Abstract
Objective:
To describe the frequency and characteristics of anticonvulsant medication treatments initiated in the neonatal period.
Study Design:
We analyzed a cohort of neonates with a seizure diagnosis who were discharged from institutions in the Pediatric Health Information System between 2007 and 2016. Adjusted risk ratios (aRRs) and 95% confidence intervals (CI) for characteristics associated with neonatal (≤ 28-days postnatal) anticonvulsant initiation were calculated via modified Poisson regression.
Results:
6245 infants from 47 institutions were included. There was a decrease in both phenobarbital initiation within the neonatal period (96.9% to 91.3%, P =0.015) and continuation at discharge (90.6% to 68.6%, P <0.001). Levetiracetam (7.9% to 39.6%, P <0.001) initiation within the neonatal period and continuation at discharge (9.4% to 49.8%, P <0.001) increased. Neonates born at ≥37-weeks’ gestation and those diagnosed with intraventricular hemorrhage, ischemic/thrombotic stroke, other hemorrhagic stroke, and hypoxic ischemic encephalopathy (HIE) had a higher probability of anticonvulsant administration. The most prevalent diagnosis was HIE (n=2223, 44.4%).
Conclusion:
Phenobarbital remains the most widely used neonatal seizure treatment. Levetiracetam is increasingly used as a second line therapy. Increasing levetiracetam use indicates a need for additional study to determine its effectiveness in reducing seizure burden and improving long-term outcomes.
Keywords: anticonvulsant, antiepileptic, fosphenytoin, hypoxic-ischemic encephalopathy, levetiracetam, neonatal, phenobarbital, phenytoin, seizures
INTRODUCTION
Seizures are a common occurrence in neonatal intensive care unit (NICU) admitted infants and often accompany neurological disorders such as hypoxic ischemic encephalopathy (HIE) and ischemic stroke.1 Frequent and/or prolonged seizures may exacerbate the underlying brain injury and place neonates at an increased risk of neurological impairment and death.1–4 Pharmacological management has been the standard of care treatment for decades,5 particularly phenobarbital.3,4,6 However, there is limited randomized clinical trial evidence that anticonvulsant therapies mitigate those outcomes.3–7 These agents themselves may also be associated with adverse neurodevelopmental sequelae.4 The decision to treat – if at all – must subsequently balance the presumed benefits of seizure treatment with potential risks of detrimental side effects.3
Current treatment practices regarding anticonvulsant medication, dosage, and duration vary widely under the circumstances of limited evidence to inform the creation of international clinical guidelines.6–8 For infants with HIE, the neonatal diagnosis most associated with seizures9, therapeutic hypothermia has emerged as a treatment to reduce seizure burden.2,10–12 Few published reports have evaluated national anticonvulsant medication prescription trends among NICU infants.5,8 There is even less examination of anticonvulsant prescription patterns for hypothermia-treated infants with HIE. A vital first step in improving neonatal seizure management will be documenting the scope of anticonvulsant prescribing.4 Therefore, our objectives for this investigation were to determine the frequency and characteristics of anticonvulsant therapy among neonates with a history of seizures and to evaluate the associations between specific clinical risk factors and anticonvulsant medication usage within the neonatal period (first 28-days postnatal).
STUDY DESIGN
Data Source
We retrospectively analyzed a cohort of infants within the Pediatric Health Information System (PHIS) database (Children’s Hospital Association, Shawnee Mission, Kansas). PHIS is an administrative database created through a partnership of over 40 nonprofit US hospitals containing information collected from participating hospitals’ medical and billing records. The data comprises demographics, International Classification of Disease, Ninth revision (ICD-9) diagnoses, ICD-9 Clinical Modification procedure codes, International Statistical Classification of Diseases and Related Health Problems, Tenth revision (ICD-10) diagnoses, ICD-10 Clinical Modification procedure codes, and daily medication billing charges. To ensure comparability between hospitals and reduce coding errors, hospital-specific charge codes were translated to a common set of Clinical Transaction Classification (CTC) Codes. The Nationwide Children Institutional Review Board approved the study and concluded this research involved minimal risk due to the use of preexisting, de-identified data.
Study Population
The cohort was inclusive of infants who were discharged during January 2007 - December 2016 and admitted to PHIS-affiliated NICUs on postnatal day 0 or 1 with a recorded gestational age (GA) and a seizure diagnosis (ICD-9 code: 779.0; ICD-10 code: P90). All infants remained in the cohort through hospital discharge.
Descriptive Variables
Gestational Age
GA at delivery was classified into multiple binary variables based on clinical levels of prematurity; extremely preterm: 22–24 weeks (ICD-9: 765.21, 765.22; ICD-10: Z3A.22- Z3A.24), very preterm: 25–28 weeks (ICD-9: 765.23, 765.24; ICD-10: Z3A.25- Z3A.28), moderately preterm: 29–34 weeks (ICD-9: 765.25–765.27; ICD-10: Z3A.29- Z3A.34), late preterm: 35–36 weeks (ICD-9: 765.28; ICD-10: Z3A.35, Z3A.36), and term: ≥37 weeks (ICD-9: 765.29; ICD-10: Z3A.37- Z3A.42, Z3A.49). If ICD codes were missing, the GA as coded in the PHIS demographic file was used. Infants were excluded if both methods of GA determination were discrepant or missing.
Anticonvulsant Therapy
We focused our investigation on those infants that were first prescribed anticonvulsant medications during the neonatal period (the first 28-days postnatal) and we also evaluated this subset of infants for continued antiepileptic medication treatment at discharge. We chose to study treatment within the neonatal period because this is the time period where the vast majority of infants that remain hospitalized following birth are first treated for seizure. Within our cohort, 5003 (95.1%) of the 5260 infants ever prescribed an antiepileptic medication were first treated within the first 28-days postnatal. The following anticonvulsant medications (CTC codes) were evaluated: pyridoxine (141221), phenobarbital (114016), levetiracetam (116001), topiramate (116061), gabapentin (116047), phenytoin/fosphenytoin (116021, 116015), oxcarbazepine (116005), carbamazepine (116035), and valproic acid and derivatives (116067). Benzodiazepines are commonly used as an anxiolytic/sedative during the neonatal period. The use of benzodiazepines was not examined in this study because the specific indication (anxiolysis vs. seizure) that prompted administration cannot be determined from the PHIS database.
Subcohort of infants that received hypothermia treatment for hypoxic ischemic encephalopathy
Since HIE was the most common diagnosis associated with antiepileptic medication treatment, we chose to, as a secondary analysis, examine treatment within the cohort of infants that were treated with hypothermia therapy. This subcohort including infants with a seizure diagnosis (ICD-9: 779.0; ICD-10: P90), an HIE diagnosis (ICD-9: 768.7, 768.70, 768.71, 768.72, or 768.73; ICD-10: P91.60, P91.61, P91.62, P91.63), and hypothermia treatment (ICD-9: 9981; ICD-10: 6A4Z0ZZ, 6A4Z1ZZ). To determine HIE-related hypothermia therapy, infants had to receive hypothermia procedure on postnatal day 0 or 1. A list of ICD codes used to create the clinical diagnoses is shown in Supplementary Table 1.
Statistical Analysis
To determine clinical characteristics independently associated with neonatal anticonvulsant medication usage, a modified Poisson regression, accounting for clustering at the hospital level, was used to estimate relative risk (RR) and 95% confidence intervals (CIs) with robust error variances.13,14 Cochran–Armitage tests were used to determine changes in anticonvulsant usage over time. All analyses were conducted using Stata 15.0 (StataCorp, College Station, Texas).
RESULTS
Of a total 216,628 infants admitted to 47 national NICU sites on postnatal day 0 or 1, a total of 6,245 (2.9%) infants with a seizure diagnosis met our inclusion criteria. HIE was the most prevalent diagnosis in our cohort (n=2,451, 39.3%. The majority of infants were born at ≥37 weeks’ gestation (n=4,335, 69.4%)(Table 1). Among the 5003 infants who received anticonvulsant therapy during the neonatal period and survived to discharge, 3524 (70.4%) were discharged on anticonvulsant therapy (Table 2).
Table 1.
Characteristics of infants diagnosed with convulsions
| Entire cohort | Hypothermia-Treated HIE Subcohort | |
|---|---|---|
| N = 6,245 | N = 561 | |
| n (%)a | n (%)a | |
| Birth gestation (weeks) | ||
| ≤24 | 273 (4.4) | 0 |
| 25–28 | 357 (5.7) | 0 |
| 29–34 | 582 (9.3) | 10 (1.8) |
| 35–36 | 698 (11.2) | 90 (16.0) |
| ≥37 | 4,335 (69.4) | 461 (82.2) |
| Sex | ||
| Female | 2,680 (42.9) | 232 (41.4) |
| Diagnoses | ||
| Hypoxic ischemic encephalopathy | 2,451 (39.3) | 561 (100.0) |
| Mild | 112 (1.8) | 16 (2.9) |
| Moderate | 325 (5.2) | 103 (18.4) |
| Severe | 662 (10.6) | 170 (30.3) |
| Unspecified | 1,352 (21.7) | 272 (48.5) |
| Ischemic or hemorrhagic stroke | 447 (7.2) | 22 (3.9) |
| Intraventricular hemorrhage | 523 (8.4) | 7 (1.3) |
| Other hemorrhagic stroke | 802 (12.8) | 51 (9.1) |
| Congenital brain abnormalities | 712 (11.4) | 14 (2.5) |
| Chromosomal abnormalities | 194 (3.1) | 2 (0.4) |
Percentages were derived using column N as the denominator.
Table 2.
Neonatal period antiepileptic medication treatment and continuation at discharge
| Anticonvulsant medication treatment within the 28-day neonatal period | Entire cohort | Hypothermia-treated HIE subcohort |
|---|---|---|
| n = 5,003 | n = 504 | |
| n (%)a,b | n (%) a,b | |
| Phenobarbital | 4,761 (95.2) | 489 (97.0) |
| Levetiracetam | 1,383 (27.6) | 130 (25.8) |
| Fosphenytoin/Phenytoin | 900 (18.0) | 75 (14.9) |
| Pyridoxine (B6) | 215 (4.3) | 11 (2.2) |
| Topiramate | 75 (1.5) | 7 (1.4) |
| Oxcarbazepine | 62 (1.2) | 6 (1.2) |
| Gabapentin | 6 (0.1) | 1 (0.2) |
| Valproic acid | 10 (0.2) | 2 (0.4) |
| Carbamazepine | 3 (0.1) | 0 |
| >1 therapy | 1,778 (35.5) | 175 (34.7) |
| Remained on any anticonvulsant at discharge following neonatal initiation | 3,524 (70.4)a,b | 346 (68.7) a,b |
| Phenobarbital | 2,845 (56.9) | 273 (54.2) |
| Levetiracetam | 1,152 (23.0) | 109 (21.6) |
| Fosphenytoin/Phenytoin | 161 (3.2) | 12 (2.4) |
| Pyridoxine (B6) | 59 (1.2) | 1 (0.2) |
| Topiramate | 60 (1.2) | 3 (0.6) |
| Oxcarbazepine | 66 (1.3) | 6 (1.2) |
| Gabapentin | 11 (0.22) | 1 (0.2) |
| Valproic acid | 0 | 0 |
| Carbamazepine | 2 (0.04) | 0 |
| >1 therapy | 719 (14.4) | 56 (11.1) |
Percentages were derived using column N as the denominator.
Total column percentage may exceed 100% due to infants receiving more than one anticonvulsant medication.
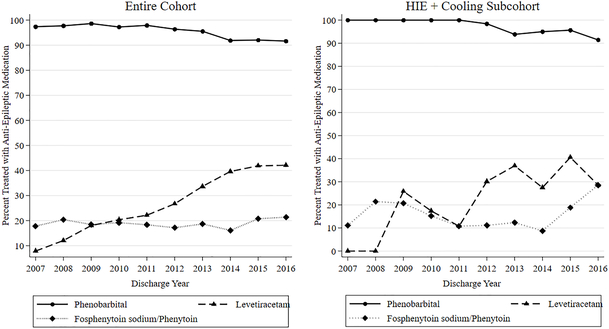
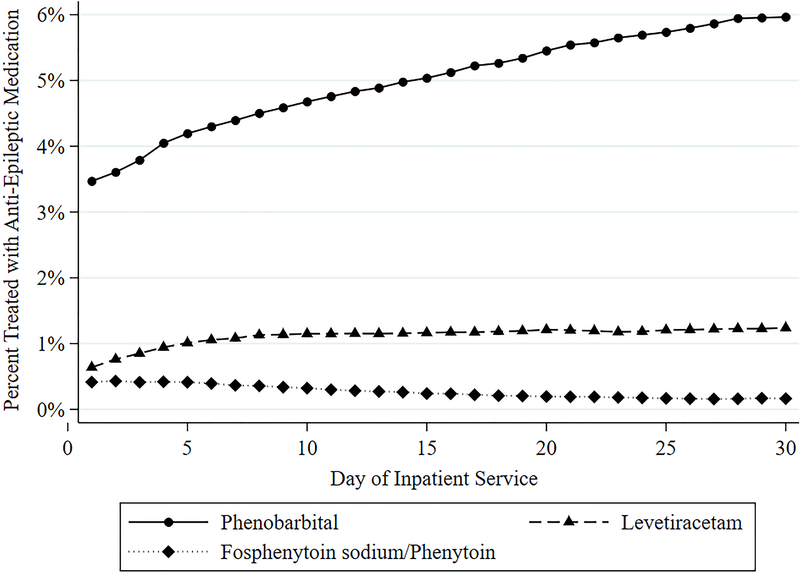
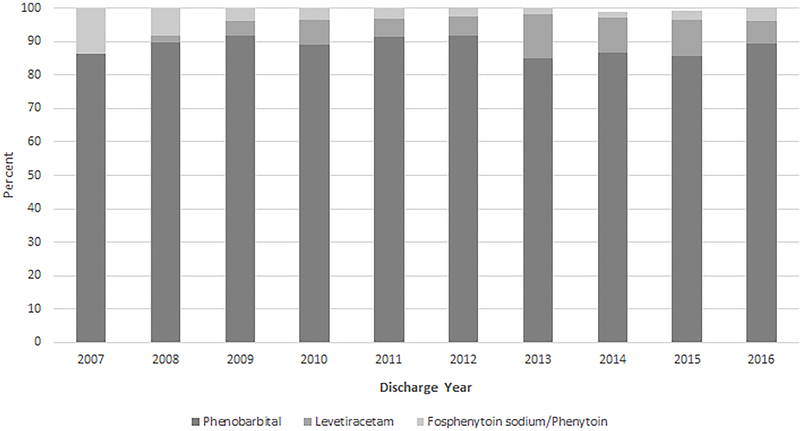
For both neonatal period and discharge prescribing, phenobarbital exposure decreased 5.6% and 23.5%, respectively, between 2007 and 2016 (P <0.05), whereas exposure to neonatal period levetiracetam therapy increased 31.7% and exposure at discharge increased 41% (P <0.001) (Table 3, Figure 2). Although total neonatal period and discharge anticonvulsant prescriptions increased throughout the study period (Figure 1), annual neonatal fosphenytoin/phenytoin treatment at discharge marginally decreased from 4.3% to 0.9% (Table 3). The collective use of other anticonvulsant medications (pyridoxine, topiramate, gabapentin, oxcarbazepine, carbamazepine, and valproic acid and derivatives) did not have a significant trend (Table 3). During the first 30 days of hospitalization, the percentage of phenobarbital and levetiracetam increased, contrary to the decreasing trend of fosphenytoin/phenytoin (Figure 3).
Table 3.
Comparison of Anti-Epileptic Medication Utilization in 2007 vs. 2016.
| Entire Cohort | Neonatal period anticonvulsant therapya |
2007 | 2016 |
P-value |
|
| n = 191 |
n = 323 |
||||
| Phenobarbital | 185 (96.9) | 295 (91.3) | 0.015 | ||
| Levetiracetam | 15 (7.9) | 128 (39.6) | <0.0001 | ||
| Fosphenytoin/Phenytoin | 33 (17.3) | 67 (20.7) | 0.337 | ||
| Otherb |
9 (4.7) |
26 (8.1) |
0.146 |
||
| n =139 |
n = 235 |
||||
| Discharged on anticonvulsant therapy | Phenobarbital | 126 (90.7) | 158 (67.2) | <0.0001 | |
| Levetiracetam | 14 (10.1) | 120 (51.1) | <0.0001 | ||
| Fosphenytoin/Phenytoin | 6 (4.3) | 2 (0.9) | 0.025 | ||
| Otherb |
6 (4.3) |
15 (6.4) |
0.401 |
||
| Fosphenytoin/Phenytoin | 0 (0) | 1 (3.7) | 0.731 | ||
| Otherb | 0 (0) | 1 (3.7) | 0.731 |
Administered within the first 28 days of life.
Pyridoxine (B6), topiramate, oxcarbazepine, gabapentin, valproic acid, and carbamazepine
Figure 2.
Percent of infants receiving phenobarbital, fosphenytoin sodium/phenytoin, and levetiractam during the neonatal period by discharge year.
Figure 1.
Antiepileptic medication utilization by discharge year.
Figure 3.
Variation of phenobarbital, fosphenytoin sodium/phenytoin, and levetiractam receipt among all hospitalized infants per service day.
Phenobarbital was the most frequently prescribed treatment among those receiving anticonvulsant medications. The second most commonly used medication was levetiracetam. Among infants who received multiple anticonvulsant therapy during the first 28 days of life (n=1778), 1,159 (65.2%) received both phenobarbital and levetiracetam. At discharge, 527 (73.3%) of infants discharged with more than one anticonvulsant therapy (n = 719) were prescribed both phenobarbital and levetiracetam. 1870 infants were administered some combination of phenobarbital, levetiracetam, and phenytoin/fosphenytoin sodium during hospitalization. 1209 began initial pharmacological seizure management with one first-line treatment (Figure 4) and 661 began with multiple on the same day; 340 began initial treatment with both phenobarbital and levetiracetam on the same day, 251 started phenobarbital and phenytoin/fosphenytoin sodium, 11 started levetiracetam and phenytoin/fosphenytoin sodium, and 59 received all three.
Figure 4.
First-line antiepileptic variation over time.
Predictors of Anticonvulsant Therapy
After accounting for clustering at the hospital level, the largest overall predictors of ever receiving anticonvulsant therapy in the first 28 postnatal days were a GA of ≥37 weeks and a diagnosis of ischemic stroke, intraventricular hemorrhage (IVH), HIE, and other stroke (Table 3).
Seizures, HIE, and Hypothermia Therapy
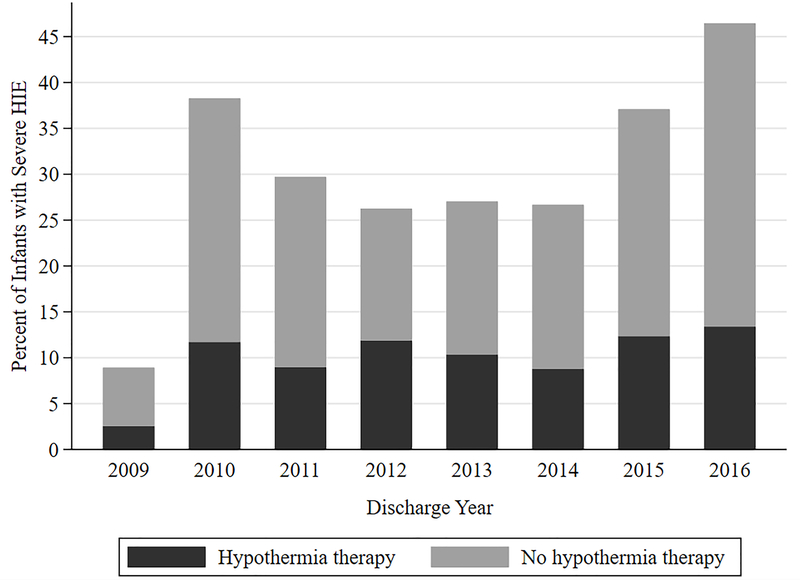
Since HIE was by far the leading neonatal diagnosis associated with seizures (Table 1), we chose to explore the subcohort of infants with HIE and a seizure diagnosis who were also treated with therapeutic cooling (n=504). ICD-9 diagnostic coding for HIE was first developed in 2006. Between 2006–2009 there was a single ICD-9 code for HIE. However ICD-9 codes for severity of HIE (mild, moderate, severe) did not exist until 2009. In addition, despite severe HIE ICD-9/ICD-10 codes after 2009 in all hospital sites, 14 did not report a code for hypothermia therapy treatment. Since the severity of HIE was not always coded (unspecified severity was not specified in 56% of patients diagnosed with HIE) and some sites did not report cooling treatment billing, we chose to focus only on infants who received cooling treatment (Figure 5). Randomized therapeutic cooling trials for HIE have primarily focused on infants with moderate and severe encephalopathy.15,16 Infants who were not cooled, but still coded with an HIE diagnosis, may have potentially been diagnosed with mild HIE. If so, they may have been less susceptible to seizures relative to cooled infants.17
Figure 5.
Reporting of HIE Severity per year among infants receiving hypothermia treatment
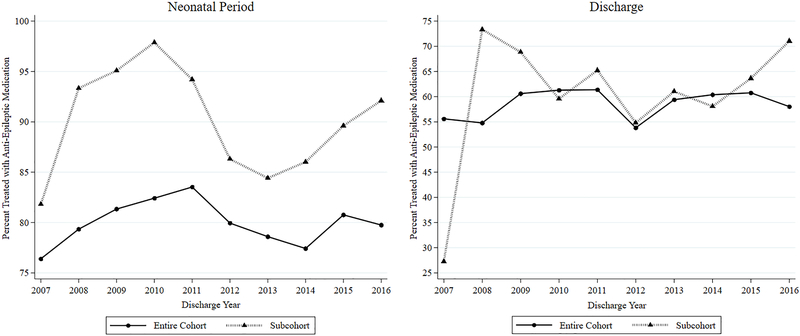
Demographically, birth at ≥37 weeks’ gestation (461, 82.2%), male sex (329, 58.6%), and a diagnosis of severe or unspecified HIE (30.3% and 48.5%, respectively) predominated (Table 1). As with the entire cohort, phenobarbital was also the most frequently prescribed therapy among those receiving anticonvulsant medications overall (Figure 2), both during the neonatal period and at discharge (Table 2). However, there were no significant individual medication trends over time. Neonatal period anticonvulsant use among the subcohort of infants with hypothermia-treated HIE and a seizure diagnosis was higher than the overall study cohort throughout the 10-year study period (Figure 1). 175 infants were prescribed more than one neonatal period medication, 116 (66.3%) of which received both phenobarbital and levetiracetam. Among 56 infants prescribed more than one anticonvulsant at the time of discharge, 41 (73.2%) received both phenobarbital and levetiracetam. Among the subcohort of infants who received cooling therapy for HIE, only IVH exposure was associated with neonatal period receipt of anticonvulsant therapy. Infants with congenital abnormalities and IVH were most likely to receive neonatal period anticonvulsant therapy (Table 3).
DISCUSSION
This multicenter analysis evaluated changes in anticonvulsant medication usage in the NICU setting over the last decade. During the 10-year study period, the frequency of phenobarbital treatment declined as the frequency of levetiracetam use simultaneously increased, reflecting similar results to a previous multicenter analysis.5 We also found that over 80% of neonates in our study treated both within the first 28 postnatal days and at discharge received phenobarbital, indicating phenobarbital is still the most common antiepileptic drug prescribed to neonates. Concerns remain regarding limited evidence of efficacy6 for seizure control and the potential for adverse, long-term neurodevelopmental effects.4
Practitioners have relied on phenobarbital for first-line neonatal seizure treatment for decades.18 Congruent with our findings, previous studies indicate phenobarbital is the most common neonatal seizure treatment.1,3–9 It and other anticonvulsants continue to be prescribed off-label in the absence of U.S. Food and Drug Administration (FDA) approvals for neonatal use.6 Phenobarbital has been attributed to apoptotic neurodegeneration in the early brain development of rats19 and a recent observational study measured an association between neonatal phenobarbital exposure and motor and cognitive impairment in early childhood.20 A single center randomized control trial found phenobarbital to have similar efficacy (< 50%) as phenytoin.21 While animal studies have suggested reduced brain damage among rodents administered prophylactic phenobarbital before cooling,22,23 those results contrast with a retrospective analysis suggesting worst neonatal outcomes, in terms of death and abnormal brain MRI, among infants who received prophylactic phenobarbital prior to cooling.24
Levetiracetam surfaced as a popular second-line treatment in recent years, a trend substantiated by our own finding of a significant increase between 2007 to 2016.5 This may potentially be due to its favorable safety and pharmacokinetic profile in older children.5,18 In contrast to phenobarbital, rodent models demonstrated that levetiracetam treatment did not have a neurodegenerative effect on the developing brain25 and was additionally associated with both neuroprotective effects against long-term outcomes and a reduction in neuronal apoptosis following hypoxic ischemic brain injury.26,27 Furthermore, a recent systematic review examining levetiracetam efficacy within observational studies measured seizure reduction or cessation following levetiracetam treatment to be between 67–75%.28 However given the paucity of clinical neonatal studies examining levetiracetam efficacy and safety, including the lack of any randomized trials,6,29 further high quality investigation is warranted. Nevertheless, levetiracetam, a newer, second-generation antiepileptic, has outpaced phenytoin as the preferred second-line treatment.5,6,21 We speculate that the significant decrease in neonatal period phenytoin treatment that we observed may be due to its limited clinical effectiveness, narrow therapeutic index, adverse side effects, and cardiac toxicity.3, 21, 30
In our study, a greater proportion of the seizure, HIE, and hypothermia treated subcohort received anticonvulsant therapies in the first 28 days of life and at discharge compared to the entire cohort of infants. Moreover, the multivariable model suggested ischemic or thrombotic stroke, IVH, HIE, and other hemorrhagic stroke predicted neonatal period anticonvulsant therapy in the entire cohort. This may be attributable to sicker, severely affected infants needing immediate and longer-term therapy for seizure management.31 Our results highlight the need for assessments concerning the antiepileptic effects of hypothermia therapy in combination with pharmacological management.
Our findings emphasize the evolving trend of anticonvulsant therapies with several limitations. The PHIS database permitted a large sample-size and detailed pharmaceutical information. However, data were collected originally for administrative purposes. As such, underreporting and recording errors may have influenced our results. We relied on less specific ICD-9 and ICD-10 discharge and procedural codes. The impact of coding errors on the results may have been mitigated by PHIS employing internal data quality audits and requirement that identifiable data errors from participating hospitals not exceed 5%.32 Moreover, we were not able to account for exclusive benzodiazepine administration, variation in diagnostic criteria for seizures due to the lack of EEG data, and we could not evaluate the severity or timing of seizures due to reliance on discharge diagnoses. Treatment and diagnosis of electrographic seizures without clinical symptoms may vary by center. Given that neonatal seizures are often subclinical among infants receiving hypothermia therapy, it is possible that seizures may have been underreported in our cohort.33 Nevertheless, our results may be applicable to other NICUs in the United States since PHIS-affiliated hospitals represent large variety metropolitan regions across the US.32
CONCLUSION
In this large, multicenter analysis of neonatal seizures among NICU infants from 2007 to 2016, phenobarbital usage decreased while levetiracetam substantially increased, despite limited evidence on safety and efficacy. The majority of infants who received neonatal period anticonvulsant therapy were subsequently discharged home on anticonvulsant medications. Further studies examining the effectiveness and safety of various anticonvulsant medications in neonates, outcomes associated with earlier weaning of antiepileptics prior to discharge, and the potential anti-epileptogenic effects of hypothermia therapy may be beneficial in mitigating the risk of adverse neurological sequelae.
Supplementary Material
Table 4.
Multivariable adjusted relative risk of ever receiving antiepileptic medication during the neonatal period. a
| Clinical Characteristics | aRR (95% CI) | P-value | |
|---|---|---|---|
| Entire cohort | Female | 1.01 (0.99, 1.04) | 0.450 |
| Gestational Age | |||
| <37 | Reference | ||
| ≥37 | 1.11 (1.06, 1.16) | <0.0001 | |
| Ischemic or thrombotic stroke | 1.16 (1.11, 1.21) | <0.0001 | |
| Intraventricular hemorrhage | 1.24 (1.17, 1.31) | <0.0001 | |
| Hypoxic ischemic encephalopathy | 1.26 (1.21, 1.32) | <0.0001 | |
| Other hemorrhagic stroke | 1.11 (1.07, 1.15) | <0.0001 | |
| Congenital brain abnormalities | 1.02 (0.96, 1.07) | 0.579 | |
| Chromosomal abnormalities | 0.94 (0.86, 1.04) | 0.268 | |
| HTE + Cooling Subcohort | Female | 1.01 (0.95, 1.07) | 0.828 |
| Gestational Age | |||
| <37 | Reference | ||
| ≥37 | 0.98 (0.89, 1.07) | 0.629 | |
| Ischemic or thrombotic stroke | 0.95 (0.78, 1.17) | 0.627 | |
| Intraventricular hemorrhage | 1.09 (1.01, 1.19) | 0.030 | |
| Other hemorrhagic stroke | 1.02 (0.97, 1.08) | 0.448 | |
| Congenital brain abnormalities | 1.11 (1.07, 1.16) | <0.0001 | |
| Chromosomal abnormalities | 1.04 (0.97, 1.12) | 0.271 | |
Administered within the first 28 days of life.
ACKNOWLEDGEMENTS
Supported in part by K08HL121182 (Slaughter) and the Research Institute at Nationwide Children’s Hospital. The authors have no conflicts of interest to disclose relevant to this manuscript.
REFERENCES
- 1.Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary Profile of Seizures in Neonates: A Prospective Cohort Study. The Journal Of Pediatrics. 2016;174:98–103.e1. doi: 10.1016/j.jpeds.2016.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uria-Avellanal C, Marlow N, Rennie JM. Outcome following neonatal seizures. Seminars In Fetal & Neonatal Medicine. 2013;18(4):224–232. doi: 10.1016/j.siny.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 3.Booth D, Evans DJ. Anticonvulsants for neonates with seizures. The Cochrane Database Of Systematic Reviews. 2004;(4):CD004218. [DOI] [PubMed] [Google Scholar]
- 4.Blume HK, Garrison MM, Christakis DA. Neonatal seizures: Treatment and treatment variability in 31 United States pediatric hospitals. Journal of Child Neurology. 2009;24(2):148–154. doi: 10.1177/0883073808321056 [DOI] [PubMed] [Google Scholar]
- 5.Ahmad KA, Desai SJ, Bennett MM, et al. Changing antiepileptic drug use for seizures in US neonatal intensive care units from 2005 to 2014. Journal of Perinatology. 2017;37(3):296–300. doi: 10.1038/jp.2016.206 [DOI] [PubMed] [Google Scholar]
- 6.Slaughter LA, Patel AD, Slaughter JL. Pharmacological treatment of neonatal seizures: a systematic review. J Child Neurol 2013;28(3):351–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatric Neurology. 2007;37(2):85–90 [DOI] [PubMed] [Google Scholar]
- 8.Shellhaas RA, Chang T, Wusthoff CJ, et al. Treatment Duration After Acute Symptomatic Seizures in Neonates: A Multicenter Cohort Study. J Pediatr 2016;181:298–301.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans D, Levene M. Neonatal seizures. Arch Dis Child Fetal Neonatal Ed 1998;78(1):F70–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low E, Boylan GB, Mathieson SR, Murray DM, Korotchikova I, Stevenson NJ, et al. Cooling and seizure burden in term neonates: an observational study. Arch Dis Child Fetal Neonatal Ed 2012, 97(4): F267–272 [DOI] [PubMed] [Google Scholar]
- 11.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr 2013, 163(2): 465–470 [DOI] [PubMed] [Google Scholar]
- 12.Guidotti I, Lugli L, Guerra MP, Ori L, Gallo C, Cavalleri F, et al. Hypothermia reduces seizure burden and improves neurological outcome in severe hypoxic-ischemic encephalopathy: an observational study. Dev Med Child Neurol 2016, 58(12): 1235–1241 [DOI] [PubMed] [Google Scholar]
- 13.Zou G, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Statistical Methods in Medical Research. 2013;22(6):661–670. doi: 10.1177/0962280211427759 [DOI] [PubMed] [Google Scholar]
- 14.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics.2000; 56:645–6 [DOI] [PubMed] [Google Scholar]
- 15.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England Journal Of Medicine. 2005;353(15):1574–1584. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013(1): Cd003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massaro AN, Murthy K, Zaniletti I, et al. Short-term outcomes after perinatal hypoxic ischemic encephalopathy: a report from the Children’s Hospitals Neonatal Consortium HIE focus group. Journal of Perinatology. 2015;35(4):290–296. doi: 10.1038/jp.2014.190. [DOI] [PubMed] [Google Scholar]
- 18.El-Dib M, Soul JS. The use of phenobarbital and other anti-seizure drugs in newborns. Seminars In Fetal & Neonatal Medicine. 2017;22(5):321–327. doi: 10.1016/j.siny.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 19.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maitre NL, Smolinsky C, Slaughter JC, Stark AR. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. J Perinatol 2013;33(11):841–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. The New England Journal Of Medicine. 1999;341(7):485–489 [DOI] [PubMed] [Google Scholar]
- 22.Davidson JO, Wassink G, van den Heuij LG, Bennet L, Gunn AJ. Therapeutic Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy - Where to from Here? Front Neurol 2015, 6: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barks JD, Liu YQ, Shangguan Y, Silverstein FS. Phenobarbital augments hypothermic neuroprotection. Pediatr Res 2010, 67(5): 532–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkar S, Barks JD, Bapuraj JR, Bhagat I, Dechert RE, Schumacher RE, et al. Does phenobarbital improve the effectiveness of therapeutic hypothermia in infants with hypoxic-ischemic encephalopathy? J Perinatol 2012, 32(1): 15–20 [DOI] [PubMed] [Google Scholar]
- 25.Manthey D, Asimiadou S, Stefovska V et al. Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brain. Exp Neurol 2005;193(2):497–503 [DOI] [PubMed] [Google Scholar]
- 26.Talos DM, Chang M, Kosaras B, et al. Antiepileptic effects of levetiracetam in a rodent neonatal seizure model. Pediatric Research. 2013;73(1):24–30. doi: 10.1038/pr.2012.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilicdag H, Daglıoglu K, Erdogan S, et al. The effect of levetiracetam on neuronal apoptosis in neonatal rat model of hypoxic ischemic brain injury. Early Human Development. 2013;89(5):355–360. doi: 10.1016/j.earlhumdev.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 28.McHugh DC, Lancaster S, Manganas LN. A Systematic Review of the Efficacy of Levetiracetam in Neonatal Seizures. Neuropediatrics. 2018;49(1):12–17. doi: 10.1055/s-0037-1608653 [DOI] [PubMed] [Google Scholar]
- 29.Glass HC. Neonatal seizures: advances in mechanisms and management. Clin Perinatol 2013;41(1):177–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeller B, Giebe J. Pharmacologic Management of Neonatal Seizures. Neonatal Network: NN. 2015;34(4):239–244. doi: 10.1891/0730-0832.34.4.239 [DOI] [PubMed] [Google Scholar]
- 31.Martinello K, Hart AR, Yap S, Mitra S, Robertson NJ. Management and investigation of neonatal encephalopathy: 2017 update. Archives Of Disease In Childhood Fetal And Neonatal Edition. 2017;102(4):F346–F358. doi: 10.1136/archdischild-2015-309639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher BT, Lindenauer PK, Feudtner C. In-hospital databases. In: Strom B, Kimmel SE, Hennessy S,editors. Pharmacoepidemiology. Oxford: John Wiley & Sons, Ltd; 2012. p. 244–258 [Google Scholar]
- 33.Boylan GB, Kharoshankaya L, Wusthoff CJ. Seizures and hypothermia: importance of electroencephalographic monitoring and considerations for treatment. Semin Fetal Neonatal Med 2015, 20(2): 103–108 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.