Abstract
Objective
A growing body of evidence suggests chronic inflammation triggers the process of endometrial carcinogenesis. Interleukin (IL) 17A is an important proinflammatory factor involved in the tumour angiogenesis processes of many solid tumours. This study aimed to characterize the function of IL17A in endometrioid-type endometrial carcinoma.
Methods
Levels of IL17A in human endometrial tissues were analysed by immunohistochemistry. In vitro proliferation and migration were analysed in Ishikawa cells treated with IL17A, using cell counting kit-8, wound healing and transwell assays. Western blots were used to analyse levels of oestrogen receptor (ER)α and ERβ proteins in Ishikawa cells treated with IL17A.
Results
IL17A levels were significantly higher in endometrial carcinoma tissues than in endometrial hyperplasic tissues. Significantly increased proliferation and migration was observed in Ishikawa cells treated with IL17A versus controls. Investigation of the molecular mechanism revealed that IL17A treatment upregulated the ERα/ERβ protein ratio in Ishikawa cells.
Conclusions
IL17A may be an important proinflammatory factor involved in promoting endometrial carcinogenesis.
Keywords: Endometrial cancer, interleukin 17A, proliferation, migration
Introduction
Endometrial cancer is the most common gynaecological tumour in developed countries, with an increasing morbidity rate as the rate of worldwide obesity rises.1 The most frequently occurring histological subtype is endometrioid-type endometrial carcinoma, which is transformed malignantly from endometrial hyperplasic diseases. Substantial epidemiologic data implicate an imbalance of oestrogens and progestogens in the aetiology of endometrial carcinoma.2 Nevertheless, emerging laboratory data show that chronic inflammation might mediate the association between obesity and endometrial cancer, and that endometrial carcinogenesis may be promoted by an inflammatory milieu,3–7 but the mechanisms involved remain unclear. Therefore, a further understanding of the mechanisms is urgently needed for effective prevention.
Interleukin (IL) 17A is an important CD4 T-cell-derived pro-inflammatory factor, which is involved in tumour angiogenesis and other pathological processes of many solid tumours, such as gastric cancer, breast cancer and cervical cancer.8–10 Therefore, targeting IL17A may be a new strategy for tumour treatment. The aims of the present study were to evaluate the expression of IL17A in endometrial hyperplasic and endometrial carcinoma tissues, then to further explore the effects of IL17A on cell proliferation and migration in vitro using the Ishikawa human endometrial cancer cell line.
Patients and methods
Study population
The present study included sequentially enrolled patients with endometrial lesions, who had undergone curettage or hysteroscopy without chemotherapy or radiotherapy at the Department of Gynaecology, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, between January 2017 and January 2019. Patients were diagnosed according to the WHO 2014 criteria.11 Patients who underwent relevant treatment within two weeks prior to study enrolment, and patients with cardiovascular, liver, kidney, immune system, haematopoietic system, systemic infection, or other serious diseases were excluded.
The study was conducted in accordance with the tenets of the Helsinki Declaration, and approved by the Medical Ethics Committee of Dongzhimen Hospital. All patients provided written informed consent prior to study inclusion.
Sample collection and immunohistochemistry
Levels of IL17A were assessed in endometrial tissue sections using immunohistochemistry, as previously described.12 Briefly, formalin-fixed tissues were embedded in paraffin using a standard protocol, then cut to 4-µm sections and deparaffinized with xylene. Sections were incubated in 0.01 mol/l citrate-tween buffer at 98°C for 20 min for antigen unmasking and then blocked in 2% goat serum/5% bovine serum albumin (BSA) in phosphate buffered saline (PBS)-tween for 2 h. Sections were then incubated with anti-IL17A mouse monoclonal primary antibody (1:500 dilution; Cat. No. ab189377; Abcam, Cambridge, UK) overnight at 4°C. Sections were washed with PBS and incubated with biotinylated anti-mouse IgG secondary antibody (1:200 dilution; Biosynthesis Biotechnology Co. Ltd, Beijing, China) for 30 min at 37°C. Sections were again washed with PBS and incubated with streptavidin–biotin complex (Biosynthesis Biotechnology Co) for 30 min at 37°C, prior to counterstaining with haematoxylin. The immunosignal was visualized using diaminobenzidine (Biosynthesis Biotechnology Co) and the presence of brown or yellow granules in the cytoplasm or nucleus signalled positive immunostaining. Relative quantification of IL17A levels was performed by randomly selecting five fields of view per section, and assessing the immunoreactive areas at 200 ×magnification, using a Nikon computer image system (Nikon, Tokyo, Japan). The optical density of resulting images was analysed using Image J software, version 1.47.13
Cell culture
The human Ishikawa endometrial adenocarcinoma cell line was purchased from the Cell Resource Centre, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (CAMS)/Peking Union Medical College (PUMC) and authenticated with short tandem repeat profiling. Cells were cultured in Gibco Dulbecco’s modified Eagles medium (DMEM)/F12 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% Gibco foetal bovine serum (FBS; Thermo Fisher Scientific) in an atmosphere of 5% CO2 at 37°C.
Cell proliferation assay
Ishikawa cells (3 × 103) were seeded into each well of 96-well plates and incubated at 37°C/5% CO2 for 24 h. Cells were then treated with IL17A (PeproTech; Cranbury, NJ, USA) at a range of concentrations from 0 to 100 ng/ml each for 24–72 h. Cell counting kit-8 (CCK8; Dojindo Molecular Technologies, Rockville, MD, USA) was used to evaluate cell proliferation, according to the manufacturer’s instructions.
Wound healing assay
Ishikawa cells (5 × 105) were seeded into each well of 6-well plates and cultured to 90% confluence. The cell monolayer was scratched with a 10-μl pipette tip and washed three times with PBS. The cells were then treated with FBS-free culture medium containing 0, 5, 50 or 100 ng/ml IL17A (Peprotech), each for 24 h at 37°C. Scratch images were obtained at 0 h and 24 h using phase-contrast inverted microscopy (Olympus, Shinjuku, Japan) at 200 × magnification. Scratched areas were analysed using Image J software, version 1.47,13 and area healing rate was calculated as: 1 – scratch area at 24 h/scratch area at 0 h.
Transwell assay
Ishikawa cells were suspended in FBS-free medium and seeded into the upper chamber of a 6.5 mm diameter transwell® insert (Lot 02618025; Corning, Glendale, AZ, USA) at a density of 4 × 104 cells/well. The lower chambers were filled with DMEM/F12 with 10% FBS that contained 0, 5, 50, or 100 ng/ml of IL17A. After incubation for 24 h at 37°C, cells that had invaded the lower surface of the upper chamber were fixed with 95% alcohol and stained with crystal violet for 15 min. The number of cells that had invaded were then counted under a light microscope.
Western blot analysis
Ishikawa cells were seeded into T-25 culture vessels at a density of 3 × 104/ml and incubated at 37°C/5% CO2 for 24 h. Cells were then treated with IL17A (PeproTech) at a range of concentrations from 0 to 100 ng/ml each for 48 h. Western blot analysis of oestrogen receptor (ER) levels was conducted as previously described.12 Briefly, cells were scraped from the culture vessel and lysed for 30 min on ice in cell extraction buffer supplemented with 1 mM phenylmethylsulphonyl fluoride and a protease inhibitor cocktail (all Applygen, Beijing, China), according to the manufacturer’s instructions. Protein concentration of the lysates was determined using a bicinchoninic acid protein assay kit (Applygen). Protein samples (20 µg of each) were separated by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis, then transferred to a poly-vinylidene fluoride membrane (Millipore, Burlington, MA, USA) using a Bio-Rad II System (Bio-Rad, Hercules, CA, USA). The membranes were blocked using 5% skimmed milk in Tris-buffered saline Tween (T-BST) and then incubated overnight at room temperature with the following primary antibodies: recombinant rabbit anti-ERα (1:500 dilution; Cat. No. ab108398; Abcam), mouse anti-ERβ (1:1 000 dilution; Cat. No. ab288; Abcam) and rabbit anti-β-actin internal loading control (1:1 000 dilution; Cat. No. ab228001; Abcam). Membranes were then washed three times for 5 min each with T-BST and incubated with goat anti-rabbit IgG/HRP (1:5 000 dilution; bs-0295G-HRP) and goat anti-mouse IgG/HRP (1:5 000 dilution; bs-0296G-HRP; both Biosynthesis Biotechnology Co) secondary antibodies for 1 h at room temperature. The protein signal was detected using an enhanced chemiluminescence kit (Millipore), according to the manufacturer’s instructions, and analysed using a ChemiDoc™ MP imaging system (Bio-Rad) and Image-Pro Plus software, version 6.0 (Media Cybernetics Inc., Rockville, MA, USA).
Statistical analyses
Data are presented as mean ± SD of n tissue samples or of triplicate in vitro experiments. One-way analysis of variance was used for determining differences among three or more groups, and all data were analysed using SPSS software, version 21.0 (IBM, Armonk, NY, USA). A P value ≤0.05 (two-tailed) was considered to be statistically significant.
Results
Endometrial carcinoma lesions showed higher levels of IL17A
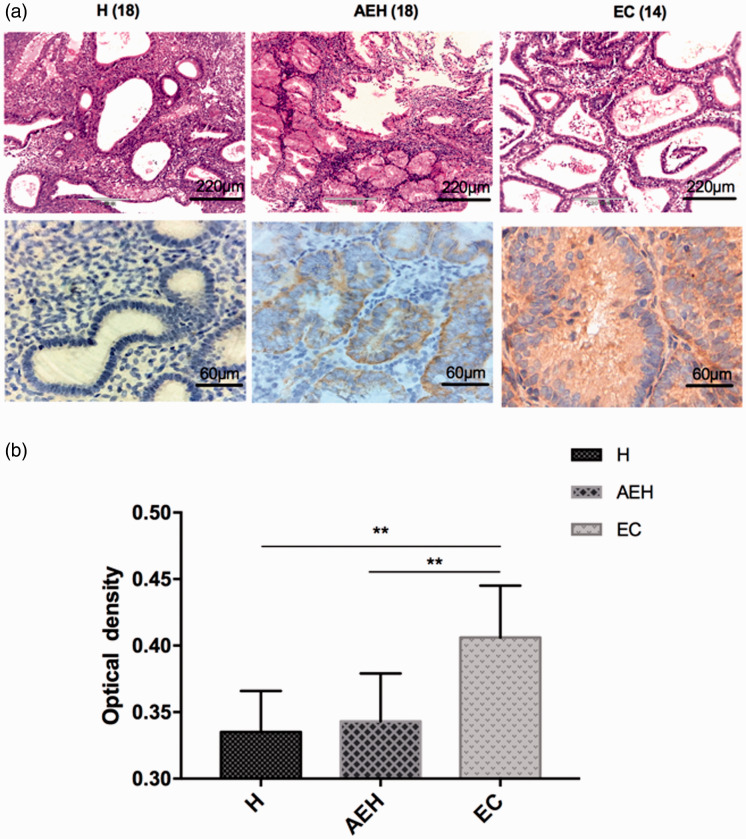
A total of 50 endometrial tissue samples were included from patients aged 27–62 years, comprising 18 cases of non-atypical endometrial hyperplasia, 18 cases of atypical endometrial hyperplasia and 14 cases of endometrial carcinoma. Levels of IL17A in the endometrial tissues were assessed using immunohistochemistry assays and were shown to be increased in the endometrial carcinoma lesions compared with non-atypical endometrial hyperplasia and atypical endometrial hyperplasia lesions (P < 0.01; Figure 1).
Figure 1.
Endometrial adenocarcinoma displayed increased levels of interleukin (IL) 17A: (a) representative photomicrographs showing immunohistochemical staining of IL17A in endometrial lesions; and (b) the staining intensity of IL17A scored by semiquantitative optical analysis. H, non-atypical endometrial hyperplasia; AEH, atypical endometrial hyperplasia; EC, endometrial carcinoma. Data presented as mean ± SD; **P < 0.01 (one-way analysis of variance).
IL17A increased Ishikawa cell proliferation
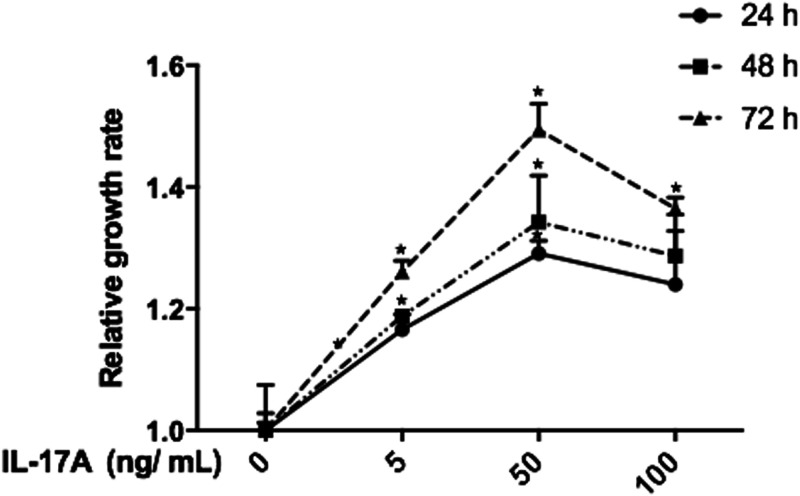
The effect of IL17A on Ishikawa cell proliferation was evaluated by CCK8 kit assay (Figure 2). IL17A, at a range of 5–100 ng/ml, was shown to significantly promote cell proliferation versus controls, and the highest proliferation rate was observed with 50 ng/ml IL17A for 72 h (P < 0.05 versus controls; Figure 2).
Figure 2.
Proliferation of Ishikawa cells was increased in response to interleukin (IL) 17A. Data presented as mean ± SD of three experiments; *P < 0.05 versus controls (one-way analysis of variance).
IL17A promoted Ishikawa cell migration
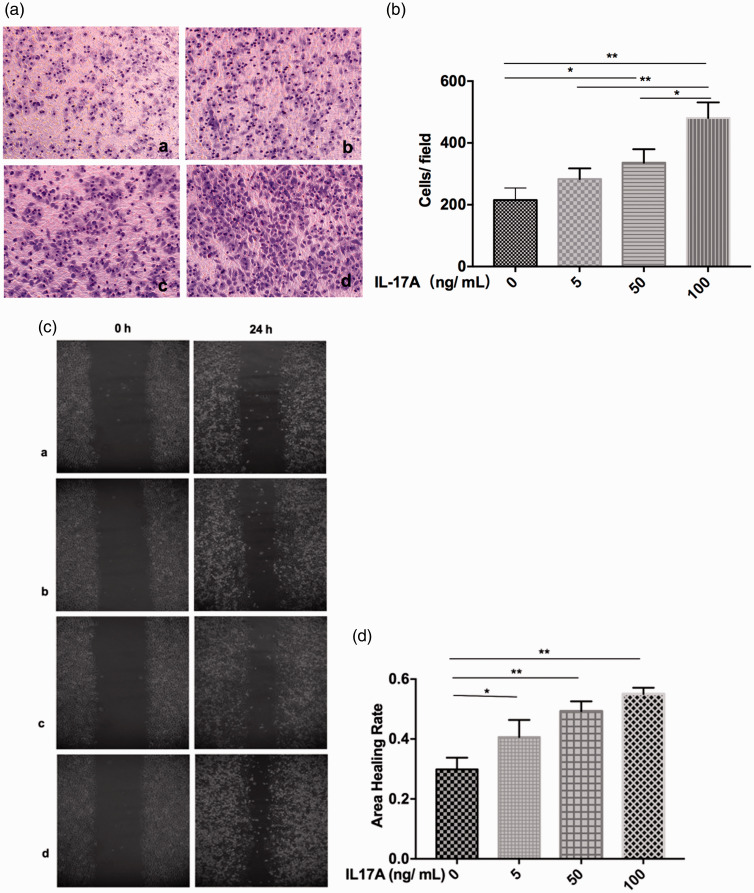
The effect of IL17A on migration of Ishikawa cells was assessed using in vitro transwell® and wound healing assays. Both assays showed that IL17A promoted Ishikawa cell migration in a dose-dependent manner (P < 0.05; Figure 3).
Figure 3.
Migration of Ishikawa cells was promoted in response to interleukin (IL) 17A: (a) representative images showing in vitro transwell® migration in response to IL17A at a, 0 ng/ml; b, 5 ng/ml; c, 50 ng/ml; and d, 100 ng/ml (original magnification, × 200); (b) cell migration quantified as cells per field of view; (c) representative images showing in vitro wound healing in response to IL17A at (a) 0 ng/ml; (b) 5 ng/ml; (c) 50 ng/ml; and (d) 100 ng/ml (original magnification, × 200); and (d) wound healing quantified as area healing rate. Data presented as mean ± SD of three experiments; *P < 0.05; **P < 0.01 (one-way analysis of variance); original magnification, × 200.
IL17A upregulated Ishikawa cell ERα/ERβ protein ratio
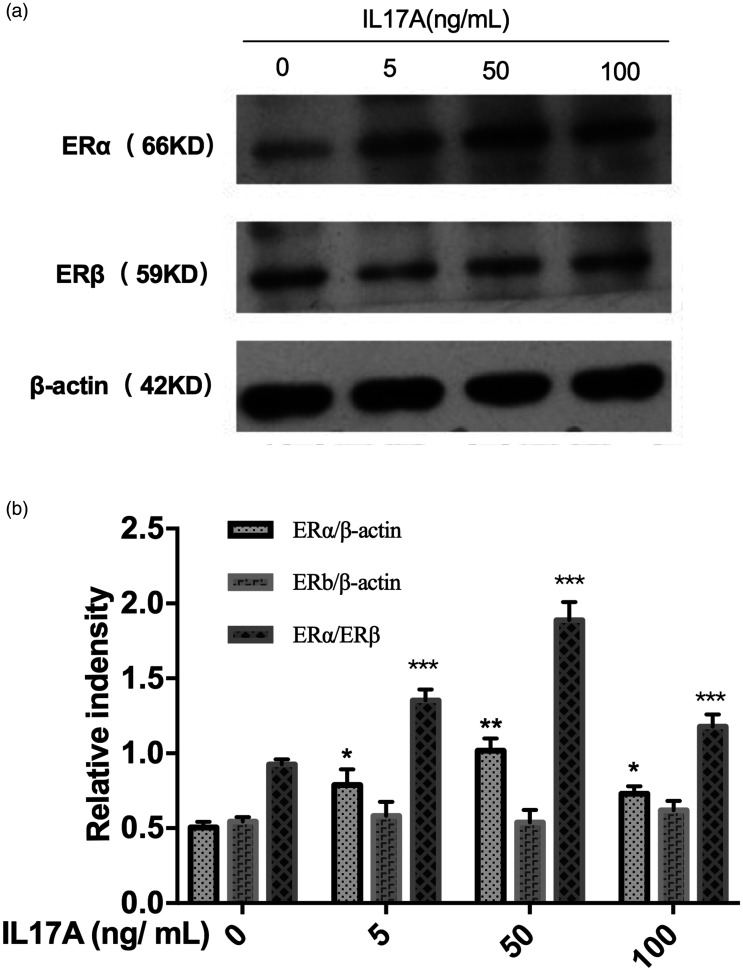
Levels of ERα and ERβ protein in Ishikawa cells treated with IL17A were assessed using Western blots. Exposure to IL17A was shown to significantly upregulate the expression of ERα protein (P < 0.05 versus controls) but had no effect on the expression of ERβ protein (Figure 4).
Figure 4.
The Ishikawa cell oestrogen receptor (ER)α/ERβ protein ratio was upregulated in response to interleukin (IL) 17A: (A) representative Western blots showing protein signal in response to different IL17A concentrations; and (B) relative signal immunosignal intensity at different IL17A concentrations. Data presented as mean ± SD of three experiments; *P < 0.05, **P < 0.01, and ***P < 0.001 versus controls (one-way analysis of variance).
Discussion
Since 2005, inflammation has been hypothesised to play a role in endometrial carcinoma development.14 Risk factors for endometrial carcinoma, that may increase endometrial exposure to inflammation, include anovulation, polycystic ovary syndrome, obesity, diabetes, excessive menstruation, early menarche, and late menopause. Chronic inflammation is known to induce rapid cell division, increasing the potential for replication error, ineffective DNA repair, and subsequent mutations that may lead to cancer development.
A pro-inflammatory milieu may also directly promote tumour growth, of which IL17A, a recently identified pro-inflammatory cytokine, may play an important tumorigenesis role.15 Lv et al.10 detected that IL17A was highly expressed in cervical cancer tissues and may promote cell proliferation and invasion via the nuclear factor (NF)-κB signalling pathway. High levels of IL17A expression are also reported to be associated with lower disease-free survival and worse prognosis in patients with invasive ductal carcinoma of the breast, and IL17A has been shown to change the gene-expression profile and behaviour of non-metastatic tumour cells, causing tumour growth in vivo.16 In the present study, IL17A expression was found to be highest in endometrial carcinoma lesions, compared with atypical and non-atypical endometrial hyperplasia lesions, suggesting that IL17A may also play a role in endometrial carcinoma development.
The present study also showed that IL17A, at doses ranging from 5 to 100 ng/ml, promoted the in vitro proliferation of Ishikawa cells, with the highest proliferation rate observed in cells treated with 50 ng/ml IL17A. Unlike the proliferation effects, IL17A induced Ishikawa cell migration in a dose-dependent manner. Thus, the effects of IL17A on the biological behaviour of Ishikawa cells was not completely dose-dependent, indicating that there may be different mechanisms behind the promotion of proliferation and migration of Ishikawa cells by IL17A.
To further investigate specific mechanisms underlying the effects of IL17A on endometrial carcinoma, levels of ERα and ERβ were assessed in Ishikawa cells following IL17A exposure in vitro. IL17A was shown to significantly upregulate ERα protein levels but had no effect on the expression of ERβ protein, which was consistent with previously reported results.17
Oestrogen receptor-α is a master transcription factor that regulates endometrial cell proliferation, while ERβ is regarded as the guardian of the endometrium that generally counteracts the ERα-promoted cell hyper-proliferation.18,19 Therefore, the ERα/ERβ ratio determines the level of proliferation to a certain degree. Levels of ERα are reported to be increased when endometrial lesions develop from hyperplasia and progress from atypical endometrial hyperplasia to endometrioid adenocarcinoma, and are decreased when the lesions progress from endometrioid adenocarcinoma G1 to G3.17 In the present study, the ERα/ERβ protein ratio was significantly elevated in response to IL17A treatment, and the response peaked at 50 ng/ml IL17A, suggesting that the mechanisms by which IL17A promote cell proliferation may be ER-dependent.
High-grade or metastatic endometrial carcinoma has long been associated with substantial changes in the extracellular microenvironment, and within this local microenvironment, immune cells and their mediators are known to facilitate metastasis formation.20 Investigations have also demonstrated that expression of pro-inflammatory cytokines, such as tumour necrosis factor-α, IL-8,21 and IL-6,22 promote tumour cell spread to secondary sites. In the present study, IL17A was found to induce Ishikawa cell migration in vitro in a dose-dependent manner, however, further studies are required to elucidate the relevant underlying mechanisms.
In conclusion, the expression of IL17A was found to be significantly increased in endometrial carcinoma tissues. Furthermore, IL17A was shown to significantly enhance the proliferation and migration of Ishikawa cells in vitro, and these effects may partly be attributed to up-regulation of the ERα/ERβ protein ratio.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Ran Cheng https://orcid.org/0000-0001-8623-4120
References
- 1.Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet 2016; 387: 1094–1108. [DOI] [PubMed] [Google Scholar]
- 2.Auclair MH, Yong PJ, Salvador S, et al. Guideline No. 392-Classification and Management of Endometrial Hyperplasia. J Obstet Gynaecol Can 2019; 41: 1789–1800. [DOI] [PubMed] [Google Scholar]
- 3.Dossus L, Rinaldi S, Becker S, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case–control study. Endocr Relat Cancer 2010; 17: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubyshkin AV, Aliev LL, Fomochkina II, et al. Endometrial hyperplasia-related inflammation: its role in the development and progression of endometrial hyperplasia. Inflamm Res 2016; 65: 785–794. [DOI] [PubMed] [Google Scholar]
- 5.Che Q, Liu BY, Liao Y, et al. Activation of a positive feedback loop involving IL-6 and aromatase promotes intratumoral 17β-estradiol biosynthesis in endometrial carcinoma microenvironment. Int J Cancer 2014; 135: 282–294. [DOI] [PubMed] [Google Scholar]
- 6.Che Q, Liu BY, Wang FY, et al. Interleukin 6 promotes endometrial cancer growth through an autocrine feedback loop involving ERK-NF-κB. Biochem Biophys Res Commun 2014; 446: 167–172. [DOI] [PubMed] [Google Scholar]
- 7.Dossus L, Becker S, Rinaldi S, et al. Tumor necrosis factor (TNF)-α, soluble TNF receptors and endometrial cancer risk: the EPIC study. Int J Cancer 2011; 129: 2032–2037. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Zeng Z, Xu L, et al. Increased expression of IL17A in human gastric cancer and its potential roles in gastric carcinogenesis. Tumour Biol 2014; 35: 5347–5356. [DOI] [PubMed] [Google Scholar]
- 9.Dawod B, Liu J, Gebremeskel S, et al. Myeloid-derived suppressor cell depletion therapy targets IL-17A-expressing mammary carcinomas. Sci Rep 2020; 10: 13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv Q, Wu K, Liu F, et al. Interleukin-17A and heparanase promote angiogenesis and cell proliferation and invasion in cervical cancer. Int J Oncol; 2018; 53: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 11.Sanderson PA, Critchley HO, Williams AR, et al. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update 2017; 23: 232–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Ma H, Yang L, et al. Silencing of the TROP2 gene suppresses proliferation and invasion of hepatocellular carcinoma HepG2 cells. J Int Med Res 2019; 47: 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Modugno F, Ness RB, Chen C, et al. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev 2005; 14: 2840–2847. [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Kolls JK. Interluekin-17A (IL17A). Gene 2017; 614: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benevides L, Da Fonseca DM, Donate PB, et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res 2015; 75: 3788–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ning C, Xie B, Zhang L, et al. Infiltrating macrophages induce ERα expression through an IL17A-mediated epigenetic mechanism to sensitize endometrial cancer cells to estrogen. Cancer Res 2016; 76: 1354–1366. [DOI] [PubMed] [Google Scholar]
- 18.Hapangama DK, Kamal AM, Bulmer JN. Estrogen receptor β: the guardian of the endometrium. Hum Reprod Update 2015; 21: 174–193. [DOI] [PubMed] [Google Scholar]
- 19.Paterni I, Granchi C, Katzenellenbogen JA, et al. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014; 90: 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahoo SS, Zhang XD, Hondermarck H, et al. The emerging role of the microenvironment in endometrial cancer. Cancers (Basel) 2018; 10: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry KK, Varney ML, Dave BJ, et al. Expression of interleukin-8 in human metastatic endometrial carcinoma cells and its regulation by inflammatory cytokines. Int J Gynecol Cancer 2001; 11: 54–60. [DOI] [PubMed] [Google Scholar]
- 22.Che Q, Xiao X, Xu J, et al. 17β-estradiol promotes endometrial cancer proliferation and invasion through IL-6 pathway. Endocr Connect 2019; 8: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]