Conclusions
This study demonstrates a model to identify cases of HAEC within a national administrative database using a combination of diagnosis and antibiotic administration codes. The incidence of recurrent post-operative HAEC is highly variable at children’s hospitals and is associated with patient history of HAEC and presence of congenital neurologic anomalies. Recurrent HAEC was not associated with chromosomal abnormalities, hospital volume or surgeon volume. Risk of re-operation and a significant increase in billed hospital charges were identified in patients with recurrent HAEC. Multicenter studies are needed to develop clinical prediction models and treatment protocols for HAEC in children with HD.
Supplementary Material
Acknowledgments
Funding: This study was funded by the Utah-Intermountain Healthcare Surgical Research Fellowship (LCCP), and a grant from the Agency for Healthcare Research and Quality (1K08HS025776, BTB).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpedsurg.2019.10.060.
References
- [1].Engum SA, Grosfeld JL. Long-term results of treatment of Hirschsprung’s disease. Semin Pediatr Surg 2004;13(4):273–85. [DOI] [PubMed] [Google Scholar]
- [2].Teitelbaum DH, Coran AG. Enterocolitis. Semin Pediatr Surg 1998;7(3):162–9. [DOI] [PubMed] [Google Scholar]
- [3].Wang J-S, Lee H-C, Huang F-Y, et al. Unexpected mortality in pediatric patients with postoperative Hirschsprung’s disease. Pediatr Surg Int 2004;20(7):525–8. [DOI] [PubMed] [Google Scholar]
- [4].Teitelbaum DH, Qualman SJ, Caniano DA. Hirschsprung’s disease. Identification of risk factors for enterocolitis. Ann Surg 1988;207(3):240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marty TL, Matlak ME, Hendrickson M, et al. Unexpected death from Enterocolitis after surgery for Hirschsprung’s disease. Pediatrics 1995;96(1):118. [PubMed] [Google Scholar]
- [6].Menezes M, Puri P. Long-term outcome of patients with enterocolitis complicating Hirschsprung’s disease. Pediatr Surg Int 2006;22(4):316–8. [DOI] [PubMed] [Google Scholar]
- [7].Rescorla FJ, Morrison AM, Engles D, et al. Hirschsprung’s disease: evaluation of mortality and long-term function in 260 cases. JAMA Surg 1992;127(8):934–42. [DOI] [PubMed] [Google Scholar]
- [8].Moore SW, Albertyn R, Cywes S. Clinical outcome and long-term quality of life after surgical correction of Hirschsprung’s disease. J Pediatr Surg 1996;31(11):1496–502. [DOI] [PubMed] [Google Scholar]
- [9].Wildhaber BE, Teitelbaum DH, Coran AG. Total colonic Hirschsprung’s disease: a 28-year experience. J Pediatr Surg2005;40(1):203–6 [discussion 6–7]. [DOI] [PubMed] [Google Scholar]
- [10].Caniano DA, Teitelbaum DH, Qualman SJ. Management of Hirschsprung’s disease in children with trisomy 21. The American Journal of Surgery 1990;159(4):402–4. [DOI] [PubMed] [Google Scholar]
- [11].Pastor AC, Osman F, Teitelbaum DH, et al. Development of a standardized definition for Hirschsprung’s-associated enterocolitis: a Delphi analysis. J Pediatr Surg 2009;44 (1):251–6. [DOI] [PubMed] [Google Scholar]
- [12].Frykman PK, Kim S, Wester T, et al. Critical evaluation of the Hirschsprung-associated enterocolitis (HAEC) score: a multicenter study of 116 children with Hirschsprung disease. J Pediatr Surg 2018;53(4):708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Demehri FR, Frykman PK, Cheng Z, et al. Altered fecal short chain fatty acid composition in children with a history of Hirschsprung-associated enterocolitis. J Pediatr Surg 2016;51(1):81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Frykman PK, Nordenskjold A, Kawaguchi A, et al. Characterization of bacterial and fungal microbiome in children with Hirschsprung disease with and without a history of Enterocolitis: a multicenter study. PLOS ONE 2015;10(4):e0124172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang EY, Tolley EA, Blakely ML, et al. Changes in hospital utilization and Management of Hirschsprung disease: analysis using the Kids’ inpatient database. Ann Surg 2013;257(2):371–5. [DOI] [PubMed] [Google Scholar]
- [16].Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat Med 1998;17(11):1261–91. [DOI] [PubMed] [Google Scholar]
- [17].Murphy AH. A new vector partition of the probability score. J Appl Meteorol 1973;12 (4):595–600. [Google Scholar]
- [18].Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kwendakwema N, Al-Dulaimi R, Presson AP, et al. Enterocolitis and bowel function in children with Hirschsprung disease and trisomy 21. J Pediatr Surg 2016;51(12):2001–4. [DOI] [PubMed] [Google Scholar]
- [20].Carneiro PMR, Brereton RJ, Drake DP, et al. Enterocolitis in Hirschsprung’s disease. Pediatr Surg Int 1992;7(5):356–60. [Google Scholar]
- [21].Elhalaby EA, Coran AG, Blane CE, et al. Enterocolitis associated with Hirschsprung’s disease: a clinical-radiological characterization based on 168 patients. J Pediatr Surg 1995;30(1):76–83. [DOI] [PubMed] [Google Scholar]
- [22].Teitelbaum DH, Cilley RE, Sherman NJ, et al. A decade of experience with the primary pull-through for hirschsprung disease in the newborn period: a multicenter analysis of outcomes. Ann Surg 2000;232(3):372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thakkar HS, Bassett C, Hsu A, et al. Functional outcomes in Hirschsprung disease: a single institution’s 12-year experience. J Pediatr Surg 2017;52(2):277–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.