Abstract
Rapid autopsy at the end of life in people with HIV (PWH) permits the preservation of valuable tissue specimens for subsequent study of HIV reservoirs. At our institution, we have developed a cohort of PWH who consent to a rapid autopsy to gather a wide range of fluids and tissues with the goal of advancing HIV cure research. The protocol for successfully performing these autopsies has required careful thought and development over months and years. We have now successfully performed six rapid autopsies and detail here our steps to build the study cohort, train and staff a team of more than a dozen personnel, and process and preserve hundreds of samples from each autopsy.
Keywords: altruism, cohort studies, HIV Cohort, HIV reservoirs, HIV Cure, rapid autopsy, tissue organ procurement
Introduction
In the modern antiretroviral (ART) era, HIV replication can be halted, but latent reservoirs remain that are able to repopulate viremia should ART be interrupted [1]. To effect an HIV cure, it will be necessary to eradicate or significantly reduce these reservoirs-wherever they are in the body-before ART can be stopped definitively [2]. Better characterization of these reservoirs is needed before we can begin to plan their eradication. It is for this reason that the ‘Last Gift’ study was designed.
The Last Gift is an end-of-life HIV research study at the University of California San Diego (UCSD), with the goal of understanding the distribution of HIV throughout the human body [3,4]. The study collects detailed clinical data and biological samples from participants before death and then performs an autopsy soon after death.
The desire to perform the autopsy in a rapid manner grew out of a need to preserve the integrity of nucleic acids, proteins, and cellular physiology for further study. For more than a decade, rapid (also previously called ‘warm’) autopsies have been performed in cancer [5–8] and neurodegenerative disease [9] research. In these prior studies, extraordinary steps were taken to rapidly process select tissues of interest (typically within 2–6 h of death) for the purpose of preserving nucleic acid sequences [10]. We sought to replicate these efforts in a systematic way with a cohort of people with HIV (PWH) to further HIV research and lay the groundwork for developing a cure.
Community involvement and selection of participants
We began preparations for building this cohort by first discussing, on multiple occasions, our plans with members of the community. We found overwhelming support from the community of HIV/AIDS survivors - particularly because the study allowed participation of PWH who might otherwise be excluded from HIV/Cure research because of their age or comorbidities [11].
Our inclusion criteria for the study are: first, Age 18 years or older and HIV positive; second, expected survival 6 months or less as determined by their primary care provider; third, capacity to provide informed consent in English; and fourth, coenrolled in the California NeuroAIDS Tissue Network and willing to agree to body donation and autopsy at time of death. Recently, the criterion for survival less than 6 months has been expanded to include anyone with a 5-year mortality of greater than 50% who is willing to undergo a rapid autopsy after death. The requirement for English fluency is due to the many study personnel and study documents/questionnaires being English-only. Our resources are limited right now but we strongly believe enrolling non-English speaking people (and minorities in general) is an important future goal to increase diversity of Last Gift study participants.
Informed consent by the participant is obtained before participation in the study. The study team also asks participants to identify their ‘Next of Kin’ (NOK - often family either by choice or chance), who then play an integral role in the study as they are primarily responsible for notification of the study team at time of participant death.
Preparation for autopsy
The preparations made before autopsy are extensive, as many moving parts need to be in place for a rapid autopsy to be performed successfully. We have developed a system of prelabeled weigh boats and cryovials and work to position supplies in advance of any potential autopsy. For more details of this system and examples, see the Supplemental materials, http://links.lww.com/QAD/B713.
On-call autopsy team
We have assembled a team of biomedical research technicians as well as clinicians and other investigators interested in the study to perform the task of quickly and efficiently conducting a rapid autopsy with very short notice. For descriptions of responsibilities and numbers of team members performing each duty, see Table S2, http://links.lww.com/QAD/B713. The role of ‘cutters’ is usually filled by medically trained personnel. Our most fastidious and organized technician has the role of Rapid Autopsy Coordinator (RAC). We are also greatly assisted in our rapid autopsies by a dedicated diener who is specially trained to perform the routine steps of dissecting a body and identifying key tissues following standard, universal precautions.
In addition, we have a study coordinator who keeps in close contact with the participant and their NOK throughout the study. With permission of the participant, there is periodic contact with family and the participant to monitor their grieving process before death. In an effort to keep the Last Gift contact information and study details in the minds of all providers, phone calls are made to hospice and facility nursing staff. After death, the NOK, hospice, or facility informs the coordinator. The body is then transported to the autopsy suite at UCSD by a contracted, 24 h on-call transportation company.
During autopsy
As arrangements are made to transport the body to the morgue, the rapid autopsy team assembles at the morgue to prepare (Fig. 1). Recently, we have added a moment of silence to thank the participant and acknowledge their gift to research. This is done after the body arrives but before the first cut is made. It was adapted from similar rituals before recovering organs for transplantation and is read aloud as follows:
From our first breath to our last, each of us tells a unique story with our lives. Today, we honor our donor, LG ## for this opportunity to further research into HIV and so many other unanswered questions about the human condition. May we take a moment of silence now to honor their gift and express our gratitude for all the discoveries this altruistic act will yield.
Fig. 1. Schematic and photos of autopsy layout.
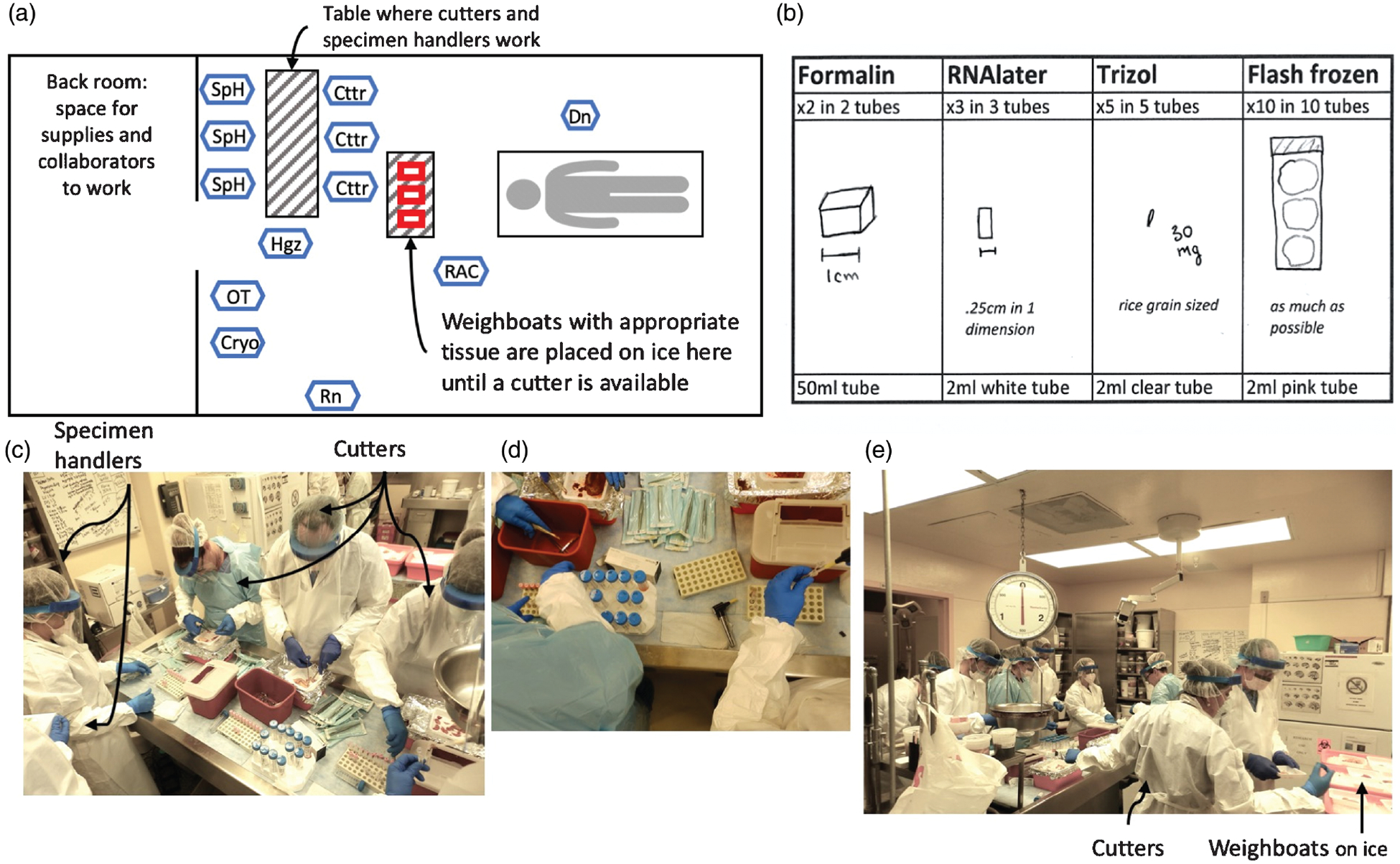
(a) Shows the general setup of the morgue during rapid autopsy. Personnel abbreviations (also given in Table S2, http://links.lww.com/QAD/B713) are as follows: Cryo, cryopreservation technician; Cttr, cutter; Dn, diener; Hgz, homegenizer; OT, optimal cutting temperature compound (OCT) technician; RAC, rapid autopsy coordinator; Rn, runner; SpH, specimen handler. (b) Shows the cutting guide used during autopsy. Tissue needs to be sectioned according to this guide for adequate penetration of preservative (e.g. RNAlater and Trizol) and tissue sectioning. Photos are of the cutting table, (c), close-up of cutting setup (d) and the general morgue setup (e).
When the body arrives, the diener begins to dissect and obtain tissues per the specifications on the weigh boats and list provided by RAC. The RAC places weigh boats on wet ice until a cutter is available to process the tissue.
The RAC and/or specimen handlers guide cutters as to how a tissue should be sectioned and/or how many pieces are required based on the weigh boat labels. After tissue is sectioned into the necessary tubes, the tubes are frozen via a dip in liquid nitrogen for 30 s and kept on dry ice until the end of the autopsy when they are moved to more permanent cold storage. The participant’s remains are cremated and the ashes are returned to the NOK or spread at sea per the donor’s wishes.
After autopsy
Immediately after an autopsy, samples are moved to permanent cold storage and collaborator samples are shipped out. Occasionally cryovials labels need to be corrected and storage locations updated. All samples are also scanned into a database for tracking.
We have found it beneficial to gather the team for a few moments of food and conversation to decompress and debrief the autopsy. With so many moving parts and occasional staff turnover, we try to stay open to constructive criticism and focus attention on any component of the rapid autopsy procedure that may not be functioning as well as it should.
Collaboration/providing tissues to outside researchers
From its inception, the study has had an objective of sharing these well characterized human tissues as widely as possible for HIV-related research. We encourage collaboration with other researchers and have already distributed tissues to nearly a dozen research teams (including collaborators at UCSD). To participate, researchers must submit concept sheets with specific requirements for tissue desired, handling requirements, what experiments are planned, and how that information will be disseminated. Thus far, 16 concept sheets have been submitted and we continue to work with groups to develop additional avenues for this important research.
Limitations
We have found that the greatest single barrier to completing the rapid autopsy protocol within the prescribed time window (i.e., <6 h) has been timely notification of the study team that a participant has passed. In the case of autopsy #4 (Table S4, http://links.lww.com/QAD/B713), study personnel were notified late and the autopsy was not able to begin until ~9 h after death, so it was simplified to obtain only flash-frozen tissue due to being outside the 6-h window. Certain situations seem to lend themselves to quick notification (e.g. inpatient hospice with around-the-clock nursing care, or attentive family members who are in regular contact with study personnel). In one case, we had a participant who elected to exercise his rights under California’s Health and Safety Code §443.1 End of Life Option Act [12] to take control over his disease and death (similar to [13]). This decision was made without input from the study team, but we were notified of his decision ~72 h before he died.
Conclusion
In our experience, we have been able to perform six rapid autopsies (see Table S4, http://links.lww.com/QAD/B713). Altogether, these have permitted the involvement of numerous collaborators and the successful storage of thousands of samples. We have been fortunate that the study enjoys widespread support from the community - both PWH and HIV-specialist providers. We believe the legacy of these precious tissues will be invaluable in contributing to understanding the reservoirs where HIV hides throughout the body and the eventual development of an HIV cure.
Our experience has taught us that this undertaking requires significant groundwork to develop the sufficient community involvement, a great deal of institutional and funding support for the research staff, and regular communication with the participants’ family members and facility staff/providers. The organ and tissue donation could not be completed without the incomparable altruism of the study participants and their loved ones. Still, we believe that this model of rapid autopsy for the preservation of important HIV-related tissue samples can be replicated wherever there is willingness to invest in creating it.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the support of the study participants and their friends and families as well as the many clinical staff who have been of assistance. The study is funded as part of P01 AI131385. The San Diego CFAR has provided immense clinical and technical support. S.A.R. was supported by the National Institutes of Health (grant number 5T32AI007384).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997; 278:1291–1295. [DOI] [PubMed] [Google Scholar]
- 2.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science 2009; 323:1304–1307. [DOI] [PubMed] [Google Scholar]
- 3.Gianella S, Taylor J, Brown TR, Kaytes A, Achim CL, Moore DJ, et al. Can research at the end of life be a useful tool to advance HIV cure? AIDS 2017; 31:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaillon A, Gianella S, Dellicour S, Rawlings SA, Schlub TE, Faria De Oliveira M, et al. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J Clin Invest 2020; 130:1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pentz RD, Cohen CB, Wicclair M, DeVita MA, Flamm AL, Youngner SJ, et al. Ethics guidelines for research with the recently dead. Nat Med 2005; 11:1145–1149. [DOI] [PubMed] [Google Scholar]
- 6.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ. Rapid (‘warm’) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res 2000; 6:1038–1045. [PubMed] [Google Scholar]
- 7.Quinn GP, Murphy D, Pratt C, Munoz-Antonia T, Guerra L, Schabath MB, et al. Altruism in terminal cancer patients and rapid tissue donation program: does the theory apply? Med Healthcare Philos 2013; 16:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duregon E, Schneider J, DeMarzo AM, Hooper JE. Rapid research autopsy is a stealthy but growing contributor to cancer research. Cancer 2019; 125:2915–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 2015; 35:354–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker DG, Whetzel AM, Serrano G, Sue LI, Lue LF, Beach TG. Characterization of RNA isolated from eighteen different human tissues: results from a rapid human autopsy program. Cell Tissue Bank 2016; 17:361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakash K, Gianella S, Dubé K, Taylor J, Lee G, Smith DM. Willingness to participate in HIV research at the end of life (EOL). PLoS One 2018; 13:e0199670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CALIFORNIA TPOTSO. Assembly Bill No. 15 2015 [12/1/2019] Available from: https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201520162AB15.
- 13.Sandstrom TS, Burke Schinkel SC, Angel JB. Medical assistance in death as a unique opportunity to advance human immunodeficiency virus cure research. Clin Infect Dis 2019; 69:1063–1067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


