Abstract
Objective
To study the effects of saikosaponin D (SSD) on proliferation and apoptosis in human non-small cell lung cancer cell lines, and to explore underlying mechanisms.
Methods
Following treatment with saikosaponin D, A549 and H1299 cells were assessed for anti-proliferation effects using cell cycle kit-8 assays, changes in nuclear morphology using 4′,6-diamidino-2-phenylindole (DAPI) staining, and cell apoptosis using annexin V/propidium iodide double staining. Proliferation- and apoptosis-related proteins were detected by immunoblotting.
Results
Saikosaponin D had dose-dependent inhibitory effects on A549 cells (IC50, 3.57 µM) and H1299 cells (IC50, 8.46 µM). DAPI staining revealed decreased cell numbers, and most H1299 cells became round after treatment with 20 µM saikosaponin D. As saikosaponin D concentration increased, the proportions of cells in G0/G1 phase, and cells undergoing apoptosis, increased. Levels of phosphorylated p44/42 and signal transducer and activator of transcription (STAT)3 were significantly downregulated in both cell lines, while total STAT3 levels were not significantly affected. The cleaved form of caspase 3 was significantly upregulated.
Conclusions
Saikosaponin D inhibits proliferation, inducing cell cycle arrest and apoptosis, in lung cancer cells in a dose-dependent manner, possibly through inhibition of STAT3 phosphorylation and activation of caspase 3.
Keywords: Saikosaponin D, STAT3 pathway, lung cancer, apoptosis
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common cancers and is a serious health threat, with current treatment based on surgery combined with chemotherapy and radiotherapy.1 Comprehensive treatment can achieve a certain therapeutic effect but causes a degree of damage to the immune system; this is particularly true of conventional chemotherapy drugs, which can cause adverse reactions.1 A present focus of research into NSCLC involves extraction of active substances from natural plants and screening them against NSCLC. As one of the important methods for treating advanced lung cancer,2 drug therapy has positive significance for slowing tumour progression and prolonging patient survival, but at present, there are few chemical drugs available for the treatment of lung cancer. In addition to classic chemotherapy drugs, such as gemcitabine, paclitaxel, cisplatin, and pemetrexed,3 small molecule targeted drugs, such as gefitinib, afatinib, osimertinib, and icotinib, are becoming first-line treatments for lung cancer.4 In clinical applications, it is important to identify more effective drugs to control lung cancer progression.
As a traditional herbal medicine with a long history, bupleurum is derived from the dry roots of plants of the genus Bupleurum and in particular, from the species Bupleurum chinense.5 Bupleurum has been shown to have anti-inflammatory, anticancer, antiviral, antibacterial, and antipyretic effects in China.6 In Asian countries, including Japan and South Korea, bupleurum is also used in the treatment of diseases such as influenza, fever, inflammation, and malaria.6 Saikosaponin is the main constituent of Bupleurum chinense, and the polyterpenoid saikosaponin D (Figure 1A) is one of the main active components in Bupleurum.6 Saikosaponin D has been shown to exhibit anticancer effects in various cancers, such as lung cancer,7,8 pancreatic cancer, breast cancer,9–11 thyroid cancer,12 ovarian cancer,13 glioma,14 and hepatoma cell cancer.15 However, the detailed mechanism behind these effects requires further exploration. The main purpose of the present study was to explore the effects of saikosaponin D on proliferation and apoptosis in NSCLC cells in vitro, and to explore the mechanism underlying these effects.
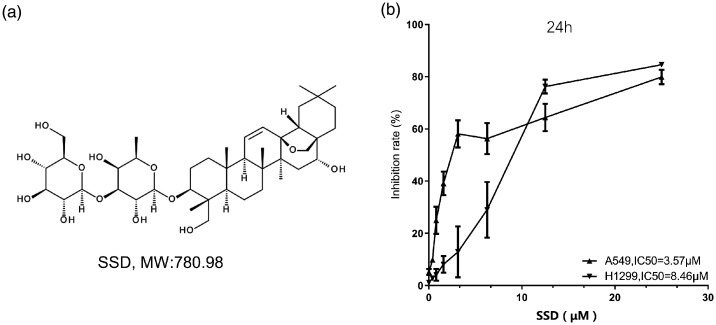
Figure 1.
(a) Diagram showing the chemical structure of saikosaponin D (SSD); and (b) The effects of 24-h treatment with varying concentrations of SSD (0, 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, and 25 μM) on inhibition of in vitro proliferation of two non-small cell lung cancer cell lines (A549 and H1299). Data presented as mean ± SD inhibition rate.
Materials and methods
Cell culture and drug treatment
Human NSCLC cell lines, A549 and H1299 (Zhejiang Ruyao Biotech Co., Ltd cell bank; Ningbo, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum and 1% penicillin-streptomycin (all Procell Biotechnology Ltd; Wuhan, China) at 37 °C in a 5% CO2 incubator. Cells cultured to approximately 80% confluence were washed with phosphate buffered saline (PBS; pH 7.4) and incubated with 0.25 % trypsin for 2 min. Detached cells were added to complete cell culture medium to terminate the digestion and centrifuged at 1000 g at room temperature for 5 min. The supernatant was discarded, and cells were immediately resuspended and passaged at a ratio of 1:3. Saikosaponin D (Shanghai Macklin Biochemical Co., Ltd; Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO) as a 20 mM stock solution, and was diluted to the desired concentration in complete medium for use in experiments. Cells were divided into four groups: treated with 5, 10 or 20 µM saikosaponin D and the control group, treated with an equal volume of complete medium containing 0.1% DMSO.
Effects on nuclear morphology
The A549 and H1299 cells were seeded at a density of 1 × 105/cm2 onto glass slides and incubated for 24 h to allow attachment and growth. After treatment with saikosaponin D or DMSO control solution for 24 h, the medium was removed from the plates, and precooled 70% ethanol was added. Next, 4′,6-diamidino-2-phenylindole (DAPI) stain solution was added to a final concentration of 1 µM, and the cells were stained for 1–2 min. Filter paper was used to absorb excess liquid, and the slides were immediately observed under a fluorescence microscope (Leica DM500; Shanghai Fenye Optoelectronic Equipment Co., LTD, Shanghai, China) to record cell morphological characteristics at an excitation wavelength of 360–400 nm.
CCK-8 cell proliferation detection
The A549 and H1299 cells that had been treated with different concentrations (0, 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, or 25 μM) of saikosaponin D for 24 h were collected. Each treatment group of cells was resuspended to 1 × 106 cells/ml with cell cycle kit (CCK)-8 solution (Dojindo Chemical Co., Ltd; Nagasaki, Japan), diluted to 10%. The cells were incubated at 37 °C for another 4 h, and the absorbance was measured at 450 nm to calculate the cell inhibition rate.
Cell cycle analysis by flow cytometry
Cell cycle was analysed by flow cytometry using propidium iodide (PI) staining. A549 and H1299 cells were plated at a density of 5 × 105 per well in a 6-well plate, followed by treatment with saikosaponin D (0, 5, 10 and 20 µM) in a humidified atmosphere of 5% CO2 for 24 h. Cells at a density of 1 × 106 were harvested and washed twice with PBS and fixed in cold ethanol (70%) at 4 °C overnight. The cells were stained with a PI solution (with 0.02% Triton X-100 [Shanghai Macklin Biochemical] and 50 mg/ml RNase [Simgen Biological Reagent Development Co. Ltd; Hangzhou, China]) for approximately 30 min in the dark. Samples were then analysed on an Invitrogen Attune flow cytometer (ThermoFisher Scientific, Wilmington, MA, USA), and data acquisition and analysis were performed using FlowJo™ software, version 10.0 (BD, Franklin Lakes, NJ, USA).
Apoptosis detection by flow cytometry
Apoptosis was analysed in A549 and H1299 cells treated with 0, 5, 10 or 20 µM saikosaponin D for 24 h, using an Annexin V/PI Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) and flow cytometry. Following treatment, cells from each group were harvested and washed with PBS, and a 1 × 106 cell/ml cell suspension was prepared with 1 × binding buffer. Cells were then incubated with Annexin V-FITC and PI for 15 min. Cells labelled with Annexin V-FITC and stained with PI were detected by flow cytometry. Unlabelled cells were used as the control group, and the proportion of cells in the different apoptotic stages was calculated.
Western blot analysis of cell cycle, apoptosis and STAT3 signalling
After treatment with different concentrations of saikosaponin D for 24 h, the A549 and H1299 cells were washed once with precooled PBS, then RIPA lysis buffer (Shanghai Macklin Biochemical) containing protease inhibitors was added to lyse the cells, and the total protein was extracted and denatured at 100 °C for 5 min. Total protein was then quantified using a bicinchonic acid assay (Beyotime Biotechnology Co., Ltd; Shanghai, China). Equal 25 µg aliquots of protein were loaded into each lane of a polyacrylamide gel for separation by SDS-PAGE, and separated proteins were then transferred to PVDF membranes. Membranes were blocked using 5% nonfat milk at room temperature for 1 h. The appropriate primary antibody was added and incubated at 4 °C overnight, as follows: anti-Cyclin D1, anti-CDK4 and anti-p27 for cell cycle analysis; anti-p-44/42 and anti-cl-Caspase 3 for apoptosis analysis; and anti-signal transducer and activator of transcription (STAT)3 and anti-p-STAT3 for analysis of STAT3 signalling, with anti-β-actin primary antibody as internal control (all diluted to 1: 2 000; Cell Signalling Technology; Danvers, MA, USA). Following incubation with primary antibodies, membranes were washed 3 times 5 min each in 1 × Tris-buffered saline Tween (TBST) and horseradish peroxidase-labelled secondary antibody (diluted to 1: 2000; Cell Signalling Technology) was added and incubated for 1.5 h at room temperature. Membranes were again washed 3 times 5 min each in 1 × TBST and finally, after adding enhanced chemiluminescent (ECL) substrate (Pierce Biotechnology, Rockford, IL, USA), the membrane was exposed and photographed under a UVP gel imager. The grey value was obtained using ImageJ software (National Institutes of Health) and relative protein levels were calculated using the grey value of the β-actin internal control as reference.
Statistical analyses
Data are presented as mean ± SD of triplicate experiments. The statistical analysis and scientific mapping of the experimental data were performed using GraphPad Prism software, version 8.0, and independent t-tests were used for pairwise comparisons. A P value < 0.05 was considered to indicate a statistically significant difference.
Results
Effect of saikosaponin D on NSCLC cell proliferation
The proliferation rate of A549 and H1299 cells was evaluated by CCK-8 assay (Figure 1B). After treatment with different concentrations of saikosaponin D for 24 h, the inhibition of cell proliferation was found to be significantly increased in a dose-dependent manner, with half minimum inhibitory concentrations (IC50) of 3.75 µM and 8.46 µM, for A549 and H1299 cells, respectively.
Effect of saikosaponin D on NSCLC cell morphology
After treatment with saikosaponin D for 24 h, DAPI stained cells were observed under phase contrast and fluorescence microscopy (Figure 2). Cells in the control groups without saikosaponin D treatment appeared normal, with no obvious apoptotic morphology. After saikosaponin D treatment, the number of A549 and H1299 cells visible in the plane of view decreased in a dose-dependent manner. In H1299 cells treated with 20 µM saikosaponin D, most cells became rounded indicating a poor cell growth status.
Figure 2.
Representative images of phase contrast and DAPI stained cells showing the effect of 24-h treatment with different concentrations of saikosaponin D (SSD), ranging from 0 to 20 μM, on the nucleus of A549 and H1299 cells. Scale bar = 75 μm.
Effect of saikosaponin D on NSCLC cell cycle and apoptosis
The A549 and H1299 cell cycle flow cytometry results showed that the ratio of cells in the G0/G1 phase in both cell lines increased after treatment with saikosaponin D (Figure 3A and 3B). As the concentration of saikosaponin D increased, the proportion of cells in G0/G1 phase gradually increased, and the proportion of cells in S and G2/M phase gradually decreased (Figure 3B). At a saikosaponin D concentration of 20 µM, the proportion of A549 cells in the G0/G1 phase increased from 33.93% in the control group to 53.46%, and the proportion of H1299 cells in the G0/G1 phase increased from 52.74% in the control group to 58.0% (Figure 3B; P < 0.05). Levels of G0/G1 phase related proteins were then analysed using Western blots (Figure 3C). Levels of Cyclin D1 and CDK4 were found to be significantly down-regulated following 24-h treatment with saikosaponin D in a dose-dependent manner (P < 0.05; Figure 3D), while P27 expression was up-regulated following saikosaponin D treatment (P < 0.05; Figure 3D).
Figure 3.
Effects of 24-h treatment with different concentrations of saikosaponin D (SSD), ranging from 0 to 20 μM, on the cell cycle of A549 and H1299 cells: (a) Representative flow cytometry images of cells in different cell cycle phases; (b) Histogram of flow cytometry results showing proportions of cells in different cell cycle phases; (c) Representative Western blots showing relative levels of the G0/G1 phase proteins Cyclin D1, CDK4 and P27, and β-actin internal controls; and (d) Histogram of Western blot results showing relative protein levels normalised to β-actin (data presented as mean ± SD). *P < 0.05 versus cells treated with 0 µM SSD (controls).
The effect of saikosaponin D on apoptosis in A549 and H1299 cells was analysed by flow cytometry. After 24 h of saikosaponin D treatment, A549 and H1299 cells were double stained with Annexin V and PI. Flow cytometry analyses showed increasing proportions of early apoptotic cells (R5 quadrant) and late apoptotic cells (R3 quadrant; Figure 4A). At a saikosaponin D concentration of 20 µM, the proportions of early and late apoptotic A549 cells reached 29.14% and 20.06%, respectively (Figure 4B), and the proportion of early and late apoptotic H1299 cells reached 64.05% and 27.86%, respectively (all P < 0.01 versus controls with 0 µM saikosaponin D). Compared with the control groups, the overall apoptosis rate was significantly increased in both cell lines following 24-h treatment with either 5, 10, or 20 µM saikosaponin D (P < 0.01).
Figure 4.
Effects of 24-h treatment with different concentrations of saikosaponin D (SSD), ranging from 0 to 20 µM, on apoptosis in A549 and H1299 cells: (a) Representative flow cytometry apoptosis profiles in cells treated with 0, 5, 10 or 20 µM SSD for 24 h (R5 quadrant, early apoptotic cells; R3 quadrant, late apoptotic cells); and (b) Distribution of cells in normal phase, or in early or late apoptosis (data presented as mean ± SD). *P < 0.05 versus cells treated with 0 µM SSD (controls).
Effect of saikosaponin D on apoptosis and the STAT3 signalling pathway
Levels of the proliferation-related protein STAT3, and expression of the apoptosis marker proteins phosphorylated p44/42 and cleaved caspase-3, in A549 and H1299 cells were analysed using Western blots (Figure 5A and B). Detection of total and phosphorylated STAT3 revealed that saikosaponin D significantly attenuated STAT3 phosphorylation (P < 0.05) but had little effect on the total STAT3 protein (P > 0.05) in both cell lines. Compared with the control groups, phosphorylation of p44/42 was significantly decreased following 24-h treatment with 10 µM and 20 µM saikosaponin D (all P < 0.01). Cleaved caspase-3 expression was significantly higher following saikosaponin D treatment at all concentrations versus controls (all P < 0.05).
Figure 5.
Effects of 24-h treatment with different concentrations of saikosaponin D (SSD), ranging from 0 to 20 µM, on the expression of proliferation-related pathway proteins in A549 and H1299 cells: (a) Representative Western blots showing phosphorylated (p)-signal transducer and activator of transcription (STAT)3, STAT3, p-p44/42 and cleaved (cl)-Caspase-3 levels with β-actin internal control; and (b) Semi-quantification of Western blot results showing relative protein levels normalised to β-actin (data presented as mean ± SD). *P < 0.05 versus cells treated with 0 µM SSD (controls).
Discussion
Lung cancer ranks first in the world for cancer-related death, and NSCLC accounts for 80–85% of all lung cancer cases.16 In China, with deterioration of the environment, the lung cancer mortality rate has ranked first among rates associated with malignant tumours.17 Chemotherapy is currently one of the most effective treatments for lung cancer, however, conventional chemotherapy drugs used to treat lung cancer often have limited clinical application due to their many severe side effects.1,3 Even with the current emergence of molecular targeted therapy, the 5-year survival rate of patients with NSCLC in China remains between 30.9 and 40.5%.18 Identification of anticancer agents from natural plants is a promising strategy in the development of cancer drugs, and these natural agents usually have complex chemical structures that are difficult to artificially synthesize. Many natural active products, such as paclitaxel, camptothecin derivatives (e.g. irinotecan), vinorelbine, lentinan, docetaxel, and elemene, have been shown to have very significant clinical anticancer effects. These drugs not only play a major role in interfering with the microtubule synthesis of cancer cells, but also have various pharmacological activities, such as inducing apoptosis and preventing angiogenesis. Natural products hold an irreplaceable position among antitumour drugs.19
Saikosaponin, obtained from the Chinese traditional medicine Bupleurum, is an extract with various pharmacological characteristics. Saikosaponins can be divided into saikosaponin A, B, C, D, M, N, P and T according to their chemical structure, among which, saikosaponin A and saikosaponin D are the main effective ingredients;20 a previous study found that after treatment with 10 µM saikosaponin D,7 the survival rate of A549 lung cancer cells decreased by 73.9%, and the proportion of cells in the G0/G1 phase significantly increased in a dose-dependent manner, while cells in S phase significantly decreased. In the present study, saikosaponin D consistently inhibited lung cancer cell proliferation, and arrested the cell cycle at the G0/G1 phase, in a dose-dependent manner, shown by increased numbers of cells in G0/G1 phase compared with controls. Numerous previous studies have revealed that saikosaponin D-induced G1 phase arrest is mainly related to the upregulation of p21,12 p27 and p53.21,22 Consistent with a previous study,7 saikosaponin D was also found to significantly induce apoptosis in lung cancer cells at a high concentration and in a dose-dependent manner. In the present study, saikosaponin D was found to induce apoptosis in H1299 cells, which are a TP53 heterozygous cell line,23 suggesting that saikosaponin D-induced apoptosis can be independent of the p53 pathway. This mechanism may offer the possibility of anticancer treatment for patients with p53 mutations.
A preliminary analysis of the signalling pathways downstream of saikosaponin D in lung cancer cells was performed in the present study. Saikosaponin D was shown to significantly attenuate STAT3 phosphorylation, which is consistent with a study on the protective effect of acetaminophen-induced liver injury and liver cancer.24,25 In a recent study of the effects of saikosaponin D on A549 cells,8 saikosaponin D was found to inhibit lung cancer and induce apoptosis, possibly through two pathways: transforming growth factor (TGF)α/c-Jun-N-terminal kinase (JNK)/p53 and TGFα/ras association domain-containing protein 1 (RASSF1a)/macrophage stimulating protein (Mst1). The results of the present study also support another proposed mechanism of action of saikosaponin D against lung cancer to some extent, which suggests that saikosaponin D may exert its inhibitory effect on lung cancer through multiple pathways. The STAT3 signalling pathway is considered to play a very important role in the development of lung cancer, and this pathway is also thought to be a point of convergence of different signalling pathways; abnormal activation of STAT3 in lung cancer can prevent apoptosis, promote cancer cell proliferation and angiogenesis, and evade immune surveillance.26 Studies have also found that this pathway plays an important role in lung cancer stem cell self-renewal and epigenetic regulation.26,27 Many studies have investigated lung cancer treatments that are based on the STAT3 pathway, such as AZD1480,28 interleukin (IL)-6 neutralizing antibody,29 and ruxolitinib.30 These studies demonstrate that targeting STAT3 is an effective and promising means of controlling lung cancer progression.26,31
The results of the present study indicated that saikosaponin D can increase the expression of cleaved caspase-3 in lung cancer cells, and simultaneously, it may promote the apoptosis of lung cancer cells by decreasing the phosphorylation of STAT3. This phenomenon further supports the application of saikosaponin D in NSCLC treatment. Future research directions for consideration may include the use of saikosaponin D as a lead material to obtain its derivatives and to evaluate its safety, efficacy, absorption and distribution characteristics in tumour-bearing animal models. Furthermore, new inhaled formulations may be considered to reduce the toxic side effects of saikosaponin D throughout the body. In addition, saikosaponin D has been reported to have strong immunomodulatory effects, such as increasing the expression of IL-2 and its receptors,32 which significantly improves overall survival during adductive immunotherapy of lung cancer.33 It is also possible to consider combining saikosaponin D with anti-programmed death (PD)-1/PD-ligand 1 and other antibodies to evaluate the effects of these combinatorial treatments on lung cancer.34
Acknowledgement
The interpretation and reporting of these data are the sole responsibility of the authors.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Medical Technology Project of Ningbo (number 2018A01).
ORCID iD
References
- 1.Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016; 5: 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remon J, Ahn MJ, Girard N, et al. Advanced-stage non-small cell lung cancer: advances in thoracic oncology 2018. J Thorac Oncol 2019; 14: 1134–1155. [DOI] [PubMed] [Google Scholar]
- 3.Saranya K, Sreejith K. and Ajaykumar . Comparison of quality of life of patients on treatment with cisplatin and gemcitabine, carboplatin and gemcitabine, carboplatin and paclitaxel, carboplatin and pemetrexed for non-small cell lung cancer. J Oncol Pharm Pract 2019; 25: 1853–1859. [DOI] [PubMed] [Google Scholar]
- 4.Kujtan L, Subramanian J. Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Expert Rev Anticancer Ther 2019; 19: 547–559. [DOI] [PubMed] [Google Scholar]
- 5.Xie JY, Di HY, Li H, et al. Bupleurum chinense DC polysaccharides attenuates lipopolysaccharide-induced acute lung injury in mice. Phytomedicine 2012; 19: 130–137. [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Dong X, Yin X, et al. Radix Bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. Biomed Res Int 2017; 2017: 7597596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu YL, Kuo PL, Lin CC. The proliferative inhibition and apoptotic mechanism of Saikosaponin D in human non-small cell lung cancer A549 cells. Life Sci 2004; 75: 1231–1242. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Liu C, Zhao R, et al. Synergetic and antagonistic molecular effects mediated by the feedback loop of p53 and JNK between saikosaponin D and SP600125 on lung cancer A549 cells. Mol Pharm 2018; 15: 4974–4984. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Qi H, Zhang X, et al. Saikosaponin D from Radix Bupleuri suppresses triple-negative breast cancer cell growth by targeting β-catenin signaling. Biomed Pharmacother 2018; 108: 724–733. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Xue HG, Feng LJ, et al. The effect of saikosaponin D on doxorubicin pharmacokinetics and its MDR reversal in MCF-7/adr cell xenografts. Eur Rev Med Pharmacol Sci 2017; 21: 4437–4445. [PubMed] [Google Scholar]
- 11.Li C, Guan X, Xue H, et al. Reversal of P-glycoprotein-mediated multidrug resistance is induced by saikosaponin D in breast cancer MCF-7/adriamycin cells. Pathol Res Pract 2017; 213: 848–853. [DOI] [PubMed] [Google Scholar]
- 12.Liu RY, Li JP. Saikosaponin-d inhibits proliferation of human undifferentiated thyroid carcinoma cells through induction of apoptosis and cell cycle arrest. Eur Rev Med Pharmacol Sci 2014; 18: 2435–2443. [PubMed] [Google Scholar]
- 13.Tsuyoshi H, Wong VKW, Han Y, et al. Saikosaponin-d, a calcium mobilizing agent, sensitizes chemoresistant ovarian cancer cells to cisplatin-induced apoptosis by facilitating mitochondrial fission and G2/M arrest. Oncotarget 2017; 8: 99825–99840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Cai T, Zhang W, et al. Effects of saikosaponin D on apoptosis in human U87 glioblastoma cells. Mol Med Rep 2017; 16: 1459–1464. [DOI] [PubMed] [Google Scholar]
- 15.Wang BF, Dai ZJ, Wang XJ, et al. Saikosaponin-d increases the radiosensitivity of smmc-7721 hepatocellular carcinoma cells by adjusting the g0/g1 and g2/m checkpoints of the cell cycle. BMC Complement Altern Med 2013; 13: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozkaya S, Findik S, Dirican A, et al. Long-term survival rates of patients with stage IIIB and IV non-small cell lung cancer treated with cisplatin plus vinorelbine or gemcitabine. Exp Ther Med 2012; 4: 1035–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang D, Liu Y, Bai C, et al. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett 2020; 468: 82–87. [DOI] [PubMed] [Google Scholar]
- 18.Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer 2019; 10: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res 2009; 59: 365–378. [DOI] [PubMed] [Google Scholar]
- 20.Li XQ, Song YN, Wang SJ, et al. Saikosaponins: a review of pharmacological effects. J Asian Nat Prod Res 2018; 20: 399–411. [DOI] [PubMed] [Google Scholar]
- 21.Yao M, Yang J, Cao L, et al. Saikosaponin-d inhibits proliferation of DU145 human prostate cancer cells by inducing apoptosis and arresting the cell cycle at G0/G1 phase. Mol Med Rep 2014; 10: 365–372. [DOI] [PubMed] [Google Scholar]
- 22.Hsu YL, Kuo PL, Chiang LC, et al. Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in induction of apoptosis and cell cycle arrest by saikosaponin D in human hepatoma cell lines. Cancer Lett 2004; 213: 213–221. [DOI] [PubMed] [Google Scholar]
- 23.Bodner SM, Minna JD, Jensen SM, et al. Expression of mutant p53 proteins in lung cancer correlates with the class of p53 gene mutation. Oncogene 1992; 7: 743–749. [PubMed] [Google Scholar]
- 24.Liu A, Tanaka N, Sun L, et al. Saikosaponin D protects against acetaminophen-induced hepatotoxicity by inhibiting NF-κB and STAT3 signaling. Chem Biol Interact 2014; 223: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He S, Lu G, Hou H, et al. Saikosaponin-d suppresses the expression of cyclooxygenase-2 through the phosphor-signal transducer and activator of transcription 3/hypoxia-inducible factor-1α pathway in hepatocellular carcinoma cells. Mol Med Rep 2014; 10: 2556–2562. [DOI] [PubMed] [Google Scholar]
- 26.Dutta P, Sabri N, Li J, et al. Role of STAT3 in lung cancer. JAKSTAT 2015; 3: e999503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan J, Zhang F, Niu R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci Rep 2015; 5: 17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 2009; 16: 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song L, Rawal B, Nemeth JA, et al. JAK1 activates STAT3 activity in non-small-cell lung cancer cells and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling. Mol Cancer Ther 2011; 10: 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Hong Y, Xu Y, et al. Inhibition of the JAK/STAT pathway with ruxolitinib overcomes cisplatin resistance in non-small-cell lung cancer NSCLC. Apoptosis 2014; 19: 1627–1636. [DOI] [PubMed] [Google Scholar]
- 31.Harada D, Takigawa N, Kiura K. The role of STAT3 in non-small cell lung cancer. Cancers (Basel) 2014; 6: 708–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato M, Pu MY, Isobe K, et al. Characterization of the immunoregulatory action of saikosaponin-D. Cell Immunol 1994; 159: 15–25. [DOI] [PubMed] [Google Scholar]
- 33.Mi D, Ren W, Yang K. Adoptive immunotherapy with interleukin-2 & induced killer cells in non-small cell lung cancer: a systematic review & meta-analysis. Indian J Med Res 2016; 143: S1–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui H, Ma N, Wang Y, et al. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res 2018; 2018: 6984948. [DOI] [PMC free article] [PubMed] [Google Scholar]