Abstract
Background:
Patient autonomy is a bioethical principle that was strengthened in the revised Declaration of Geneva. Shared decision making (SDM) is particularly relevant in the management of multiple sclerosis (MS) because many preference-sensitive decisions have to be made during the disease course. We aimed to summarize the available evidence on SDM in the MS field and to inform future research and practice.
Methods:
We performed a scoping review by searching MEDLINE (past 5 years). Studies were included if they reported primary/secondary research and focused on SDM related to people with MS. Data were grouped into topics, with results presented in narrative form.
Results:
From 865 references, we included 55 studies conducted mostly in Europe. Half of the studies were observational, followed by qualitative (20%), mixed-methods (17%), randomized controlled trials (RCTs, 5%), quasi-experimental (5%), and reviews (4%). Most studies addressed people with relapsing-remitting MS (85%); the remaining addressed health care professionals, patients’ significant others, or a combination. We identified five main topics: decisions on disease-modifying drugs, decisions on chronic cerebrospinal venous insufficiency treatment, information provision and patient education, health literacy, and risk knowledge.
Conclusions:
The high proportion of included studies on SDM in MS in Europe suggests an earlier adoption of these concepts in this area. Decisions on disease-modifying drugs was the prevalent topic. Only 5% of studies were RCTs, indicating that more research is needed to study the effectiveness of SDM interventions. Studies addressing people with primary and secondary progressive MS are also needed.
Keywords: Decision aids, Multiple sclerosis (MS), Patient Autonomy, Rehabilitation, Shared decision making (SDM)
One of the most important ethical principles of the Declaration of Geneva1 is the respect of a patient’s autonomy and dignity. In this context, the concept of patient empowerment can be applied at the patient level as one of the underpinning ethos that patients have the rights and responsibilities, as well as opportunities, related to patient autonomy in health care.2 So far, there is no agreed-on definition of patient empowerment, but important aspects include regarding patient empowerment as a transformative process for patients to gain control of their health and health care and adapt to their disease. This involves information and education as well as active participation in treatment decisions.2 Indicators of empowerment include knowledge, health literacy, participation in shared decision making (SDM), and self-management.2
At the health care provider level, health care professionals (HCPs) should support and integrate the autonomy of the patient. Coaching interventions and SDM could activate patient empowerment, thus increasing patient autonomy.2 Based on the ethical principle of patient autonomy and on patient preferences, SDM is an approach in which the patient and the clinician participate actively and responsibly in the decision-making process.
Multiple sclerosis (MS) is a chronic degenerative disease affecting approximately 2,300,000 people worldwide. It initially presents with relapses in approximately 85% of those affected. During the disease course, several preference-sensitive decisions have to be made, including starting, switching, or stopping immunotherapies; relapse treatment; motherhood and raising children; and lifestyle changes. Several studies show that persons with MS prefer an active and collaborative role when making decisions about their health,3,4 with some differences across cultures.4 However, in the context of information provision, interventions to promote informed choice and improve patient-relevant outcomes are still rare.5
As the Special Interest Group on Patient Autonomy of the European Rehabilitation in Multiple Sclerosis (RIMS) network, we collaborated for 10 years to assess the implementation of SDM in MS across Europe and to develop and evaluate interventions and outcome measures in the context of patient autonomy (see the SIGS section at www.eurims.org for more information about the Patient Autonomy group).
The aims of this scoping review were to 1) “map” the extent, range, and nature of the available evidence on (shared) decision making in MS during the past 5 years and 2) summarize findings to inform future research and practice.
Methods
Following the Arksey and O’Malley framework,6,7 stages of the scoping review included formulation of the research question; identification and selection of the relevant studies; data charting, collating, and summarizing; and reporting results. We adhered to the PRISMA-ScR (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews) checklist for scoping review conduct and reporting (Appendix S1, which is published in the online version of this article at ijmsc.org).
Our research question was as follows: What is the current stage of research concerning SDM in MS? We were interested in studies on patient autonomy and/or (shared) decision making in MS (eg, SDM in general, decision aids, decision coaching).
We searched all relevant studies published in the past 5 years (date of the search: August 6, 2019; language: English; database: MEDLINE). The search strategy is provided in Appendix S2.
Two reviewers (A.C.R. and A.G.) conducted a pilot test on ten articles to refine the eligibility criteria. Next, the results were split, and the same two reviewers screened titles and abstracts of studies for eligibility. Full-text screening of studies assessed as “relevant” or “unclear” followed the same way using a standardized form, which was revised after the pilot full-text screening test. Inclusion of unclear articles was resolved by consensus. Studies were included if they reported either primary (quantitative or qualitative) or secondary research focused on MS SDM in general, provided that such studies reported processes related to a health decision. We excluded studies on counseling, studies on physician education, and studies in which persons with MS were less than 50% of the total sample.
Data were extracted using standardized forms by one reviewer (A.C.R.) and checked for accuracy and completeness by another (A.G.).8 Reviewers resolved unclear issues through consensus. Extracted data included study author (year of publication), methods, participants’ characteristics, aims, main results, and funding.
Data were synthesized descriptively to map different aspects of the literature as outlined in our key question. Studies were grouped according to identified topics. The results of the review are presented in a narrative form.
Results
Of 865 references identified, two duplicates were excluded, and after initial screening, another 736 citations were excluded. Of the 127 full-text articles retained for further screening, 72 were excluded because they were not focused on SDM or were opinion articles/commentaries, abstracts, or studies of mixed populations where persons with MS were less than 50%.
We included 55 studies (Table S1, Figure S1), mostly conducted in Europe (63%), followed by the United States and Canada (29%), Australia (4%), and Asia (4%). Among European countries, figures were higher for Germany (22%), the United Kingdom (16%), and Italy (13%) (Figure 1). Most of the studies (60%) were published between 2017 and 2019, with a peak of 28% (15 of 55) in 2017. Overall, three studies (5%) were randomized controlled trials (RCTs), with the others being observational (27 of 55 [49%]), qualitative (11 of 55 [20%]), mixed-methods (9 of 55 [17%]), quasi-experimental (3 of 55 [5%]), and reviews (2 of 55 [4%]).
Figure 1.
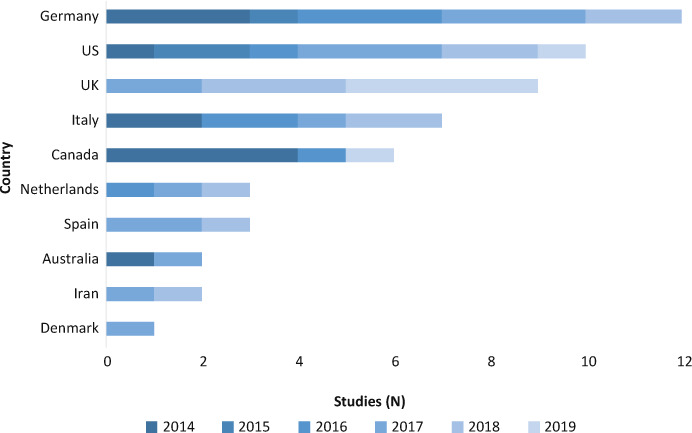
Countries and years of publication of included studies
UK, United Kingdom; US, United States.
Participants were mostly people with relapsing-remitting MS (RRMS) (47 of 55 [85%]), people with MS and HCPs (3 of 55 [5%]), persons with MS and significant others (3 of 55 [5%]), and HCPs only (3 of 55 [5%]).
Of the 55 studies included, 51 (92%) reported the source of funding; 13 (24%) received funding from pharmaceutical companies.
For the purpose of this review, studies were grouped into the following topics: 1) decisions on disease-modifying drugs (DMDs), 2) decisions on chronic cerebrospinal venous insufficiency (CCSVI) treatment,9 3) information provision and patient education, 4) health literacy, and 5) risk knowledge. Some studies reporting educational interventions were grouped to the specific DMD decision-making topic.
Decisions on DMDs
In total, 27 studies (28 publications) reported on decisions related to DMDs. Study designs comprised mostly surveys, qualitative studies, and discrete-choice experiments, whereas interventional studies were lacking.
Eskyte et al10 performed a critical interpretive synthesis review to explore the perspective of people with RRMS on DMD decision making. Their analysis revealed that contextual factors are relevant next to medical and individual reasoning. Furthermore, it was found that there was no consensus among persons with RRMS regarding the meaning of DMD efficacy (eg, more relapses, magnetic resonance imaging [MRI] lesions). A few studies11–13 also explored factors influencing DMD preferences and distinguished between different efficacy outcomes (eg, disease progression, relapses, and quality of life). Although data from two European surveys (the Netherlands, 185 persons with RRMS; Spain, 37 persons with RRMS) showed that people with RRMS considered disease progression, a US survey (129 persons with RRMS) identified out-of-pocket costs as the most important outcome,12 supporting the importance of contextual factors as outlined by Eskyte et al.10 However, in a US study (135 people with RRMS), the most important DMD attribute was treatment effectiveness, and costs were ranked sixth.14 Herein, persons with RRMS ranked avoidance of cognitive impairment (eg, memory problems) as the most important treatment goal, followed by motor impairment. Interestingly, HCPs in the Netherlands rated safety as the most important factor, followed by progression.15 Tintore et al16 performed a survey of the views of 900 neurologists and 982 people with RRMS. Potential adverse effects and safety of DMDs were rated as the most important factors in decision making by both persons with RRMS and neurologists, and the most important goals were to reduce relapse frequency and slow down disability progression. A German study in 156 persons with RRMS showed that the route of administration (oral vs parenteral) and treatment frequency played an important role.17
Seven studies used hypothetical treatment options to explore patient preferences for DMD attributes showing different results concerning preferences.18–24 Two studies identified severe adverse effects as the most important attribute.22,23 Two studies reported that the likelihood of patients taking a DMD decreased with an increase in adverse effects and a decrease in treatment efficacy.19,22 Bruce et al19 further found that nonadherent patients devalued treatment efficacy and exaggerated the risks of DMDs. In a subsequent study, Bruce et al20 found that the risk evaluation of patients was associated with MS knowledge and treatment adherence. Fox et al21 showed higher risk tolerance in more disabled patients and patients not on medication, and Bottomley et al18 found that the method of taking medication was most important.
Köpke et al25 assessed the effectiveness of an evidence-based patient information program to increase informed choice in people with RRMS. The intervention had a significant effect on informed choice and risk knowledge, with no adverse events. In addition, Köpke et al26 investigated the effectiveness of a 6-hour HCP-led interactive group education program and an information brochure compared with standard information. The intervention increased the number of informed choices, and the proportion of persons with adequate risk knowledge was significantly higher in the intervention group 2 weeks after the intervention, but not after 6 months. In another study by the same group, Rahn et al27 conducted a pilot RCT on a nurse-led decision coaching program based on an SDM approach to support persons with RRMS in DMD decision making. The study showed the feasibility of the program and indicated more informed choices in the intervention group.27,28 Notably, in another study the feasibility of a decision aid on DMDs was assessed via a focus group and survey.29
Another important topic in this category was the role of patient preferences regarding DMD decision making, showing that patients generally prefer an active or shared approach in decision making30–32 and that DMD decisions for a first-line therapy were jointly made with a shift to decreased involvement in later decisions.33 Furthermore, Wilkie et al34 looked at decisional conflict and decisional regret in the process of DMD decision making and found that 53% of people with RRMS had decisional conflict. Both decisional conflict and regret varied in the different stages of decision making.
Finally, the process of and perspectives on DMD decision making were explored in three qualitative studies,35–37 indicating that important factors related to DMD decision making are treatment factors, individual factors, social support, and quality of life.
Decisions on CCSVI Treatment
Six studies focused on CCSVI treatment.38–43 Notably, four qualitative studies (all conducted in Canada) and two studies that analyzed YouTube videos (mostly produced in Canada and the United States) were included. Whereas Ghahari et al42 showed that the most recurrent message on the videos was that “CCSVI is not a miracle but worth trying,” Hynes et al43 reported that there is still advocacy for the method, but the interest has declined.
Information Provision and Patient Education
Overall, 14 studies (16 publications) addressed the topic of information provision and patient education. Two studies reported on the effectiveness of different information aids for persons with MS.44,45 Giordano et al44 assessed the effectiveness of an information aid (personal interview and take-home booklet/website) in 159 newly diagnosed persons with RRMS, showing that it was not superior to the comparator (booklet/website alone). The study was the late phase of a project that developed and tested the “Sapere Migliora” information aid for newly diagnosed people with MS using a multicenter, multiphased approach in Italy.46,47 Mohamadirizi et al45 compared the effect of an electronic educational program with that of a booklet. The educational program increased patient knowledge. Brand et al48 developed and pilot tested an evidence-based patient education program on MRI in MS, showing some important gaps in knowledge of MRI.
Information on disease prognosis was investigated by Dennison et al,49 who emphasized the importance for persons with MS to discuss long-term prognosis with HCPs and that most persons with MS considered prognostication useful for decision making.
The family has a crucial role in helping persons with MS to manage their disease also through the use of health information. In a qualitative study, Mazanderani et al 201950 explored the interrelational dynamics of information practices in families of persons with MS. Managing information is a form of work for the family, and health information can be considered per se a distinctive form of care. Factors influencing the management of such information can be the skills of the person with MS, the form and stage of MS, family preferences and dynamics, health care environment, and systems for providing health information.
Three studies reported on an international collaborative project, IN-DEEP (Integrating and Deriving Evidence, Experiences, and Preferences), which aimed to develop and test a website containing relevant, unbiased, up-to-date health information for people with MS.51–53 In the preparatory work, Colombo et al51 and Synnot et al52 showed that the internet was considered useful by persons with MS and that, at the same time, it was difficult for them to identify reliable information there. Colombo et al53 assessed the newly developed section of the Italian website on interferon-β for persons with RRMS using an online survey. The website was judged clear and understandable, with most persons with RRMS being more confident when making decisions about the interferons.
Four studies54–57 reported on information provided via social media. Della Rosa et al54 investigated the information available on the social network sites. Patient support, information, and awareness were the categories that obtained more engagement. Lavorgna et al56 assessed the role of appointed influencers in a medically supervised Italian Web-based community (SMsocialnetwork.com) for people with MS. Findings indicated that the community was a safe environment where correct medical information was posted and “fake news” was not.
In their review, Giunti et al58 evaluated MS apps. Of the 30 apps included, most were on disease management and disease and treatment information. Apps were generally created by small- and medium-sized enterprises or pharmaceutical companies. Personal data management (ie, access to personal information and data defined by the user) and patient education were the most common features.
Riemann-Lorenz59 designed and pilot tested an interactive 2-hour evidence-based patient education program on the influence of diet on MS. Although the program was considered comprehensible, persons with MS were disappointed by the limited evidence base for dietary approaches.
Health Literacy
Five studies were on health literacy.60–64 In an RCT, Kasper et al60 evaluated newly developed bar graphs for persons with MS risk communication showing that they were understood as well as standard pictographs. Reen et al61 showed improved understanding in persons with MS when treatment effects were communicated in absolute terms compared with relative terms and numbers needed to treat/harm. Adding baseline information on clinical trials significantly improved understanding. Rahn et al63 developed and pilot tested different types of information materials exemplifying confidence intervals to persons with RRMS. Patient information materials were considered acceptable. In a pilot RCT, the “average weight version”—where a farmer wants to estimate the average weight of his apples—proved more effective compared with the standard information version.63 Gaissmaier et al62 showed that the numeracy of persons with MS treated with natalizumab was comparable with that of a German national sample, with higher numeracy levels in men and in the highly educated in both samples. Disease variables were not associated with numeracy. Dehghani et al64 developed a new health literacy questionnaire for persons with MS consisting of 22 items grouped into four factors (appraisal of and ability to search health information, knowledge of caring for MS, and successful practices in health conditions).
Risk Knowledge
Three studies reported on risk knowledge in MS.3,65,66 Two studies described the development and validation of a risk knowledge questionnaire to be used as a patient-reported outcome measure in educational interventions. The third study3 was an international online survey (1939 persons with MS) investigating risk knowledge and role preferences across eight European countries, and factors associated with risk knowledge. Risk knowledge differed across countries. Higher education, previous experience with DMDs, and correct answer to a medical data interpretation question were positively associated with risk knowledge, whereas higher fear of wheelchair dependency was negatively associated with it.
Discussion
During the past few years, several organizations and governments worldwide have acknowledged the importance of SDM, which has been described as the pinnacle of patient-centered care.67 Despite this, its implementation has received only limited assessment, and reliable and sensitive outcome measures are still missing.68
In the present scoping review, we aimed to map the evidence on SDM in MS during the past 5 years, particularly focusing on patient autonomy and decision making. From more than 800 references, we included 55 studies conducted mostly in Europe. Participants were mostly persons with RRMS. Identified topics were decisions on DMDs, decisions on CCSVI treatment, information provision and education, health literacy, and risk knowledge.
As for the country in which studies were conducted, figures were higher for Germany (22%), the United Kingdom (16%), and Italy (13%) (Figure 1). This mirrors the early acceptance and adoption of SDM concepts in these countries, particularly in Germany, where since 2001 several governmental research programs have been funded and HCP training programs developed. This is at odds with the other European countries, where, to our knowledge, no specific SDM programs have been launched thus far.69 In the United States, where 18% of the studies were conducted, some programs were developed to foster SDM using financial and legal incentives, by implementing patient decision aids.70
The present review shows that only 5% of the included studies were RCTs, indicating the need to assess the effectiveness of SDM interventions at the international level. This could be due to several reasons, including the fact that conducting experimental studies is demanding, with limited funding opportunities. Furthermore, most SDM interventions are complex, and it is acknowledged that the design, conduct, and reporting of trials on such interventions are challenging.71
Of the identified topics, some are related to specific health care decisions, such as DMDs and CCSVI, and the others are studies on the prerequisites of SDM in MS. The high number of studies addressing decisions on DMDs is not surprising because it reflects a key decision in clinical practice for people with MS and HCPs, with 12 licensed DMDs so far. Notably, some studies were on contextual factors related to DMD decision making, and some explored patient preferences for DMD attributes using advanced statistical methods. Only three studies (two RCTs and one quasi-experimental study) showed the efficacy of decision support programs, and all were conducted in Germany.25–27
The other topic regarding a health care decision is CCSVI treatment, an example of how a treatment without scientific validation spread quickly through the internet and led to persons with MS paying for procedures, at high cost and risk. Information provision and evidence-based education can be vital in such cases for the patients. Studies included in this topic analyzed videos from YouTube, mostly from Canada and the United States. Despite the lack of efficacy of the CCSVI treatment, and that experts cautioned against the “liberation therapy,” more than $20 million was spent in CCSVI research in Canada and the United States. Such studies reflect the impact of this unprecedented venture72 on social media.
As outlined in the conceptual map of patient empowerment by Bravo et al,2 SDM is one of the empowering interventions focusing on the individual, directly derived from the HCP ethos (ie, responsibilities to respect patient autonomy, adopt a partnership style within the health care relationship). At the same time, SDM acts on the level of empowerment, moderated by providers’ characteristics, values, preferences, and training. Information and education are prerequisites for SDM to occur. Within this conceptual map, the other identified topics in the present review (ie, knowledge and health literacy) are all indicators of patient empowerment. These indicators are intertwined and interact with each other, having also a bidirectional relationship with the patient (eg, adaptation to chronic illness, quality of life, well-being), as well as with clinical outcomes (ie, health status).
Within the information provision and patient education topic, we included studies on information aids and on information programs on MRI and disease prognosis. Importantly, some studies addressed the information provided via the Web, social media, and apps. The vast amount of (health) information available on the Web, and on other platforms, could potentially overwhelm persons with MS. Persons with MS need access to unbiased, up-to-date, evidence-based information. This is in line with the recent published literature showing that informing people with MS increases disease-related knowledge, with no adverse effects.5 Furthermore, some studies included in the decisions on DMDs would also fit here, indicating a clear overlap between the two topics.
Health literacy is an important part of the information provision process and can contribute to patient empowerment and possibly to the SDM process. Notably, some studies in the present review assessed patient understanding of medical data, and one reported on the development of a new MS-specific questionnaire on health literacy.64 Also, HCPs should be trained in understanding the health literacy of their patients and how it might interact with providing their therapies.
The present study has some limitations. Because this was a scoping review, the search strategy was not comprehensive, as it was restricted to MEDLINE only and did not include other databases. Also, we did not search the grey literature, and we limited the results to the past 5 years and English language. Therefore, it is likely that publication and language biases have occurred, limiting the generalizability of these results.
Another limitation is that we did not assess the quality of the included studies.73 Furthermore, based on the available evidence, no recommendation for HCPs can be made because the methods of the scoping review do not envisage any grading of the literature.
More research is needed in this field using rigorous designs (eg, RCTs) and focusing on all the MS forms (including primary and secondary progressive MS) and on all the phases of the disease trajectory (from diagnosis disclosure to the late phases). Researchers are strongly encouraged to use the Medical Research Council framework for complex interventions71 to develop and test such “SDM-proved” interventions.
As MS clinicians and researchers who are part of the RIMS Special Interest Group on Patient Autonomy, we aim to foster SDM in MS. In doing so, we suggest a few steps forward. First, it would be advisable to involve all relevant stakeholders in the SDM process, including the patients, and to work in a multidisciplinary (and interdisciplinary) way. Second, training programs dedicated to HCPs should be developed on SDM, patient-centered care, risk communication, and relation-building so that they are fully aware of the SDM process and can communicate effectively with persons with MS. Finally, patient decision aids, conversation aids, and decision coaching programs should be tested. All the previously mentioned steps can surely enhance the quality of care in MS.
PRACTICE POINTS
Shared decision making is a key factor in the management of MS because many preference-sensitive decisions have to be made over the lifetime of the disease.
Results of this scoping review show that shared decision making and patient autonomy are studied mostly in those requiring immunotherapy and using observational methods rather than testing interventions.
More research is required using interventional designs and addressing also people with primary and secondary progressive MS.
Supplementary Material
Footnotes
Financial Disclosures: Dr Solari has received speaker honoraria from Biogen, Merck Serono, Novartis, Almirall, and EXCEMED. Dr Nicholas has disclosed consulting fees from Biogen, Roche, and Novartis and being principal investigator on MS trials for Novartis, Biogen, Roche, and Sanofi. The other authors declare no conflicts of interest.
Funding/Support: None.
References
- 1.Parsa-Parsi RW. The Revised Declaration of Geneva: a modern-day physician’s pledge. JAMA. 2017;318:1971–1972. doi: 10.1001/jama.2017.16230. [DOI] [PubMed] [Google Scholar]
- 2.Bravo P, Edwards A, Barr PJ, Scholl I, Elwyn G, McAllister M. Conceptualising patient empowerment: a mixed methods study. BMC Health Serv Res. 2015;15:252. doi: 10.1186/s12913-015-0907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giordano A, Liethmann K, Köpke S et al. Risk knowledge of people with relapsing-remitting multiple sclerosis: results of an international survey. PLoS One. 2018;13:e0208004. doi: 10.1371/journal.pone.0208004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solari A, Giordano A, Kasper J et al. Role preferences of people with multiple sclerosis: image-revised, computerized self-administered version of the Control Preference Scale. PLoS One. 2013;8:e66127. doi: 10.1371/journal.pone.0066127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Köpke S, Solari A, Rahn A, Khan F, Heesen C, Giordano A. Information provision for people with multiple sclerosis. Cochrane Database Syst Rev. 2018;10:CD008757. doi: 10.1002/14651858.CD008757.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Method. 2005;8:19–32. [Google Scholar]
- 7.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59:697–703. doi: 10.1016/j.jclinepi.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Zamboni P, Galeotti R, Menegatti E et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:392–399. doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskyte I, Manzano A, Pepper G et al. Understanding treatment decisions from the perspective of people with relapsing remitting multiple sclerosis: a critical interpretive synthesis. Mult Scler Relat Disord. 2019;27:370–377. doi: 10.1016/j.msard.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Kremer IEH, Evers SMAA, Jongen PJ, van der Weijden T, van de Kolk I, Hiligsmann M. Identification and prioritization of important attributes of disease-modifying drugs in decision making among patients with multiple sclerosis: a nominal group technique and best-worst scaling. PLoS One. 2016;11:e0164862. doi: 10.1371/journal.pone.0164862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hincapie AL, Penm J, Burns CF. Factors associated with patient preferences for disease-modifying therapies in multiple sclerosis. J Manag Care Spec Pharm. 2017;23:822–830. doi: 10.18553/jmcp.2017.23.8.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sempere AP, Vera-Lopez V, Gimenez-Martinez J, Ruiz-Beato E, Cuervo J, Maurino J. Using a multidimensional unfolding approach to assess multiple sclerosis patient preferences for disease-modifying therapy: a pilot study. Patient Prefer Adherence. 2017;11:995–999. doi: 10.2147/PPA.S129356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Col NF, Solomon AJ, Springmann V et al. Evaluation of a novel preference assessment tool for patients with multiple sclerosis. Int J MS Care. 2018;20:260–267. doi: 10.7224/1537-2073.2017-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kremer IEH, Evers SMAA, Jongen PJ, Hiligsmann M. Comparison of preferences of healthcare professionals and MS patients for attributes of disease-modifying drugs: a best-worst scaling. Health Expect. 2018;21:171–180. doi: 10.1111/hex.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tintore M, Alexander M, Costello K et al. The state of multiple sclerosis: current insight into the patient/health care provider relationship, treatment challenges, and satisfaction. Patient Prefer Adherence. 2017;11:33–45. doi: 10.2147/PPA.S115090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Utz KS, Hoog J, Wentrup A et al. Patient preferences for disease-modifying drugs in multiple sclerosis therapy: a choice-based conjoint analysis. Ther Adv Neurol Disord. 2014;7:263–275. doi: 10.1177/1756285614555335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottomley C, Lloyd A, Bennett G, Adlard N. A discrete choice experiment to determine UK patient preference for attributes of disease modifying treatments in multiple sclerosis. J Med Econ. 2017;20:863–870. doi: 10.1080/13696998.2017.1336099. [DOI] [PubMed] [Google Scholar]
- 19.Bruce JM, Bruce AS, Catley D et al. Being kind to your future self: probability discounting of health decision-making. Ann Behav Med. 2016;50:297–309. doi: 10.1007/s12160-015-9754-8. [DOI] [PubMed] [Google Scholar]
- 20.Bruce JM, Bruce AS, Lynch S et al. Probability discounting of treatment decisions in multiple sclerosis: associations with disease knowledge, neuropsychiatric status, and adherence. Psychopharmacology. 2018;235:3303–3313. doi: 10.1007/s00213-018-5037-y. [DOI] [PubMed] [Google Scholar]
- 21.Fox RJ, Salter A, Alster JM et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4:241–249. doi: 10.1016/j.msard.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Jarmolowicz DP, Bruce AS, Glusman M et al. On how patients with multiple sclerosis weigh side effect severity and treatment efficacy when making treatment decisions. Exp Clin Psychopharmacol. 2017;25:479–484. doi: 10.1037/pha0000152. [DOI] [PubMed] [Google Scholar]
- 23.Wicks P, Brandes D, Park J, Liakhovitski D, Koudinova T, Sasane R. Preferred features of oral treatments and predictors of non-adherence: two web-based choice experiments in multiple sclerosis patients. Interact J Med Res. 2015;4:e6. doi: 10.2196/ijmr.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson L, Loucks A, Bui C et al. Patient centered decision making: use of conjoint analysis to determine risk-benefit trade-offs for preference sensitive treatment choices. J Neurol Sci. 2014;344:80–87. doi: 10.1016/j.jns.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Köpke S, Kern S, Ziemssen T et al. Evidence-based patient information programme in early multiple sclerosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2014;85:411–418. doi: 10.1136/jnnp-2013-306441. [DOI] [PubMed] [Google Scholar]
- 26.Köpke S, Kasper J, Flachenecker P et al. Patient education programme on immunotherapy in multiple sclerosis (PEPIMS): a controlled rater-blinded study. Clin Rehabil. 2016;31:250–261. doi: 10.1177/0269215516639734. [DOI] [PubMed] [Google Scholar]
- 27.Rahn AC, Köpke S, Backhus I et al. Nurse-led immunotreatment DEcision Coaching In people with Multiple Sclerosis (DECIMS): feasibility testing, pilot randomised controlled trial and mixed methods process evaluation. Int J Nurs Stud. 2018;78:26–36. doi: 10.1016/j.ijnurstu.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Rahn AC, Köpke S, Kasper J, Vettorazzi E, Mühlhauser I, Heesen C. Evaluator-blinded trial evaluating nurse-led immunotherapy DEcision Coaching In persons with relapsing-remitting Multiple Sclerosis (DECIMS) and accompanying process evaluation: study protocol for a cluster randomised controlled trial. Trials. 2015;16:106. doi: 10.1186/s13063-015-0611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bansback N, Chiu JA, Carruthers R et al. Development and usability testing of a patient decision aid for newly diagnosed relapsing multiple sclerosis patients. BMC Neurol. 2019;19:173. doi: 10.1186/s12883-019-1382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cofield SS, Thomas N, Tyry T, Fox RJ, Salter A. Shared decision making and autonomy among US participants with multiple sclerosis in the NARCOMS Registry. Int J MS Care. 2017;19:303–312. doi: 10.7224/1537-2073.2016-091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Amico E, Leone C, Patti F. Disability may influence patient willingness to participate in decision making on first-line therapy in multiple sclerosis. Funct Neurol. 2016;31:21–23. doi: 10.11138/FNeur/2016.31.1.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heesen C, Kleiter I, Meuth SG et al. Benefit-risk perception of natalizumab therapy in neurologists and a large cohort of multiple sclerosis patients. J Neurol Sci. 2017;376:181–190. doi: 10.1016/j.jns.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Brown H, Gabriele S, White J. Physician and patient treatment decision-making in relapsing-remitting multiple sclerosis in Europe and the USA. Neurodegener Dis Manag. 2018;8:371–376. doi: 10.2217/nmt-2018-0023. [DOI] [PubMed] [Google Scholar]
- 34.Wilkie DD, Solari A, Nicholas R. Initiating disease-modifying treatments in multiple sclerosis: measuring the decision process using decisional conflict and decisional regret scales. Mult Scler J Exp Transl Clin. 2019;5 doi: 10.1177/2055217319833006. 2055217319833006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Mortensen G, Rasmussen PV. The impact of quality of life on treatment preferences in multiple sclerosis patients. Patient Prefer Adherence. 2017;11:1789–1796. doi: 10.2147/PPA.S142373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowden D, Lee V, Ritchie JA. Redefining self: patients’ decision making about treatment for multiple sclerosis. J Neurosci Nurs. 2014;46:E14–E24. doi: 10.1097/JNN.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 37.Ceuninck van Capelle Ad, Meide Hvd, Vosman FJH, Visser LH. A qualitative study assessing patient perspectives in the process of decision-making on disease modifying therapies (DMT’s) in multiple sclerosis (MS) PLoS One. 2017;12(8):e0182806. doi: 10.1371/journal.pone.0182806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Driedger SM, Maier R, Marrie RA, Brouwers M. Caught in a no-win situation: discussions about CCSVI between persons with multiple sclerosis and their neurologists: a qualitative study. BMC Neurol. 2017;17:176. doi: 10.1186/s12883-017-0954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray CL, Ploughman M, Harris C, Hogan S, Murdoch M, Stefanelli M. The liberation procedure decision-making experience for people with multiple sclerosis. Glob Qual Nurs Res. 2014;1:2333393614551413. doi: 10.1177/2333393614551413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ploughman M, Harris C, Hogan SH et al. Navigating the “liberation procedure”: a qualitative study of motivating and hesitating factors among people with multiple sclerosis. Patient Prefer Adherence. 2014;8:1205–1213. doi: 10.2147/PPA.S65483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder J, Adams K, Crooks VA, Whitehurst D, Vallee J. “I knew what was going to happen if I did nothing and so I was going to do something”: faith, hope, and trust in the decisions of Canadians with multiple sclerosis to seek unproven interventions abroad. BMC Health Serv Res. 2014;14:445. doi: 10.1186/1472-6963-14-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghahari S, Forwell SJ. Social media representation of chronic cerebrospinal venous insufficiency intervention for multiple sclerosis. Int J MS Care. 2016;18:49–57. doi: 10.7224/1537-2073.2014-073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hynes SM, Ghahari S, Forwell SJ. “Waiting for science to catch up with practice”: an examination of 10-year YouTube trends in discussions of chronic cerebral spinal venous insufficiency treatment for multiple sclerosis. Inform Health Soc Care. 2019;44:327–337. doi: 10.1080/17538157.2019.1582052. [DOI] [PubMed] [Google Scholar]
- 44.Giordano A, Lugaresi A, Confalonieri P et al. Implementation of the ‘Sapere Migliora’ information aid for newly diagnosed people with multiple sclerosis in routine clinical practice: a late-phase controlled trial. Mult Scler. 2014;20:1234–1243. doi: 10.1177/1352458513519180. [DOI] [PubMed] [Google Scholar]
- 45.Mohamadirizi S, Shaygannejad V, Mohamadirizi S, Tolou-Ghamari Z. The effect of electronic education on knowledge of patients with multiple sclerosis. J Educ Health Promot. 2017;6:10. doi: 10.4103/jehp.jehp_144_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borreani C, Giordano A, Falautano M et al. Experience of an information aid for newly diagnosed multiple sclerosis patients: a qualitative study on the SIMS-Trial. Health Expect. 2014;17:36–48. doi: 10.1111/j.1369-7625.2011.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solari A, Martinelli V, Trojano M et al. An information aid for newly diagnosed multiple sclerosis patients improves disease knowledge and satisfaction with care. Mult Scler. 2010;16:1393–1405. doi: 10.1177/1352458510380417. [DOI] [PubMed] [Google Scholar]
- 48.Brand J, Köpke S, Kasper J et al. Magnetic resonance Imaging in multiple sclerosis: patients’ experiences, information interests and responses to an education programme. PLoS One. 2014;9:e113252. doi: 10.1371/journal.pone.0113252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennison L, Brown M, Kirby S, Galea I. Do people with multiple sclerosis want to know their prognosis? a UK nationwide study. PLoS One. 2018;13:e0193407. doi: 10.1371/journal.pone.0193407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazanderani F, Hughes N, Hardy C, Sillence E, Powell J. Health information work and the enactment of care in couples and families affected by multiple sclerosis. Sociol Health Illn. 2019;41:395–410. doi: 10.1111/1467-9566.12842. [DOI] [PubMed] [Google Scholar]
- 51.Colombo C, Mosconi P, Confalonieri P et al. Web search behavior and information needs of people with multiple sclerosis: focus group study and analysis of online postings. Interact J Med Res. 2014;3:e12. doi: 10.2196/ijmr.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Synnot AJ, Hill SJ, Garner KA et al. Online health information seeking: how people with multiple sclerosis find, assess and integrate treatment information to manage their health. Health Expect. 2016;19:727–737. doi: 10.1111/hex.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colombo C, Filippini G, Synnot A et al. Development and assessment of a website presenting evidence-based information for people with multiple sclerosis: the IN-DEEP project. BMC Neurol. 2016;16:30. doi: 10.1186/s12883-016-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Della Rosa S, Sen F. Health topics on Facebook groups: content analysis of posts in multiple sclerosis communities. Interact J Med Res. 2019;8:e10146. doi: 10.2196/10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavorgna L, Lanzillo R, Brescia Morra V, Abbadessa G, Tedeschi G, Bonavita S. Social media and multiple sclerosis in the posttruth age. Interact J Med Res. 2017;6:e18. doi: 10.2196/ijmr.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavorgna L, De Stefano M, Sparaco M et al. Fake news, influencers and health-related professional participation on the Web: a pilot study on a social-network of people with multiple sclerosis. Mult Scler Relat Disord. 2018;25:175–178. doi: 10.1016/j.msard.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 57.Rath L, Vijiaratnam N, Skibina O. Assessing understanding of individual risk and symptoms of progressive multifocal leukoencephalopathy in patients prescribed natalizumab for multiple sclerosis. Intern Med J. 2017;47:194–199. doi: 10.1111/imj.13318. [DOI] [PubMed] [Google Scholar]
- 58.Giunti G, Guisado Fernández E, Dorronzoro Zubiete E, Rivera Romero O. Supply and demand in mHealth apps for persons with multiple sclerosis: systematic search in app stores and scoping literature review. JMIR Mhealth and Uhealth. 2018;6:e10512. doi: 10.2196/10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riemann-Lorenz K, Eilers M, von Geldern G, Schulz K-H, Kopke S, Heesen C. Dietary interventions in multiple sclerosis: development and pilot-testing of an evidence based patient education program. PLoS One. 2016;11:e0165246. doi: 10.1371/journal.pone.0165246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasper J, van de Roemer A, Pöttgen J et al. A new graphical format to communicate treatment effects to patients: a web-based randomized controlled trial. Health Expect. 2017;20:797–804. doi: 10.1111/hex.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reen GK, Silber E, Langdon DW. Best methods of communicating clinical trial data to improve understanding of treatments for patients with multiple sclerosis. Value Health. 2018;21:762–766. doi: 10.1016/j.jval.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 62.Gaissmaier W, Giese H, Galesic M et al. Numeracy of multiple sclerosis patients: a comparison of patients from the PERCEPT study to a German probabilistic sample. Patient Educ Couns. 2018;101:74–78. doi: 10.1016/j.pec.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Rahn AC, Backhus I, Fuest F et al. Comprehension of confidence intervals: development and piloting of patient information materials for people with multiple sclerosis: qualitative study and pilot randomised controlled trial. BMC Med Inform Decis Mak. 2016;16:122. doi: 10.1186/s12911-016-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dehghani A, Keshavarzi A. Development and validation of a multidimensional health literacy questionnaire for multiple sclerosis patients. Mult Scler Relat Disord. 2018;25:156–162. doi: 10.1016/j.msard.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 65.Heesen C, Kasper J, Fischer K et al. Risk knowledge in relapsing multiple sclerosis (RIKNO 1.0): development of an outcome instrument for educational interventions. PLoS One. 2015;10:e0138364. doi: 10.1371/journal.pone.0138364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heesen C, Pottgen J, Rahn AC et al. What should a person with relapsing-remitting multiple sclerosis know? focus group and survey data of a risk knowledge questionnaire (RIKNO 2.0) Mult Scler Relat Disord. 2017;18:186–195. doi: 10.1016/j.msard.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Barry MJ, Edgman-Levitan S. Shared decision making: pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 68.Colligan E, Metzler A, Tiryaki E. Shared decision-making in multiple sclerosis. Mult Scler. 2017;23:185–190. doi: 10.1177/1352458516671204. [DOI] [PubMed] [Google Scholar]
- 69.Härter M, Moumjid N, Cornuz J, Elwyn G, van der Weijden T. Shared decision making in 2017: international accomplishments in policy, research and implementation. Z Evid Fortbild Qual Gesundheitswes. 2017;123–124:1–5. doi: 10.1016/j.zefq.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 70.Montori VM, Kunneman M, Brito JP. Shared decision making and improving health care: the answer is not in. JAMA. 2017;318:617–618. doi: 10.1001/jama.2017.10168. [DOI] [PubMed] [Google Scholar]
- 71.Craig P, Dieppe P, Macintyre S et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benjaminy S, Schepmyer A, Illes J, Traboulsee A. Resilience, trust, and civic engagement in the post-CCSVI era. BMC Health Serv Res. 2018;18:366. doi: 10.1186/s12913-018-3130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higgins JPT, editor. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell; 2011. (Cochrane book series). Reprinted ed. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


