Abstract
Objective
Primary hyperparathyroidism (PHPT) is relatively common in China and results in severe damage to the skeletal system. This study aimed to investigate changes in bone mineral density (BMD) over 2 years in patients with PHPT after parathyroidectomy.
Methods
This retrospective cohort study included patients with PHPT who underwent parathyroidectomy between January 2010 and December 2015. BMD and T-scores and Z-scores of the lumbar spine (L1, L2, L3, and L4) and total hip (femoral neck, great trochanter, and Ward’s triangle) at baseline and 2 years after surgery were measured by dual-energy X-ray absorptiometry.
Results
Thirty patients with moderate to severe PHPT (17 men and 13 women) aged 38.90±15.48 years were included. BMD, and T-score and Z-score values at the lumbar spine and total hip at 6 months, 1 year, and 2 years after parathyroidectomy were significantly improved compared with preoperative values. Improvement in BMD was largest at L4 (46.7%) and smallest at L1 (37.4%) in the lumbar spine 2 years after parathyroidectomy. For the total hip, the increase in BMD was largest at Ward’s triangle (42.6%) and smallest at the femoral neck (37.5%).
Conclusions
BMD of the lumbar spine and total hip is improved after parathyroidectomy in patients with PHPT.
Keywords: Bone mineral density, parathyroidectomy, primary hyperparathyroidism, lumbar spine, hip, dual-energy X-ray absorptiometry
Introduction
Primary hyperparathyroidism (PHPT) is a relatively common endocrine disorder characterized by abnormally elevated parathyroid hormone secretion mostly due to sporadic solitary parathyroid adenoma.1,2 PHPT is widely found in postmenopausal women, and is frequently diagnosed through incidental hypercalcemia and damage to the skeletal system.3 Currently, patients with PHPT are diagnosed at early stages owing to the wide application of biochemical screening strategies.4 However, hyperparathyroidism in Asia is often characterized by markedly elevated serum calcium levels and various systemic organ disorders related to symptomatic hyperparathyroidism compared with Europe and the USA.5–7 In Western countries, overt kidney stone disease occurs in less than 20% of patients with PHPT and radiologically evident bone disease is even less common.8 However, there are patients with multi-system involvement in symptomatic PHPT in China.
Increased parathyroid hormone levels act on target organs, such as the bones and kidneys, resulting in elevated or abnormal calcium levels.9 Persistent abnormal elevated serum intact parathyroid hormone (iPTH) levels can damage organs in various parts of the body.10 This results in loss of bone minerals and can cause osteopenia or osteoporosis. Reduction of bone mineral density (BMD) is related to the risk of fracture and affects bone health.11 Therefore, patients with ineffective treatment or osteoporosis need to undergo surgical removal of the lesion’s parathyroid tissue. After an effective operation, the patients’ biochemical indicators, osteoporosis, urinary calculi, kidney function, and other symptoms gradually improve.12
Most previous studies focused on primary hyperthyroidism and did not distinguish patients with the severe form of this disease.13,14 The present study performed a comprehensive analysis of patients with moderate to severe PHPT in our hospital. BMD measurements were performed using dual-energy X-ray absorptiometry (DXA) to analyze changes in BMD at different sites, including the lumbar spine and total hip, before parathyroidectomy, and 6 months, 1 year, and 2 years after parathyroidectomy. These findings provide information for the effective treatment of patients with PHPT to prevent adverse events, such as hip fracture.
Materials and methods
Patients
This retrospective cohort study included consecutive patients who were treated in the General Surgery Department of Beijing Jishuitan Hospital from January 2010 to December 2015. The inclusion criteria were as follows: (1) iPTH levels >700 pg/mL (normal: 15–65 pg/mL) and serum calcium ion levels exceeding the normal range (2.25–2.75 mmol/L); (2) successful surgery (after parathyroidectomy, pathological diagnosis of a specimen was used for further confirmation and serum calcium and iPTH levels were normal for at least 6 months postoperatively15) performed by experienced physicians working in the same department of our hospital; (3) stable and continuous preoperative and postoperative biochemical and BMD follow-up with measurements performed in our hospital; and (4) traditional therapy (non-surgical treatment, such as drug therapy) was not effective (biochemical indicators and symptoms were not improved). The exclusion criteria were as follows: (1) secondary hyperparathyroidism; (2) incomplete clinical data, including BMD data; (3) the presence of conditions that may affect hormone levels, such as a history of malignancy, active infection, pregnancy, and lactating/nursing; and (4) calcium ion and iPTH levels above the normal range following effective resection of parathyroid lesions.16 The study was approved by the Institutional Review Board of Beijing Jishuitan Hospital (review batch number: 201905-01), which waived the requirement for informed consent.
Clinical data collection and examination methods
The baseline characteristics included demographic data (sex, age, height, weight, and body mass index [BMI]), smoking, drinking, deformity, skeletal pain, fatigue, fracture, urinary calculi, hypertension, and basic information on parathyroid specimens. All patients were operated on by one of three surgeons. The indications for surgery were as defined by the National Institutes of Health consensus conference guidelines.17 After the specimens were removed, they were sent for analysis and pathological interpretation was performed by professional pathologists. Data collected from the specimens included parathyroid properties, wet weight (g), and volume (cm3). Blood samples were taken in the early morning before surgery when all patients were in a fasted state. The blood samples were centrifuged at 3000 to 5000 ×g and processed within 2 hours after phlebotomy and stored at −80°C. Biochemical indicators were measured using automated techniques. Serum calcium (normal values: 2.25–2.75 mmol/L) and phosphorus (normal values: 0.8–1.6 mmol/L) levels were measured by an autoanalyzer (Hitachi H7600; Hitachi Corp., Tokyo, Japan). Corrected calcium levels were based on the serum albumin level. Serum iPTH levels were determined using a chemiluminescent immunometric assay (E601; Roche Diagnostics, Basel, Switzerland; normal range 15–65 pg/mL). The intra- and inter-assay coefficients of variance were <10%. The normal range of values for serum alkaline phosphatase (Hitachi H7600) is 40–150 IU/L.
After surgical removal of the lesions of the parathyroid tissue, the same biochemical indices were tested at 6 months, 1 year, and 2 years postoperatively according to the requirements of preoperative biochemical tests. BMD measurements were performed using DXA with a Lunar DPX (GE Healthcare, Madison, WI, USA) in the array (fan beam) mode, including the lumbar spine (L1, L2, L3, and L4) and total hip (femoral neck, great trochanter, and Ward’s triangle), with a precision error of ≤1% at all sites. BMD values are expressed as absolute values (g/cm2), and the T-score and Z-score. The Z-score is the number of standard deviations (SDs) that a given measurement differs from the mean for a sex-, age-, and race-matched reference population. The T-score is the number of SDs that a given measurement differs from the mean for a normal young adult reference population. According to World Health Organization (WHO) criteria, the patients were classified as having osteoporosis (T-score ≤−2.5), osteopenia (T-score of >−2.5 to −1), and normal (T-score >−1).18 BMD measurements were taken before surgery and 6 months, 1 year, and 2 years after surgery, and the data were saved.
Statistical analysis
The data were analyzed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 4 (GraphPad Inc., San Diego, CA, USA) was used to produce graphs. Demographic characteristics are shown using descriptive analysis. Continuous variables are expressed as mean ± SD. Pre- and post-test data were compared using the t-test or Wilcoxon test on the basis of distribution of data. The inspection level was two-sided and P<0.05 was considered statistically significant.
Results
Among 105 patients with abnormalities in serum calcium and/or iPTH concentrations, 67 met the diagnostic criteria of moderate to severe PHPT.19 An additional 37 patients were excluded owing to the absence of follow-up data and lack of complete effective parathyroidectomy. Thirty patients with successful parathyroidectomy and longitudinal BMD follow-up were included in the final analysis. The baseline characteristics of the included patients are summarized in Table 1.
Table 1.
Baseline characteristics of patients with moderate to severe primary hyperparathyroidism.
| Baseline characteristics | Value |
|---|---|
| Demographic data | |
| Men | 17 (57%) |
| Women | 13 (43%) |
| Age (years) | 38.90 ± 15.48 |
| Height (m) | 1.68 ± 0.11 |
| Weight (kg) | 62.53 ± 13.13 |
| BMI | 22.00 ± 2.84 |
| Habits and conditions | |
| Smoking | 10 (33%) |
| Drinking | 4 (13%) |
| Deformity | 4 (13%) |
| Skeletal pain | 25 (83%) |
| Fatigue | 11 (37%) |
| Fracture | 15 (50%) |
| Urinary calculi | 11 (37%) |
| Hypertension | 3 (10%) |
| Parathyroid specimens | |
| Hyperplasia | 2 (7%) |
| Adenoma | 27 (90%) |
| Carcinoma | 1 (3%) |
| Wet weight (g) | 7.44 ± 9.16 |
| Volume (cm3) | 16.04 ± 20.56 |
Continuous variables are presented as mean ± standard deviation and categorical data are presented as the percentage of analyzed patients (n, %). BMI, body mass index.
Comparison of BMD before and after surgery
Preoperative measurements showed severe bone loss, with preoperative lumbar spine and total hip T-scores of −3.42±1.48 and −3.39±1.12, respectively. Both of these values were below −2.5 (i.e., osteoporosis). BMD of the total hip, which is mainly cortical bone, was lower than that of L1–L4, which is mainly cancellous bone.
Mean BMD of L1–l4 gradually increased over time and showed significant increases at the 6-month, 1-year, and 2-year follow-ups compared with the preoperative value (all P<0.05, Table 2). We observed the same trend for T-scores and Z-scores (all P<0.05). The postoperative BMD, T-scores, and Z-scores of the total hip also gradually increased and were significantly higher at the 6-month (all P<0.001), 1-year (all P<0.01), and 2-year (all P<0.05) follow-ups compared with before surgery. These two locations showed a more significant increase in the second year after surgery than in the first year. Moreover, stratified analyses based on sex showed similar trends in the changes of BMD, the T-score, and the Z-score at L1–L4 and the total hip in male and female patients at the 6-month follow-up. However, changes in BMD at L1–L4 at the 1- and 2-year follow-ups were not significantly different in female patients, but they were significant in male patients (both P<0.05). Furthermore, changes in BMD at the total hip at the 1- and 2-year follow-ups were not significantly different in male patients.
Table 2.
Changes in BMD before and after surgery in patients with severe hyperparathyroidism.
| Site | Parameter | Both sexes and total | Before surgery | 6 months after surgery | 1 year after surgery | 2 years after surgery | P valuea | P valueb | P valuec |
|---|---|---|---|---|---|---|---|---|---|
| L1–L4* | BMD (g/cm2) | Men | 0.74 ± 0.14 | 0.90 ± 0.15 | 1.02 ± 0.09 | 1.09 ± 0.07 | 0.003 | 0.039 | 0.007 |
| Women | 0.62 ± 0.18 | 0.87 ± 0.19 | 1.04 ± 0.35 | 1.33 ± 0.32 | 0.031 | 0.156 | 0.117 | ||
| All | 0.69 ± 0.17 | 0.89 ± 0.16 | 1.03 ± 0.21 | 1.21 ± 0.24 | <0.001 | 0.011 | 0.012 | ||
| T-score | Men | −2.83 ± 1.21 | −1.60 ± 1.43 | −0.20 | 0.20 | 0.011 | – | – | |
| Women | −4.26 ± 1.48 | −2.03 ± 1.81 | −0.55 ± 2.62 | 1.75 ± 2.76 | 0.041 | 0.143 | 0.122 | ||
| All | −3.42 ± 1.48 | −1.77 ± 1.51 | −0.43 ± 1.86 | 1.23 ± 2.15 | <0.001 | 0.032 | 0.046 | ||
| Z-score | Men | −2.72 ± 1.31 | −1.37 ± 1.63 | 0.30 | 0.60 | 0.018 | – | – | |
| Women | −3.89 ± 1.23 | −1.58 ± 1.62 | −0.10 ± 2.26 | 2.35 ± 2.47 | 0.036 | 0.139 | 0.131 | ||
| All | −3.20 ± 1.37 | −1.45 ± 1.53 | 0.03 ± 1.62 | 1.77 ± 2.02 | <0.001 | 0.022 | 0.040 | ||
| Total hip | BMD (g/cm2) | Men | 0.55 ± 0.13 | 0.69 ± 0.14 | 0.89 ± 0.04 | 0.92 ± 0.01 | 0.003 | 0.139 | 0.081 |
| Women | 0.52 ± 0.17 | 0.65 ± 0.10 | 0.75 ± 0.10 | 0.84 | 0.012 | 0.016 | – | ||
| All | 0.54 ± 0.14 | 0.67 ± 0.12 | 0.82 ± 0.10 | 0.89 ± 0.05 | <0.001 | 0.002 | 0.008 | ||
| T-score | Men | −3.19 ± 0.93 | −2.13 ± 1.16 | −0.90 | −0.40 | 0.016 | – | – | |
| Women | −3.64 ± 1.35 | −2.68 ± 0.88 | −1.80 ± 0.85 | −1.10 | 0.022 | 0.021 | – | ||
| All | −3.39 ± 1.12 | −2.35 ± 1.04 | −1.50 ± 0.79 | −0.75 ± 0.49 | <0.001 | 0.002 | <0.001 | ||
| Z-score | Men | −3.01 ± 0.90 | −1.93 ± 1.25 | −0.60 | 0.00 | 0.017 | – | – | |
| Women | −3.40 ± 1.22 | −2.45 ± 0.85 | −1.55 ± 0.92 | −0.40 | 0.027 | 0.021 | – | ||
| All | −3.18 ± 1.03 | −2.14 ± 1.09 | −1.23 ± 0.85 | −0.20 ± 0.28 | <0.001 | 0.007 | 0.010 |
Data are shown as mean ± standard deviation. Values without a standard deviation only represent one patient.
Mean of L1–L4; a6 months after surgery vs before surgery; b1 year after surgery vs before surgery; c2 years after surgery vs before surgery. T-score of >−2.5 to −1 indicates osteopenia; T-score of <−2.5 indicates osteoporosis.
BMD, bone mineral density.
Comparison of BMD in different parts of the femur and spine
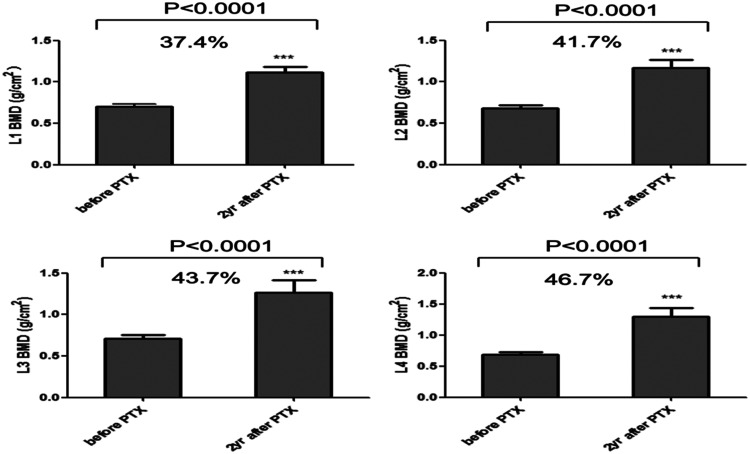
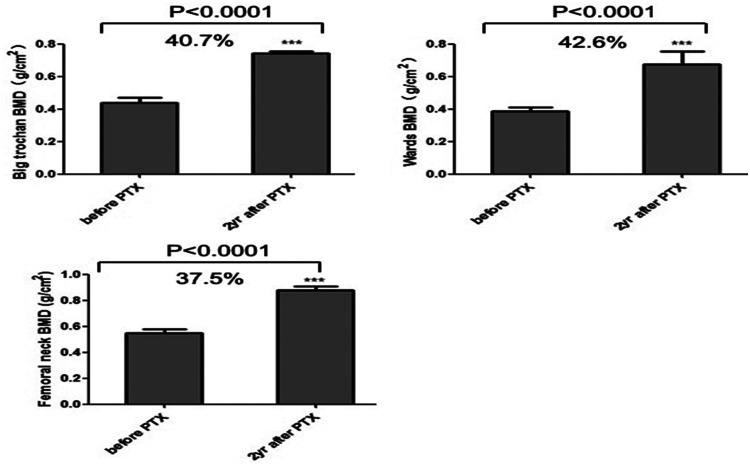
BMD of different parts of the lumbar spine (cancellous bone) are shown in Figure 1. BMD of L3 in the lumbar spine was 0.71 ± 0.17 g/cm2 and that for L2 was 0.68 ± 0.17 g/cm2. BMD of L1, L2, L3, and L4 were significantly higher at 2 years after surgery compared with before surgery (all P<0.001). L4 showed the largest increase (46.7%) and L1 the smallest increase (37.4%). BMD of the femoral neck, Ward’s triangle, and great trochanter were significantly higher 2 years after surgery compared with before surgery (all P<0.001). Among them, the femoral neck had the highest BMD and Ward’s triangle the lowest before surgery. However, BMD of Ward’s triangle increased by 42.6% and the femoral neck by 37.5% (Figure 2).
Figure 1.
Changes in bone mineral density (BMD) in different parts of the lumbar spine from before surgery to the 2-year follow-up. The graphs show mean (±standard error of the mean) BMD before and 2 years after surgery at L1, L2, L3, and L4. P values were obtained by the Wilcoxon test between pre- and post-parathyroidectomy (PTX).
Figure 2.
Changes in bone mineral density (BMD) in different parts of the hip from before surgery to the 2-year follow-up. The graphs show the mean (±standard error of the mean) BMD before and 2 years after surgery at the femoral neck, Ward’s triangle, and the great trochanter. P values were obtained by the Wilcoxon test between pre- and post-parathyroidectomy (PTX).
Discussion
This study investigated changes in BMD over 2 years in patients with moderate to severe PHPT who had undergone successful parathyroidectomy. These findings provide important information on the changes in BMD in a patient population that is rarely covered in the literature. We found that there was a slightly higher number of male patients with moderate to severe PHPT than female patients, and that this disease greatly affected the skeletal, urinary, and other systems. Moreover, BMD, T-scores, and Z-scores at the lumbar spine and total hip in patients gradually improved during the postoperative follow-up after parathyroidectomy.
Although this study included more male than female patients, the difference between sexes was small. Women account for the majority of patients with PHPT, with a female to male ratio as high as 3 or 4: 1.20 In this study, bone pain was present in 83% of patients, 50% of patients presented fractures, and 37% had fatigue and were uncomfortable. Because of the long course of the disease, 13% of patients had severe bone damage and skeletal deformities. Symptomatic PHPT, characterized by damaged bones and kidneys, remains the main feature in countries, such as China and India, owing to a lack of recognition of this disease and appropriate screening.21 Typical signs in imaging of PHPT include cortical bone absorption of the phalanx and “black skull sign” of the skull when the skeletal system is destroyed to a certain extent. When the kidney becomes the main target organ, urinary calculus and decreased renal function are observed.22 Similar to previous studies, we found multiple systemic invasions other than in the skeletal system; 37% of patients had urinary stones and 10% had hypertension.
Resection of parathyroid lesions is the only means for treating hyperparathyroidism. Resection of lesions refers to removal of highly functional parathyroid tissue.23 The imaging location of abnormal parathyroid tissue before surgery is important. Accurate positioning is an effective guarantee for a successful operation and surgical treatment of hyperparathyroidism.24 Patients with symptomatic hyperparathyroidism should undergo surgical intervention unless contraindications for inoperable surgery or other medical procedures are present.25 Surgical intervention is necessary for patients with bone pain, skeletal deformities, and soft tissue calcification.26 Even if hyperparathyroidism is asymptomatic, surgery should be performed if the skeletal system is affected. Surgical intervention can even benefit patients with normal calcium and hyperparathyroidism.27 Considering the benefits, some scholars believe that patients with hyperparathyroidism should undergo surgical intervention as long as there are no clear surgical contraindications and the patients are in good condition and agree to this treatment.28 Similar to a previous study,29 the most common cause of PHPT in the present study was adenoma (90%), with 7% of patients with hyperplasia and 3% with cancer.
The incidence of elevated iPTH levels in patients with PHPT continues to increase, resulting in abnormal bone transformation and bone metabolism, increased bone loss, and decreased bone density.30 Patients with hyperparathyroidism have increased risks of fracture at all sites.31 VanderWalde et al.32 reported a 13% reduction in the risk of fracture after surgical removal of lesions. Skeletal involvement in patients with hyperparathyroidism occurs mainly in areas comprising cortical bone, such as the distal radius of the humerus.33 However, remodeling and improvement of the skeletal system mainly occur in cancellous bone after parathyroidectomy.34 Therefore, improvement in BMD is more rapid and longer-lasting in areas dominated by cancellous bone, such as the lumbar spine. A long-term follow-up study by Rubin et al.35 found that BMD of the lumbar spine was increased by up to 8% at 1 year after parathyroidectomy, while that of the femoral neck increased by 5%. Increased BMD in cortical and cancellous bones can be maintained even 15 years after effective surgery. Similar to these previous findings, our study showed higher BMD and T-scores for L1–L4 (cancellous bone) before surgery in patients with moderate to severe PHPT compared with those in the total hip. Therefore, this finding suggested that cortical bone was the main affected site in this group of patients.
Nordenstrom et al.36 reported improved BMD after surgery in 50% of 126 patients with hyperparathyroidism who were followed up. Additionally, a long-term follow-up showed that BMD in the lumbar spine and femoral neck was increased by up to 12% after surgery.35 Similar results were reported in randomized and prospective studies. Vestergaard et al.37 found reliable evidence of increased BMD in the lumbar spine and femoral neck 1 year after parathyroidectomy. Dy et al.38 divided the improvement of BMD in patients with hyperparathyroidism into three categories, including a decrease in BMD (<0%), a moderate increase (1% to 5%), and significant improvement (>5%). Their follow-up results showed that 62% of patients showed a moderate increase in BMD after surgery and that the improvement in BMD was most obvious in the sites where BMD had decreased the most. Long-term postoperative follow-up analysis of BMD in patients with hyperparathyroidism is limited in the literature.13 The present study assessed patients with moderate to severe PHPT for 2 years following surgery. We observed that BMD of the total hip and lumbar spine was significantly improved after 2 years. In the total hip, BMD of the femoral neck showed the smallest increase (37.5%), while that for Ward’s triangle showed the largest increase (42.6%). We also observed a mean increase in BMD after surgery of 37%, with a maximum increase in L4 (46.7%). We hypothesize that bone destruction in patients with moderate to severe PHPT is severe and that BMD improves after surgery. Moreover, a previous study showed that after removing the parathyroid gland, bone turnover markers and iPTH gradually returned to normal levels.39
This study has some limitations. First, the number of patients with moderate to severe PHPT was small. Therefore, the results might be variable and require further validation. Larger studies recruiting patients from multiple centers would increase the number of patients. Second, this was a retrospective cohort study. Therefore, selection and recall biases, as well as potential confounders, were inevitable. Although the follow-up period was 2 years, data related to changes in BMD through long-term follow-up were not available. Third, background therapies for PHPT and related secondary osteoporosis could affect the changes in BMD after parathyroidectomy. This issue should be addressed in future studies. Fourth, changes in BMD might be affected by hypovitaminosis D and geographical location, which were not considered in the present study.40,41 Fifth, BMD at 1/3 radius was not assessed because it is not a conventional inspection site in China. Finally, all patients were treated in our hospital. Therefore, this was a single-center study. Consequently, the representativeness of our findings is limited. Future multicenter, large-sample, prospective studies are warranted to verify our findings.
In conclusion, our study shows that, in patients with moderate to severe PHPT, BMD of the total hip, which is mostly cortical bone, and that of the lumbar spine, which is mostly cancellous bone, is greatly improved after parathyroidectomy at a 2-year follow-up. Before surgery, BMD of the total hip is lower than that of the lumbar spine, but the greatest increase in BMD occurs in cancellous bone regions during follow-up. These conclusions require further research and confirmation.
Acknowledgements
We thank all other staff members who contributed to the study.
Footnotes
Authors' contributions: Conception and design: SL, WT, and XJ. Administrative support: WT and XJ. Provision of study materials or patients: SL, MG, YZ, AC, CC, HY, WS, and KH. Collection and assembly of data: SL, MG, YZ, AC, CC, HY, WS, and KH. Data analysis and interpretation: SL, MG, YZ, AC, CC, HY, WS, and KH. Manuscript writing: All authors. Final approval of manuscript: All authors.
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding from the Beijing Municipal Administration of Hospitals Ascent Plan, (DFL20150401) and Beijing Jishuitan Hospital’s Discipline new star Plan (XKXX201604).
ORCID iD: Wei Tian https://orcid.org/0000-0002-0132-3392
References
- 1.Aster J, Abbas A, Robbins K. Pathologic basis of disease. 9th ed Philadelphia: Elsevier Health Sciences, 2014, pp.58–59. [Google Scholar]
- 2.Madkhali T, Alhefdhi A, Chen H, et al. Primary hyperparathyroidism. Ulus Cerrahi Derg 2016; 32: 58–66. DOI: 10.5152/ucd.2015.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser WD. Hyperparathyroidism. Lancet 2009; 374: 145–158. DOI: 10.1016/s0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 4.Makras P, Anastasilakis AD. Bone disease in primary hyperparathyroidism. Metabolism 2018; 80: 57–65. DOI: 10.1016/j.metabol.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers 2016; 2: 16033. DOI: 10.1038/nrdp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abood A, Vestergaard P. Increasing incidence of primary hyperparathyroidism in Denmark. Dan Med J 2013; 60: A4567. [PubMed] [Google Scholar]
- 7.Pradeep PV, Jayashree B, Mishra A, et al. Systematic review of primary hyperparathyroidism in India: the past, present, and the future trends. Int J Endocrinol 2011; 2011: 921814. DOI: 10.1155/2011/921814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilezikian JP. Primary hyperparathyroidism In: Feingold KR, Anawalt B, Boyce A, et al. (eds). Endotext [Internet]. South Dartmouth: MDText.com Inc, 2017. PMID: 25905161 [Google Scholar]
- 9.Bandeira F, Griz L, Chaves N, et al. Diagnosis and management of primary hyperparathyroidism–a scientific statement from the Department of Bone Metabolism, the Brazilian Society for Endocrinology and Metabolism. Arq Bras Endocrinol Metabol 2013; 57: 406–424. [DOI] [PubMed] [Google Scholar]
- 10.Masi L. Primary Hyperparathyroidism . Front Horm Res 2019; 51: 1–12. DOI: 10.1159/000491034. [DOI] [PubMed] [Google Scholar]
- 11.Bandeira F, Cusano NE, Silva BC, et al. Bone disease in primary hyperparathyroidism. Arq Bras Endocrinol Metabol 2014; 58: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan AA, Hanley DA, Rizzoli R, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int 2017; 28: 1–19. DOI: 10.1007/s00198-016-3716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caliskan M, Beysel S, Kizilgul M, et al. The effect of parathyroidectomy on bone mineral density in primary hyperparathyroidism. Turk J Med Sci 2019; 49: 1674–1680. DOI: 10.3906/sag-1904-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miguel GA, Carranza FH, Rodriguez JCR, et al. Trabecular bone score, bone mineral density and bone markers in patients with primary hyperparathyroidism 2 years after parathyroidectomy. Horm Metab Res 2019; 51: 186–190. DOI: 10.1055/a-0850-8679. [DOI] [PubMed] [Google Scholar]
- 15.Ishay A, Herer P, Luboshitzky R. Effects of successful parathyroidectomy on metabolic cardiovascular risk factors in patients with severe primary hyperparathyroidism. Endocr Pract 2011; 17: 584–590. DOI: 10.4158/ep10321.or. [DOI] [PubMed] [Google Scholar]
- 16.Chen CL, Chen NC, Hsu CY, et al. An open-label, prospective pilot clinical study of denosumab for severe hyperparathyroidism in patients with low bone mass undergoing dialysis. J Clin Endocrinol Metab 2014; 99: 2426–2432. DOI: 10.1210/jc.2014-1154. [DOI] [PubMed] [Google Scholar]
- 17.Bilezikian JP, Potts JT, Jr, Fuleihan Gel H, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 2002; 87: 5353–5361. DOI: 10.1210/jc.2002-021370. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Scientific Group Research on Menopause. WHO Technical Service Report Series 670. Geneva: WHO, 1998, pp.1–107. [Google Scholar]
- 19.Bhadada SK, Bhansali A, Shah VN, et al. Changes in serum leptin and adiponectin concentrations and insulin resistance after curative parathyroidectomy in moderate to severe primary hyperparathyroidism. Singapore Med J 2011; 52: 890–893. [PubMed] [Google Scholar]
- 20.Seib CD, Chomsky-Higgins K, Gosnell JE, et al. Patient Frailty Should Be Used to Individualize Treatment Decisions in Primary Hyperparathyroidism. World J Surg 2018; 42: 3215–3222. DOI: 10.1007/s00268-018-4629-3. [DOI] [PubMed] [Google Scholar]
- 21.Pawlowska M, Cusano NE. An overview of normocalcemic primary hyperparathyroidism. Curr Opin Endocrinol Diabetes Obes 2015; 22: 413–21.DOI: 10.1097/MED.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 22.Thier M, Daudi S, Bergenfelz A, et al. Predictors of multiglandular disease in primary hyperparathyroidism. Langenbecks Arch Surg 2018; 403: 103–109. DOI: 10.1007/s00423-017-1647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab 2014; 99: 3561–3569. DOI: 10.1210/jc.2014-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg 2011; 253: 585–591. DOI: 10.1097/SLA.0b013e318208fed9. [DOI] [PubMed] [Google Scholar]
- 25.Papier A, Kenig J, Barczynski M. [Evaluation of different intraoperative iPTH assay criteria in monitoring of minimally invasive parathyroidectomy for primary hyperparathyroidism]. Przegl Lek 2014; 71: 14–18. [PubMed] [Google Scholar]
- 26.Kravets I. Hyperthyroidism: Diagnosis and Treatment. Am Fam Physician 2016; 93: 363–370. [PubMed] [Google Scholar]
- 27.Koumakis E, Souberbielle JC, Payet J, et al. Individual site-specific bone mineral density gain in normocalcemic primary hyperparathyroidism. Osteoporos Int 2014; 25: 1963–1968. DOI: 10.1007/s00198-014-2689-2. [DOI] [PubMed] [Google Scholar]
- 28.Koumakis E, Souberbielle JC, Sarfati E, et al. Bone mineral density evolution after successful parathyroidectomy in patients with normocalcemic primary hyperparathyroidism. J Clin Endocrinol Metab 2013; 98: 3213–3220. DOI: 10.1210/jc.2013-1518. [DOI] [PubMed] [Google Scholar]
- 29.Kitada M, Yasuda S, Nana T, et al. Surgical treatment for mediastinal parathyroid adenoma causing primary hyperparathyroidism. J Cardiothorac Surg 2016; 11: 44. DOI: 10.1186/s13019-016-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonewald LF. The amazing osteocyte. J Bone Miner Res 2011; 26: 229–238. DOI: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein EM, Silva BC, Boutroy S, et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res 2013; 28: 1029–1040. DOI: 10.1002/jbmr.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWalde LH, Liu IL, Haigh PI. Effect of bone mineral density and parathyroidectomy on fracture risk in primary hyperparathyroidism. World J Surg 2009; 33: 406–411. DOI: 10.1007/s00268-008-9720-8. [DOI] [PubMed] [Google Scholar]
- 33.Bandeira F, Cassibba S. Hyperparathyroidism and Bone Health. Curr Rheumatol Rep 2015; 17: 48. DOI: 10.1007/s11926-015-0523-2. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen P, Steiniche T, Brixen K, et al. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone 1999; 25: 237–244. [DOI] [PubMed] [Google Scholar]
- 35.Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab 2008; 93: 3462–3470. DOI: 10.1210/jc.2007-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordenstrom E, Westerdahl J, Lindergard B, et al. Multifactorial risk profile for bone fractures in primary hyperparathyroidism. World J Surg 2002; 26: 1463–1467. DOI: 10.1007/s00268-002-6433-2. [DOI] [PubMed] [Google Scholar]
- 37.Vestergaard P, Mollerup CL, Frokjaer VG, et al. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ 2000; 321: 598–602. DOI: 10.1136/bmj.321.7261.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dy BM, Grant CS, Wermers RA, et al. Changes in bone mineral density after surgical intervention for primary hyperparathyroidism. Surgery 2012; 152: 1051–1058. DOI: 10.1016/j.surg.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Khan AA. Medical management of primary hyperparathyroidism. J Clin Densitom 2013; 16: 60–63. DOI: 10.1016/j.jocd.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Minisola S, Romagnoli E, Scillitani A, et al. Hypovitaminosis D in primary hyperparathyroidism: to treat or not to treat? That is the question. J Endocrinol Invest 2014; 37: 413–414. doi:10.1007/s40618-014-0060-2 [DOI] [PubMed] [Google Scholar]
- 41.De Lucia F, Minisola S, Romagnoli E, et al. Effect of gender and geographic location on the expression of primary hyperparathyroidism. J Endocrinol Invest 2013; 36: 123–126. doi:10.3275/8455 [DOI] [PubMed] [Google Scholar]