Abstract
Objective
This study compared the diagnostic performance of alpha-fetoprotein (AFP) and des-gamma-carboxyprothrombin (DCP) in early-stage hepatitis B virus-related hepatocellular carcinoma (HBV-HCC) under different backgrounds.
Methods
Patients were enrolled and divided in four groups: chronic HBV infection (CHB), liver cirrhosis (LC), early-stage CHB-HCC, and early-stage LC-HCC. Serum AFP and DCP levels were measured. Receiver-operating characteristic (ROC) curve and area under the curve (AUC) analyses were applied to compare the diagnostic performance of DCP and AFP for HCC.
Results
In total, 200 patients were enrolled, including 48, 64, 33, and 55 patients with CHB, LC, CHB-HCC, and LC-HCC, respectively. ROC curve analysis revealed that the AUCs of AFP, DCP, and their combination in differentiating early-stage LC-HCC from LC were 0.776, 0.758, and 0.786, respectively. The values of these markers in discriminating early-stage CHB-HCC from CHB were 0.828, 0.731, and 0.862, respectively.
Conclusions
DCP was inferior to AFP in differentiating early-stage CHB-HCC from CHB. However, AFP and DCP displayed similar performance in distinguishing early-stage LC-HCC and LC.
Keywords: Des-gamma-carboxyprothrombin, alpha-fetoprotein, diagnostic performance, hepatocellular carcinoma, hepatitis B virus, liver cirrhosis, chronic hepatitis B
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor in adults globally and the third most common cause of cancer-related death. Approximately 470,000 new cases of HCC are diagnosed in China annually, accounting for 55% of the total incidence worldwide.1,2 The incidence of HCC in China is mainly related to hepatitis B virus (HBV) infection, hepatic fibrosis, and cirrhosis.3,4 Approximately 2% to 5% of cases of liver cirrhosis per year progress to HCC.5 At present, strategies for treating HCC include surgical resection, interventional embolization, radiofrequency ablation, liver transplantation, and chemotherapy.6 However, postoperative recurrence and metastasis rates remain high, and only approximately 10% of patients survive for more than 5 years. By contrast, early diagnosis has been accepted as an effective strategy for improving the long-term survival rate of HCC. The current diagnostic methods for HCC are imaging examinations, including B-scan ultrasound, CT, and MRI,7 and the detection of serum tumor markers.8,9
Alpha-fetoprotein (AFP) is the most commonly used serum biomarker in the diagnosis and monitoring of HCC globally, especially in China.10 However, several benign liver diseases, such as chronic hepatitis and liver cirrhosis (LC), can also elevate serum AFP levels.11 Furthermore, AFP levels may be normal in some patients with HCC. Thus, the sensitivity and specificity of AFP in diagnosing HCC is inadequate.12 Des-gamma-carboxyprothrombin (DCP), a prothrombin precursor, was first reported to be associated with liver cancer by Liebman et al. in 1984.13 Then, later research indicated that DCP could be overexpressed in HCC, suggesting its utility as a serum biomarker for liver cancer diagnosis.14–16 However, whether serum DCP has better accuracy than AFP, a widely applied biomarker, in diagnosing early-stage HBV-related HCC (HBV-HCC) is unclear.
In this study, we compared the diagnostic performance of AFP and DCP for early-stage HBV-HCC under different backgrounds.
Materials and methods
Study design and patients included
The patients included in this cross-sectional study were enrolled at Beijing Youan Hospital between June 2010 and March 2020 and divided in four groups: chronic HBV infection (CHB), LC, early-stage CHB-HCC, and early-stage LC-HCC. The inclusion criteria for participants in this study were as follows: chronic HBV infection for more than 6 months; age of 18 to 80 years; serum AFP and DCP level measurements before surgery; and the absence of other viral infections or cancer types. CHB was diagnosed according to the Guideline of Prevention and Treatment for Chronic Hepatitis B (2015 update). LC was diagnosed according to the diagnostic criteria for chronic hepatitis B (2019 edition) of the Chinese Medical Association. These criteria included current positivity for HBsAg or anti-HBc and a clear history of chronic HBV infection as well as liver biopsy pathology consistent with LC or the presence of at least two of the following items: (1) signs of cirrhosis or portal hypertension on imaging, (2) esophageal and gastric varices on endoscopy, (3) presence of liver stiffness consistent with cirrhosis, (4) reduced blood albumin levels and prolongation of the prothrombin time, and (5) a platelet count of less than 100 × 109/L. Moreover, patients with LC should be followed up for more than 6 months to exclude the possibility of HCC. In this study, only patients with early-stage HBV-HCC were included. Early-stage HCC was defined by a total diameter of up to three lesions of ≤3 cm without metastasis. Although the initial diagnosis of HCC might have been made using B-scan ultrasound or dynamic contrast imaging, all cases were confirmed via postoperative histopathological examination. In addition, patients with intrahepatic cholangiocarcinoma, mixed HCC, or HCC caused by other factors such as hepatitis C virus, alcoholic liver disease, and biliary cirrhosis were excluded. The study was approved by the ethics committee of Beijing Youan Hospital, and written informed consent was obtained from all participants.
Measurements of serum AFP and DCP
The LUMIPULSE® G1200 (Fujirebio Inc., Tokyo, Japan), an automated chemiluminescent enzyme immunoassay system, was applied to measure serum AFP and DCP levels according to the manufacturer’s instructions.
Statistical analysis
SPSS version 22.0 (IBM, Armonk, NY, USA), MedCalc version 15.2.2 (MedCalc Software Ltd, Ostend, Belgium), and GraphPad Prism version 7 (GraphPad Software, San Diego, CA, USA) were used to analyze the data. Between-group differences were compared using Student’s t-test (for normally distributed data) or the Mann–Whitney test (for non-normally distributed data). Clinical characteristics were compared using Pearson’s χ2 test and Fisher’s exact test. The diagnostic value was evaluated using receiver operating characteristic (ROC) curve and area under the curve (AUC) analyses. In all instances, P < 0.05 denoted statistical significance.
Results
Clinical characteristics of the enrolled patients
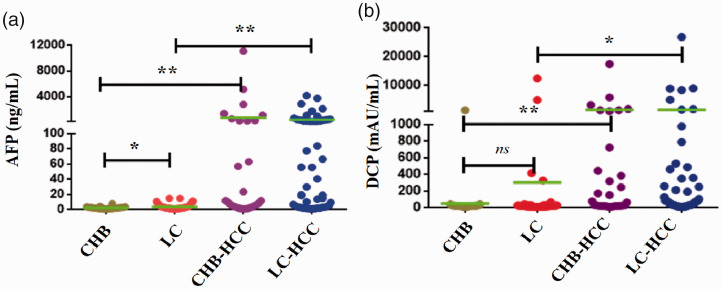
In total, 200 patients were included in this study, including 48, 64, 33, and 55 patients with CHB, LC, early-stage CHB-HCC, and early-stage LC-HCC, respectively. The detailed characteristics of the patients are summarized in Table 1. Gender and aspartate aminotransferase levels were well matched among the groups. Age, Child–Pugh grade, alanine aminotransferase levels, and total bilirubin levels significantly differed among the groups (all P < 0.05). The median (interquartile range) serum AFP levels in the CHB, LC, early-stage CHB-HCC, and early-stage LC-HCC groups were 2.47 (1.54, 2.99), 2.82 (1.70, 4.76), 8.80 (3.24, 214.1), and 19.46 ng/mL (3.21, 194.5), respectively. Serum AFP levels were significantly lower in the CHB group than in the LC and CHB-HCC groups (both P < 0.05, Figure 1a). Meanwhile, AFP levels were significantly higher in the LC-HCC group than in the LC group (P < 0.05, Figure 1a). The median serum (interquartile range) DCP levels in the CHB, LC, early-stage CHB-HCC, and early-stage LC-HCC groups were 223 (18, 27.75), 26 (16, 32), 36 (22.5, 25.86), and 34 mAU/mL (17.5, 114.3), respectively. Serum DCP levels were significantly lower in the CHB group than in the CHB-HCC group (P < 0.05), whereas its levels were significantly higher in the LC-HCC group than in the LC group (P < 0.05, Figure 1b).
Table 1.
The clinical characteristics of the included patients.
| Items | CHB | LC | Early-stage CHB-HCC | Early-stage LC-HCC | P |
|---|---|---|---|---|---|
| N | 48 | 64 | 33 | 55 | |
| Sex | |||||
| Male | 35 | 42 | 25 | 45 | 0.252 |
| Female | 13 | 22 | 8 | 10 | |
| Age (years) | |||||
| ≤50 | 30 | 55 | 24 | 35 | 0.017* |
| >50 | 18 | 9 | 9 | 20 | |
| Child–Pugh | |||||
| A | 48 | 47 | 30 | 45 | 0.001* |
| B | 0 | 14 | 3 | 9 | |
| C | 0 | 3 | 0 | 1 | |
| AST (U/L) | |||||
| ≤40 | 37 | 48 | 24 | 43 | 0.940 |
| >40 | 11 | 16 | 9 | 12 | |
| ALT (U/L) | |||||
| ≤50 | 42 | 61 | 25 | 42 | 0.003* |
| >50 | 6 | 3 | 8 | 13 | |
| TBil (µmol/L) | |||||
| ≤21 | 40 | 33 | 23 | 41 | 0.002* |
| >21 | 8 | 31 | 10 | 14 | |
| AFP (ng/mL), median (interquartile range) | 2.47 (1.54, 2.99) | 2.82 (1.70, 4.76) | 8.80 (3.24, 214.1) | 19.46 (3.21, 194.5) | |
| DCP (mAU/mL), median (interquartile range) | 23 (18, 27.75) | 26 (16, 32) | 36 (22.5, 2586) | 34 (17.5,114.3) | < |
Data are presented as numbers unless otherwise indicated.
*, P < 0.05.
CHB, chronic hepatitis B virus infection; LC, liver cirrhosis; HCC, hepatocellular carcinoma; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TBil, total bilirubin; AFP, alpha-fetoprotein; DCP, des-gamma-carboxyprothrombin.
Figure 1.
Serum AFP and DCP in patients with CHB, LC, and early-stage HCC. a, AFP. b, DCP. *, P < 0.05. **, P < 0.01. ns, not significant.
AFP, alpha-fetoprotein; DCP, des-gamma-carboxyprothrombin; CHB, chronic hepatitis B virus infection; LC: liver cirrhosis; HCC, hepatocellular carcinoma.
Diagnostic value of AFP and DCP for distinguishing early-stage HBV-HCC from CHB and LC
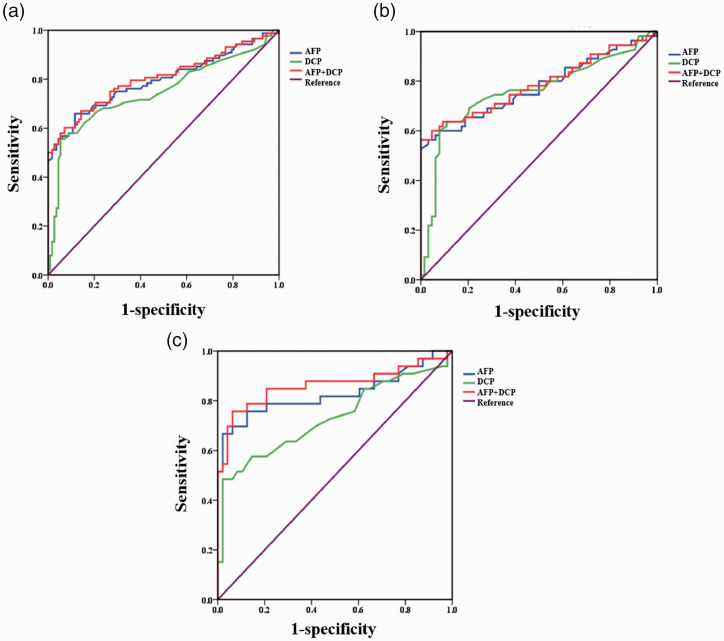
ROC curve analysis illustrated that the AUCs of AFP and DCP for distinguishing early-stage HCC from CHB and LC were 0.794 (95% confidence interval [CI] = 0.727 to 0.861) and 0.747 (95% CI = 0.674 to 0.821), respectively (Table 2, Figure 2a). When AFP and DCP were combined, the AUC was 0.804 (95% CI = 0.739 to 0.870, Table 2, Figure 2a). The comparison of ROC curves revealed no significant differences among the AUCs of AFP, DCP, and their combination in distinguishing early-stage HCC from CHB and LC.
Table 2.
The value of AFP, DCP, and their combination in diagnosing early-stage HBV-related HCC.
| Items | Cutoff | AUC (95% CI) | Sen (%) | Spe (%) | PPV (%) | NPV (%) | +LR | −LR | Youden index (%) |
|---|---|---|---|---|---|---|---|---|---|
| Early-stage HCC vs. LC, CHB | |||||||||
| AFP (ng/mL) | 5 | 0.794 (0.727 to 0.861) | 65.9 | 88.4 | 95.8 | 79.9 | 5.7 | 0.39 | 54.3 |
| DCP (mAU/mL) | 44 | 0.747 (0.674 to 0.821) | 55.7 | 94.6 | 95.0 | 75.4 | 10.3 | 0.47 | 50.3 |
| AFP + DCP | 0.46 | 0.804 (0.739 to 0.870) | 60.2 | 92.9 | 95.4 | 77.3 | 8.5 | 0.43 | 53.1 |
| Early-stage LC-HCC vs. LC | |||||||||
| AFP (ng/mL) | 11.6 | 0.776 (0.687 to 0.865) | 56.4 | 96.9 | 95.8 | 72.3 | 18.2 | 0.45 | 53.2 |
| DCP (mAU/mL) | 38.0 | 0.758 (0.665 to 0.850) | 63.6 | 89.1 | 96.3 | 75.8 | 5.8 | 0.41 | 52.7 |
| AFP+DCP | 0.58 | 0.786 (0.698 to 0.873) | 56.4 | 100 | 95.8 | 72.3 | NA | 0.44 | 56.4 |
| Early-stage CHB-HCC vs. CHB | |||||||||
| AFP (ng/mL) | 4.7 | 0.828 (0.722 to 0.934) | 66.7 | 97.9 | 95.6 | 81.0 | 31.7 | 0.34 | 64.6 |
| DCP (mAU/mL) | 44 | 0.731 (0.611 to 0.851) | 48.5 | 97.9 | 94.1 | 73.4 | 23.1 | 0.53 | 46.4 |
| AFP+DCP | 0.34 | 0.862 (0.767 to 0.958) | 75.8 | 93.8 | 89.4 | 84.9 | 12.2 | 0.26 | 69.5 |
AFP, alpha-fetoprotein; DCP, des-gamma-carboxyprothrombin; HPB, hepatitis B virus; HCC, hepatocellular carcinoma; LC, liver cirrhosis; CHB, chronic hepatitis B virus infection; AUC, area under the curve; CI, confidence interval; Sen, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; −LR, negative likelihood ratio; NA, not available, could not calculate.
Figure 2.
Receiver operating characteristic curve analysis of AFP, DCP, and their combinations in diagnosing early-stage hepatitis B virus-related HCC. a, early-stage HCC vs. CHB and LC. b, early-stage LC-HCC vs. LC. c, early-stage CHB-HCC vs. CHB.
AFP, alpha-fetoprotein; DCP, des-gamma-carboxyprothrombin; HCC, hepatocellular carcinoma; CHB, chronic hepatitis B virus infection; LC: liver cirrhosis.
Diagnostic value of AFP and DCP in distinguishing early-stage LC-HCC from LC
ROC curve analysis illustrated that the AUCs of AFP and DCP for differentiating early-stage LC-HCC from LC were 0.776 (95% CI = 0.687 to 0.865) and 0.758 (95% CI = 0.665 to 0.850), respectively (Table 2, Figure 2b). When AFP and DCP were combined, the AUC was 0.786 (95% CI = 0.698 to 0.873, Table 2, Figure 2b). The comparison of ROC curves revealed no statistical differences among the AUCs of AFP, DCP, and their combination in differentiating early-stage LC-HCC from LC.
Diagnostic value of AFP and DCP in distinguishing early-stage CHB-HCC and CHB
ROC curve analysis revealed that the AUCs of AFP and DCP in discriminating early-stage CHB-HCC from CHB were 0.828 (95% CI = 0.722 to 0.934) and 0.731 (95% CI = 0.611 to 0.851), respectively (Table 2, Figure 2c). When AFP and DCP were combined, the AUC was 0.862 (95% CI = 0.767 to 0.958, Table 2, Figure 2c). The comparison of ROC curves revealed that the AUC of AFP was not significantly larger than that of DCP. Conversely, the AUC of the combination of AFP and DCP was significantly larger than that of DCP (P = 0.005) but not AFP.
Discussion
HCC is a serious health problem worldwide, especially in eastern Asian countries such as China and Korea, in which hepatitis virus infection is endemic.17 HCC also has several clinical characteristics, including diagnosis at an advanced stage, a lack of effective treatments, and poor prognosis. Early diagnosis is important for improving the prognosis of patients with HCC. Serum tumor markers are commonly used for tumor diagnosis, efficacy assessments, prognosis prediction, and postoperative monitoring of recurrence and metastasis.18 Thus, many studies have focused on serum tumor markers for the early diagnosis of tumors.
To diagnose HCC, emphasis has been placed on serum tumor markers. Basic research has identified more than 100 markers related to HCC,19 and several markers are generally considered to have diagnostic value, including AFP,11 DCP, glypican-3,20 Golgi type II transmembrane protein,21 Lens culinaris agglutinin-reactive fraction of AFP,22–24 vascular endothelial growth factor, and hepatocyte growth factor. AFP is a commonly used biomarker in the diagnosis of HCC. However, the sensitivity and specificity of serum AFP remain relatively low.25,26 Thus, new diagnostic biomarkers are urgently needed for diagnosing HCC.
It was reported that DCP is significantly overexpressed in HCC.14 For instance, Song et al.27 found that serum DCP levels were significantly higher in HCC than in CHB and LC, and DCP levels might associated with the tumor diameter as well as vascular invasion and metastasis in HCC.28,29 The diagnostic value of serum DCP in HCC had been intensively investigated. Most researchers believed that DCP might be a favorable biomarker for diagnosing HCC with potentially better performance than AFP.30 For instance, a meta-analysis determined that the summary sensitivity and specificity of AFP were 63% and 59%, respectively, compared with 91% and 86%, respectively, for DCP.31 Serum DCP has also been used to diagnose AFP-negative HCC, especially HBV-related HCC.30,32,33 However, some studies found that the diagnostic value of serum DCP might be inferior that that of AFP and that DCP might be a complementary biomarker to AFP for diagnosing HCC. Moreover, whether the combination of DCP and AFP resulted in significantly better diagnostic results than AFP alone in HCC remained unclear.12,16,34
In China, most cases of HCC are associated with chronic HBV infection. Several previous studies evaluated the diagnostic value of AFP and DCP by comparing mixed HCC with LC or CHB.31,35 However, the value of biomarkers in diagnosing HCC might differ in the presence or absence of LC. Serum AFP levels were significantly lower in the CHB group than in the LC and HCC groups. Differing from previous studies, this study investigated and compared the value of DCP and AFP in diagnosing HBV-related HCC under different backgrounds. Our results illustrated that AFP was the best biomarker for distinguishing CHB and early-stage CHB-HCC, and the addition of DCP did not improve the diagnostic performance. However, when differentiating LC and early-stage LC-HCC, AFP, DCP, and their combination had similar diagnostic performance. Thus, serum DCP might have similar performance as AFP in discriminating LC and early LC-HCC.
This study had some limitations. First, the included patients were enrolled from one center, and the total number of participants was relatively small. Second, this study only focused on the diagnostic value of AFP and DCP in HBV-associated HCC, and other etiologies were excluded. Thus, more studies are needed to validate the results of this study.
In conclusion, we analyzed and compared the diagnostic performance of serum DCP and AFP in the diagnosis of early-stage HBV-HCC under different backgrounds. This study revealed that DCP might have lower value than AFP in discriminating early-stage CHB-HCC from CHB. In distinguishing early-stage LC-HCC and LC, AFP and DCP had similar performance, but the combination of AFP and DCP could not significantly improve the diagnostic value.
Footnotes
Author contributions: T.S., L.Z., and Y.T. conceived and designed the protocol and study. T.S., R.X., L.W., and L.Z. identified the studies to be screened. T.S. and L.W. identified the eligible studies, extracted data, and assessed the methodological quality of the included studies. Y.T., T.S., L.W., and L.Z. wrote the manuscript.
Availability of data and materials: The datasets supporting the conclusion of this study are included in the article.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was supported by the Natural Science Foundation of Shanghai (Grant No. 19ZR1447100) and Foundation of Shanghai Dermatology Hospital (Grant Nos. 17HBDS08 and 2018KYQD02).
ORCID iDs
Ting Song https://orcid.org/0000-0003-2810-471X
References
- 1.Wang FS, Fan JG, Zhang Z, et al. The Global Burden of Liver Disease: The Major Impact of China. Hepatology 2014; 60: 2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2017; 67: 7. [DOI] [PubMed] [Google Scholar]
- 3.Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: an update for clinicians. Mayo Clin Proc 2007; 82: 967–975. [DOI] [PubMed] [Google Scholar]
- 4.Nagaratnam N, Nagaratnam K, Cheuk G. Hepatocellular Carcinoma. In: Geriatric Diseases. Berlin: Springer. 2017.
- 5.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012; 57: 167–185. DOI: 10.1016/j.jhep.2012.02.010 S0168-8278(12)00167-5 [pii]. [DOI] [PubMed] [Google Scholar]
- 6.Marquardt JU, Galle PR, Teufel A. Molecular diagnosis and therapy of hepatocellular carcinoma (HCC): an emerging field for advanced technologies. J Hepatol 2012; 56: 267–275. [DOI] [PubMed] [Google Scholar]
- 7.Choi BI, Lee JM. Advancement in HCC imaging: diagnosis, staging and treatment efficacy assessments: imaging diagnosis and staging of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2010; 17: 369. [DOI] [PubMed] [Google Scholar]
- 8.Song P, Gao J, Inagaki Y, et al. Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and china. Liver Cancer 2013; 2: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakamoto M. Early HCC: diagnosis and molecular markers. J Gastroenterol 2009; 44: 108–111. [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Liu H, Liu Z, et al. Analysis of serum alpha-fetoprotein (AFP) and AFP-L3 levels by protein microarray. J Int Med Res 2018; 46: 4297–4305. DOI: 10.1177/0300060518789304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomasi TB., Jr. Structure and function of alpha-fetoprotein. Annu Rev Med 1977; 28: 453–465. DOI: 10.1146/annurev.me.28.020177.002321. [DOI] [PubMed] [Google Scholar]
- 12.Park SJ, Jang JY, Jeong SW, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 2017; 96: e5811. DOI: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebman HA, Furie BC, Tong MJ, et al. Desgamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med 1984; 310; 1427–1431. [DOI] [PubMed] [Google Scholar]
- 14.Svobodova S, Karlikova M, Topolcan O, et al. PIVKA-II as a Potential New Biomarker for Hepatocellular Carcinoma - A Pilot Study. In Vivo 2018; 32: 1551–1554. DOI: 10.21873/invivo.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan C, Hu J, Yang J, et al. Serum ARCHITECT PIVKA-II reference interval in healthy Chinese adults: Sub-analysis from a prospective multicenter study. Clin Biochem 2018; 54: 32–36. DOI: 10.1016/j.clinbiochem.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Saitta C, Raffa G, Alibrandi A, et al. PIVKA-II is a useful tool for diagnostic characterization of ultrasound-detected liver nodules in cirrhotic patients. Medicine (Baltimore) 2017; 96: e7266. DOI: 10.1097/md.0000000000007266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka M, Katayama F, Kato H, et al. Hepatitis B and C Virus Infection and Hepatocellular Carcinoma in China: A Review of Epidemiology and Control Measures. J Epidemiol 2011; 21: 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirabe K, Itoh S, Yoshizumi T, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol 2010; 95: 235–240. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Satomura S. Biomarkers for Hepatocellular Carcinoma (HCC): An Update. Netherlands: Springer, 2015, pp.179–193. [DOI] [PubMed] [Google Scholar]
- 20.Di Tommaso L, Franchi G, Park YN, et al. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology 2010; 45: 725–734. [DOI] [PubMed] [Google Scholar]
- 21.Mao Y, Yang H, Xu H, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010; 59: 1687–1693. [DOI] [PubMed] [Google Scholar]
- 22.Kumada T, Toyoda H, Tada T, et al. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol 2014; 49: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto K, Imamura H, Matsuyama Y, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol 2010; 45: 1272–1282. [DOI] [PubMed] [Google Scholar]
- 24.Aoyagi Y, Suzuki Y, Isemura M, et al. The fucosylation index of alpha-fetoprotein and its usefulness in the early diagnosis of hepatocellular carcinoma. Cancer 1988; 61: 769–774. [DOI] [PubMed] [Google Scholar]
- 25.Carr BI, Guerra V, Giannini EG, et al. Low alpha-fetoprotein HCC and the role of GGTP. Int J Biol Markers 2014; 29: 395–402. [DOI] [PubMed] [Google Scholar]
- 26.Gan W, Huang JL, Zhang MX, et al. New nomogram predicts the recurrence of hepatocellular carcinoma in patients with negative preoperative serum AFP subjected to curative resection. J Surg Oncol 2018; 117: 1540–1547. [DOI] [PubMed] [Google Scholar]
- 27.Song P, Feng X, Inagaki Y, et al. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-γ-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: A multi-center case-controlled study of 1,153 subjects. Biosci Trends 2014; 8: 266–273. [DOI] [PubMed] [Google Scholar]
- 28.Sumi A, Akiba J, Ogasawara S, et al. Des-γ-carboxyprothrombin (DCP) and NX-DCP expressions and their relationship with clinicopathological features in hepatocellular carcinoma. PLoS One 2015; 10: e0118452. DOI: 10.1371/journal.pone.0118452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsubara M, Shiraha H, Kataoka J, et al. Des-γ-carboxyl prothrombin is associated with tumor angiogenesis in hepatocellular carcinoma. J Gastroenterol Hepatol 2012; 27: 1602–1608. DOI: 10.1111/j.1440-1746.2012.07173.x. [DOI] [PubMed] [Google Scholar]
- 30.Ji J, Wang H, Li Y, et al. Diagnostic Evaluation of Des-Gamma-Carboxy Prothrombin versus α-Fetoprotein for Hepatitis B Virus-Related Hepatocellular Carcinoma in China: A Large-Scale, Multicentre Study. PLoS One 2016; 11: e0153227. DOI: 10.1371/journal.pone.0153227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Zhang Z, Zhang P, et al. Diagnostic Accuracy of Des-gamma-carboxy Prothrombin versus Alpha-fetoprotein for Hepatocellular Carcinoma: A Systematic Review. Hepatol Res 2013; 44: E11–E25. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Wu M, Lin D, et al. Des-gamma-carboxyprothrombin is a favorable biomarker for the early diagnosis of alfa-fetoprotein-negative hepatitis B virus-related hepatocellular carcinoma. J Int Med Res 2020; 48: 300060520902575. DOI: 10.1177/0300060520902575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Zhang W, Liu Y, et al. Diagnostic value of prothrombin induced by the absence of vitamin K or antagonist-II (PIVKA-II) for early stage HBV related hepatocellular carcinoma. Infect Agent Cancer 2017; 12: 47. DOI: 10.1186/s13027-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi J, Kim GA, Han S, et al. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early-Stage Hepatocellular Carcinoma. Hepatology 2019; 69: 1983–1994. DOI: 10.1002/hep.30233. [DOI] [PubMed] [Google Scholar]
- 35.Yu R, Xiang X, Tan Z, et al. Efficacy of PIVKA-II in prediction and early detection of hepatocellular carcinoma: a nested case-control study in Chinese patients. Sci Rep 2016; 6: 35050. DOI: 10.1038/srep35050. [DOI] [PMC free article] [PubMed] [Google Scholar]