Abstract
Introduction:
Many evidence indicates that Carcinoembryonic antigen (CEA) and Carbohydrate antigen 19-9 (CA 19-9) have strong reactivity with tumor cells and may serve as a useful marker in identifying patients with colorectal cancer (CRC).
Objectives:
The goal of this study was to evaluate the relationship between preoperative concentration of serum levels of CEA and CA 19-9 and progression of colorectal cancer.
Methods:
The retrospective study included 80 patients operated for colorectal cancer at the Clinic for General and Abdominal Surgery, Clinical Center of University of Sarajevo, from 2013 to 2018. The following clinical and laboratory parameters were observed: age, sex, preoperatively measured concentrations of CEA and CA 19-9 antigens, CRC localization, postoperative histopathological findings and CRC stage (TNM classification). All of the data above were processed by relevant statistical methods, with an accepted level of statistical significance of p <0.05.
Results:
The highest serum levels of CEA and CA 19-9 were observed in stage IV of CRC. Average CEA and CA 19-9 values did not differ significantly between tumor stages (p>0.05). Preoperatively measured serum concentrations of CEA and CA 19-9 in patients with CRC were significantly correlated (rho = 0.328, p = 0.001). An increase in the depth of tumor invasion of the intestinal wall tumor (pT) is followed by an increase in the serum value of the CEA marker, but this ratio was not statistically significant (rho=0.194, p=0.080), while the relationship between depth of intestinal wall invasion and serum level of CA 19-9 was significantly positive correlation (rho = 0.252, p = 0.024). However, the linear regression analysis model showed that serum levels of CEA and CA 19-9 could not be predictors of CRC stage and depth of tumor invasion of the intestinal wall (p> 0.05).
Conclusion:
Preoperatively measured serum values of CEA and CA 19-9 cannot indicate the specific stage and histopathological size of the CRC.
Keywords: colorectal cancer, carcinoembryonic antigen, carbohydrate antigen
1. INTRODUCTION
Colon cancer is globally the third most commonly diagnosed cancer in both men and women (1). About 1.65 million new cases and nearly 855,000 deaths were reported in 2015 (2). Colorectal cancer (CRC) is expected to increase globally by 60% by 2030 to over 2.2 million new cases and 1.1 million deaths (3).
The fact is that current clinical and pathological indicators cannot predict the outcome of the disease. Therefore, significant efforts have been made in recent years to find new markers for timely identification and a more selective approach to the therapy.
Carcinoembryonic antigen (CEA) is a protein normally found in embryonic or fetal tissue. It is almost absent in the serum after birth, but small amounts may be present in the colon. In adults, CEA may be elevated in malignant disorders which produce proteins, especially mucinous cancers associated with the gastrointestinal tract or ovaries. A meta-analysis found that the CEA sensitivity for the diagnosis of CRC was only 46%, while the specificity was also limited (4). Carbohydrate antigen 19-9 (CA 19-9) or cancer antigen 19-9 is an oncofetal tumor marker which was isolated and reported by Koprowski et al. for the first time in 1979 (5). It is elevated in 20-40% patients with gastric cancer (6), 20-40% patients with CRC (7) and 70-93% patients with pancreatic tumor (8, 9).
Serum levels of CA 19-9 and CEA are well known tumor markers that are used in the diagnosis of CRC and are also reported to correlate with tumor progression, the degree of metastasis, and as well as the overall prognosis for colon cancer (4). However, they cannot be used in the diagnosis of non-metastatic colon cancer because of their non-specificity for early detection of colon cancer. The results of a study conducted by Japanese authors indicate that elevated preoperative serum levels of CA 19-9 may serve as a useful marker in identifying patients with lymph node-negative colorectal cancers at high risk for recurrence after surgery (10).
An expert panel on breast and colon cancer markers established by the American Society of Clinical Oncology (ASCO) recommended that neither serum CEA nor any other marker, including CA 19-9, should be used as screening or diagnostic test for CRC (11). Some researchers conclude that preoperative serum CEA and CA 19-9 values should be associated with mandatory procedures for determining the stage of CRC patients, while the values of other tumor markers is yet to be evaluated in future studies (12).
2. AIM
The aim of this study was to analyze the relationship between preoperative concentration of CA 19-9 and CEA in the serum of patients with colorectal cancer and to determine whether the monitored biomarkers have predictive significance in assessing the stage of colorectal cancer and the depth of bowel wall invasion (pT).
3. MATERIAL AND METHODS
The study was designed as a retrospective, observational study. It was performed at the Clinic for General and Abdominal Surgery, Clinical Center University of Sarajevo (CCUS). The study involved 80 patients undergoing colorectal cancer surgery from 2014 to 2018. The study included: patients of both genders, older than 18 years, with clinically and histopathologically confirmed colorectal cancer, patients with histopathologic findings of resected tumor tissue, patients which have undergone tumor staging, and patients with colorectal cancer which had preoperatively measured serum concentrations of CA 19-9 and CEA antigen. Patients with proven neoplasm of another organ not related to colorectal cancer were not included in the study.
Analysis of serum concentrations of CEA and CA 19-9 antigen were performed at the Department of Clinical Chemistry CCUS. Reference values used for CEA in serum were 0-5 mcg/L, and for CA 19-9 0-35 U/mL.
Histopathologic examination of tissue samples was performed at the Department of Clinical Pathology CCUS. CRC staging was determined according to the TNM classification by the American Association of Cancer (AJCC) from 2010 (13).
4. RESULTS
A total of 80 subjects diagnosed with colorectal cancer (CRC) were enrolled in the study. The ratio of male and female respondents is 36 to 44 (45% : 55%). The age difference shown was not statistically significant, p = 0.324 (Graph 1). Most men and women are between 60 and 80 years old.
Graph 1. Age structure of respondents in relation to gender.

Preoperatively measured concentrations of carcinoembryonic antigen (CEA) and carbohydrate antigen (CA 19-9) in the serum of patients with colorectal cancer were correlated with each other (rho = 0.328, p = 0.001) (Graph 2).
Graph 2. Ratio of serum CEA and CA 19-9 in subjects with colorectal cancer.

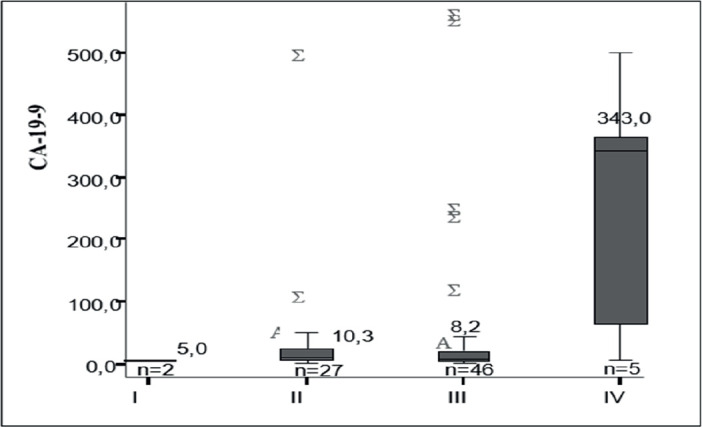
Serum concentrations of CEA and CA 19-9 from subjects with different stages of CRC are shown in Table 1. The most extreme serum CEA values were found in five subjects with stage IV CRC, but the mean values (median) of this marker did not statistically significantly differ between different tumor stages (p> 0.05).
Table 1. Serum concentration of CEA and CA 19-9 in colorectal cancer patients in relation to cancer stage. Data are presented as median and interquartile range q1-q3. *p<0.05 between stage II and IV; ǂp<0.05 between stage III and IV.
| Stage of CRC | ||||
|---|---|---|---|---|
| Variabe | I (n=2) |
II (n=27) |
III (n=46) |
IV (n=5) |
| CEA (mcg/L) | 0.77 (0.37-0.78) |
3.60 (2.42-13.90) |
4.34 (1.77-20.44) |
29.3 (2.05-606.5) |
| CA 19-9 (U/mL) |
5.03 (2.74-4.80) |
10.33 (6.54-26.34) |
8.21 (3.67-20.14) |
343,00*ǂ (35.9-1675.4) |
Extremely elevated CA 19-9 concentrations were found in stage IV of CRC (343.0 (35.98–1675.43) U/mL). Mean values (median) of this marker were not significantly different between the first, second and third stages of colorectal cancer (p>0.05), while the differences between stages II and IV, as well as between stages III and IV, were statistically significant (p<0.05) (Graph 3).
Graph 3. Serum values of CA 19-9 markers relative to tumor stage.
The histopathological size of colon tumors is accompanied by an increase in serum CEA, but this ratio is not statistically significant (rho = 0.194, p = 0.08). In contrast, the histopathological size of the colorectal tumor, that is the depth of tumor invasion of the intestinal wall (pT), was accompanied by a statistically significant increase in the serum value of the CA 19-9 marker (rho = 0.252, p = 0.024).
A linear regression analysis model was used to investigate the mode of independent association of the monitored variables with the stage of CRC and the depth of tumor invasion of the bowel wall. In this model, serum values of CEA and CA 19-9 showed no statistically significant effect on the histopathological size of CRC (p> 0.05) (Table 2).
Table 2. Independent predictors of colorectal cancer stage and bowel wall infiltration.
| Unstandardized Coefficients | Standardized Coefficients | ||||
|---|---|---|---|---|---|
| B | Std. Error | Beta | t | p | |
| (Constant) | 2.193 | 0.374 | 5.859 | 0.0001 | |
| CEA | 0.000 | 0.000 | 0.091 | 1.062 | 0-292 |
| CA 19-9 | -0.001 | 0.000 | -0.117 | -1.317 | 0.192 |
| Stage of CRC | |||||
| (Constant) | 4.128 | 0.725 | 5.691 | 0.0001 | |
| CEA | 0.000 | 0.001 | -0.033 | -0.232 | 0.817 |
| CA-19-9 | 0.002 | 0.001 | 0.252 | 1.725 | 0.089 |
| Depth of bowel wall infiltration (pT) | |||||
5. DISCUSSION
Colorectal cancer is one of the major health problems in most countries of the world. More than one million people suffer from this disease every year (14). The primary goal of all health systems is the early detection of this cancer, because then surgical and oncological treatment will allow longer survival and better quality of life for the patient.
Some studies have shown that CEA and CA 19-9 markers are not specific for early detection of colorectal cancer (15, 16). However, the determination of these antigens in colorectal tissue is an important prognostic marker, and some researchers conclude that preoperative serum levels of CEA and CA 19-9 should be associated with mandatory procedures in determining the stage of CRC (16, 17) .
Our study involved examining the relationship between preoperative serum concentration of CA 19-9 and CEA in patients with CRC, and histopathological tumor size. Serum CA 19-9 concentration is markedly increased in stage IV CRC and is statistically significantly different from the concentration of this biomarker in the second and third stages of this tumor disease. Serum CEA values do not differ significantly between CRC stages although the highest values are in stage IV disease. The results of the study showed that preoperatively measured concentrations of CEA and CA 19-9 in the serum of patients with CRC were correlated whereby higher serum CEA markers were observed in subjects with higher serum CA 19-9 levels. The depth of tumor invasion of the colon wall is accompanied by an increase in the serum value of the CEA marker, but this ratio is not statistically significant, whereas the serum CA 19-9 value is statistically significant in relation to the histopathological size of the tumor.
The CEA marker values in our study are similar to those reported by Al-Shuneigat et al, where the average CEA value was 3.56 mcg/L, while the average CA 19-9 value was slightly higher than our study and was 28 U/mL (17). Similar results were obtained by Zhang SY et al. in a study of 138 patients, where the average CEA value was 5.60 mcg/L, or in the range 2.08-11.25, while the average CA 19-9 value was 21.60 U/mL, i.e. in the range of 11.45–46.00 U/mL (18).
The highest average values of CEA markers (29.30 mcg/L) in our study were observed in stage IV of the tumor, followed by stage III 4.34 mcg/L, and stage II 3.60 mcg/L, while the lowest values were on average in stage I 0.77 mcg/L. Our results are partly in line with those of Tomasevic SR. and their study of 183 subjects, where the mean CEA values in the first and second stages were 4.12 mcg/L, values in the third stage were 5.19 mcg/L, and the average marker values in the fourth stage were 37.22 mcg/L, while CEA values were significantly elevated in stage IV, whereas in other stages the values of this biomarker were approximately the same. Similar results were obtained for the tumor marker CA 19-9, where in the first and second stages CA 19-9 values were 13.66 U/mL, values in the third stage were 17.35 U/mL, while in the fourth stage they found significantly elevated values of this marker 77.72 U/mL (19).
In our study, the serum values of CA 19-9 markers were significantly increased in stage IV of CRC compared to serum values of markers in other CRC stages. A statistically significant difference in the CA 19-9 tumor marker values between II and IV stages was found, as well as between III and IV stages (p <0.05).
The increase of CA 19-9 has demonstrated a significantly higher frequency of metastasis and distinctly lower survival rate, making it an adverse prognostic factor for CRC patients (20). The results of retrospective-prospective study by Vukobrat-Bijedic et al. further confirms the important role of significantly elevated levels of CA 19-9 and CEA as later markers in metastatic colon cancer (21).
The results of Polat et al. indicate that serum CEA and CA 19-9 values do not correlate with pT magnitude of intestinal tumor invasion (p> 0.05), which is partially consistent with our results (22). Specifically, in our study, the histopathological size of colon tumors was accompanied by an increase in the serum value of the CEA marker, but this ratio was not statistically significant (p = 0.080). In contrast to CEA, the ratio of CA 19-9 markers to histopathological tumor size was statistically significant (p = 0.024). However, the linear regression model did not indicate the predictive importance of either of these two biomarkers in predicting the stage or depth of tumor invasion of the bowel wall.
6. CONCLUSION
A combination of CA 19-9 and CEA can be utilized as diagnostic markers for advanced stages of CRC. However, the results obtained do not give predictive significance to either of the two biomarkers in distinguishing different CRC stages and depth of tumor invasion in the bowel wall.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent forms. Author’s contributions: Study concept and design: E.H; I.R; A.S; A.M; A.R; E.H; E.K;
Acquisition of data:
E.H; I.R; A.S;
Analysis and interpretation of data:
E.H; I.R; A.S; A.M; A.R; E.H; E.K;
Drafting of the manuscript:
E.H; I.R; A.S;
Critical revision of the manuscript for intellectual content:
E.H; I.R; A.S; A.M; A.R; E.H; E.K;
Conflict of interest:
There are no conflict of interest.
Financial support and sponsorship:
None.
REFERENCES
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Mcrae FA. Colorectal cancer: Epidemiology, risk factors and protective factors. In: Savarese DMF, editor. UpToDate. Waltham, MA: UpToDate Inc; 2018. [Google Scholar]
- 3.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Zhang Y, Niu Y, Li K, Liu X, Chen H, Gao C. A systematic review and meta-analysis of diagnostic and prognostic serum biomarkers of colorectal cancer. PLoS One. 2014;9(8):e103910. doi: 10.1371/journal.pone.0103910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212:53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 6.Kato K, Taniguchi M, Kawakami T, Nagase A, Matsuda M, Onodea K, Yamaguchi H, Higuchi M, Furukawa H. Gastric Cancer with a Very High Serum CA 19-9 Level. Case Rep Gastroenterol. 2011;5(1):258–261. doi: 10.1159/000327984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forones NM, Tanaka M. CEA and CA 19-9 as prognostic indexes in colorectal cancer. Hepatogastroenterology. 1999 Mar-Apr;46(26):905–908. [PubMed] [Google Scholar]
- 8.Al-Shamsi HO, Alzahrani M, Wolff RA. The clinical utility of normal range carbohydrate antigen 19-9 level as a surrogate marker in evaluating response to treatment in pancreatic cancer–a report of two cases. JGO. 2016;7(3):E45–E51. doi: 10.21037/jgo.2016.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Nakagoe T, Sawai T, Tsuji T, Jibiki MA, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Arisawa K. Preoperative serum level of CA19-9 predicts recurrence after curative surgery in node-negative colorectal cancer patients. Hepatogastroenterology. 2003;50(51):696–699. [PubMed] [Google Scholar]
- 11.Kuebler JP, Wieands S, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 12.Reiter W, Stieber P, Reuter C, Nagel D, Lau-Werner U, Lamerz R. Multivariate analysis of the prognostic value of CEA and CA 19-9 serum levels in colorectal cancer. Anticancer Res. 2000;20(6D):5195–5198. [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology. 2010;17:1471–1374. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J, Steliarov-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europa: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Reiter W, Stieber P, Reuter C, Nagel D, Lau-Werner U, Lamerz R. Multivariate analysis of the prognostic value of CEA and CA19-9 serum levels in colorectall cancer. Anti cancer Res. 2000;20(6D):5195–5198. [PubMed] [Google Scholar]
- 16.Deng J, Shi Q, Mirza W, Jecmenica M, Yoder M, Mulloth R, Gordon E, Sothwal A, Thanneeru S, Dharmapuri S. Extremely high serum level of carbohydrate antigen 19-9 in a patient with colon adenocarcinoma. Cancer Rep Rev. 2017;1(3):1–3. [Google Scholar]
- 17.Al-Shuneigat JM, Mahgoub SS, Huq F. Colorectal carcinoma: nucleosomes, carcinoembryonic antigen and ca 19-9 as apoptotic markers; a comparative study. J Biomed Sci. 2011;18:50. doi: 10.1186/1423-0127-18-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang SY, Lin M, Zhang HB. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinoma. Int J Clin Exp Pathol. 2015;8:9404–9409. [PMC free article] [PubMed] [Google Scholar]
- 19.Tomašević SR. Doktorska disertacija. Beograd: 2016. Procena individualnih prediktivnih faktora za nastanak carcinoma kolona. [Google Scholar]
- 20.Levy M, Visokai V, Lipska L, Topolcan O. Tumor markers in staging and prognosis of colorectal carcinoma. Neoplasma. 2008;55:138–142. [PubMed] [Google Scholar]
- 21.Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, Bijedic N, Bjelogrlic I, Gogov B, Mehmedovic A. Cancer Antigens (CEA and CA 19-9) as Markers of Advanced Stage of Colorectal Carcinoma. Med Arch. 2013;67(6):397–401. doi: 10.5455/medarh.2013.67.397-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polat E, Duman U, Duman M, Atici AE, Reyhan E, Dalgic T, Bostanci EB, Yol S. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1–e7. doi: 10.3747/co.21.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]


