Abstract
Objectives:
Evaluate the relationship between endothelial activation, malaria complications, and long-term cognitive outcomes in severe malaria survivors.
Design:
Prospectively cohort study of children with cerebral malaria, severe malarial anemia, or community children.
Setting:
Mulago National Referral Hospital in Kampala, Uganda.
Subjects:
Children 18 months to 12 years old with severe malaria (cerebral malaria, n = 253 or severe malarial anemia, n = 211) or community children (n = 206) were followed for 24 months.
Interventions:
None.
Measurements and Main Results:
Children underwent neurocognitive evaluation at enrollment (community children) or a week following hospital discharge (severe malaria) and 6, 12, and 24 months follow-up. Endothelial activation was assessed at admission on plasma samples (von Willebrand factor, angiopoietin-1 and angiopoietin-2, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1, soluble E-Selectin, and P-Selectin). False discovery rate was used to adjust for multiple comparisons. Severe malaria was associated with widespread endothelial activation compared with community children (p < 0.0001 for all markers). Acute kidney injury was independently associated with changes in von Willebrand factor, soluble intercellular adhesion molecule-1, soluble E-Selectin, P-Selectin, and angiopoietin-2 (p < 0.0001 for all). A log10 increase in angiopoietin-2 was associated with lower cognitive z scores across age groups (children < 5, β −0.42, 95% CI, −0.69 to −0.15, p = 0.002; children ≥ 5, β −0.39, 95% CI, −0.67 to −0.11, p = 0.007) independent of disease severity (coma, number of seizures, acute kidney injury) and sociodemographic factors. Angiopoietin-2 was associated with hemolysis (lactate dehydrogenase, total bilirubin) and inflammation (tumor necrosis factor-α, interleukin-10). In children with cerebral malaria who had a lumbar puncture performed, angiopoietin-2 was associated with blood-brain barrier dysfunction, and markers of neuroinflammation and injury in the cerebrospinal fluid (tumor necrosis factor-α, kynurenic acid, tau).
Conclusions:
These data support angiopoietin-2 as a measure of disease severity and a risk factor for long-term cognitive injury in children with severe malaria.
Keywords: child health, cognition, critical illness, kidney, malaria, vascular endothelium
Plasmodium falciparum is responsible for significant global morbidity and mortality. In 2017, an estimated 219 million malaria cases and 435,000 deaths were reported (1). Cerebral malaria (CM) and severe malarial anemia (SMA) are leading causes of severe malaria. Long-term deficits in cognition have been described in children following both CM and SMA (2–4). Risk factors for cognitive impairment following severe malaria are largely clinical (3, 5, 6). A number of cerebrospinal fluid (CSF) markers of neuroinflammation, neurotoxicity, and axonal injury have been described as risk factors for cognitive impairment following severe malaria (7–10). To date, no markers of cognitive impairment malaria have been described in blood.
As the cellular interface that lines blood and lymphatic vessels, the endothelium actively regulates blood flow to tissue beds and modulates coagulation, inflammation, and vascular permeability. Endothelial activation is a common feature in critical illness (reviewed in [11]). Endothelial activation contributes to severe malaria pathogenesis by upregulating cell adhesion molecules (CAMs) that mediate cytoadherence of parasitized erythrocytes in microvasculature, contributing to tissue hypoperfusion, ischemia, and metabolic acidosis (12), as well as blood-brain barrier dysfunction (13). As the endothelium directly interacts with parasitized erythrocytes and represents the cellular barrier between the parasite and the brain, we hypothesize endothelial activation and increased endothelial permeability may contribute to brain injury and impaired cognition in survivors.
Ectodomain shedding of CAMs rapidly changes a cells ability to respond to ligands through the release of surface-bound proteins. Cytokine-inducible CAMs are upregulated in malaria and shed by activated endothelium to generate soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), and soluble E-Selectin (sE-Selectin). Severe malaria is associated with elevations of surface-bound and soluble forms of CAMs (14). Activated endothelium also release the contents of specialized storage granules called Weibel-Palade bodies that contain proteins involved in hemostasis and inflammation. Weibel-Palade body constituents include von Willebrand factor (vWF), P-Selectin, and angiopoietin-2. vWF is elevated in severe malaria (15–17) and has been implicated in platelet-mediated binding of parasitized erythrocytes (18). P-Selectin is involved in leukocyte trafficking, elevated in severe malaria (19), and endothelial-derived P-Selectin has been implicated in experimental CM pathogenesis (20, 21). The angiopoietin-TEK tyrosine kinase (Tie-2) system regulates endothelial quiescence through angiopoietin-1 signaling through its receptor, Tie-2, expressed on endothelium. Angiopoietin-2 release results in reduced Tie-2 phosphorylation (22), and disrupted angiopoietin-1-Tie-2 signaling that can destabilize blood vessels and increase vascular permeability (23). Severe malaria is characterized by decreased angiopoietin-1 and increased angiopoietin-2 and angiopoietin-2:angiopoietin-1 ratio (24–27).
We hypothesize endothelial activation is central to the pathophysiology of severe malaria and a risk factor for long-term cognitive deficits in children. We evaluated a panel of previously described markers of endothelial activation in malaria in a prospective cohort study assessing the relationship between endothelial activation and severe malaria complications, including acute kidney injury (AKI), which is a complication of emerging importance in children with severe malaria (28–31). Our primary outcome was the relationship between endothelial activation and cognition over 2 years follow-up using tools validated for use in Ugandan children.
MATERIALS AND METHODS
Study Design
This prospective cohort study was conducted at Mulago National Referral and Teaching Hospital, Kampala, Uganda, from 2008 to 2013. All eligible children between 18 months and 12 years old were enrolled. Two groups of children with severe malaria were enrolled: children with CM and SMA. CM was defined as follows: coma (Blantyre Coma Score ≤ 2); P. falciparum on blood smear; and no known cause of coma. SMA was defined as the presence of P. falciparum on blood smear in children with a hemoglobin level less than or equal to 5 g/dL. Children with CM and SMA were classified as CM. Children with severe malaria were managed according to the Ugandan Ministry of Health treatment guidelines at the time of the study.
Healthy community children (CC) were recruited from the nuclear family, extended family, or household compound area of children with severe malaria. Exclusion criteria for all children were as follows: known chronic illness requiring medical care; known developmental delay; or prior history of coma, head trauma, hospitalization for malnutrition, or cerebral palsy. Additional exclusion criteria for children with SMA were as follows: impaired consciousness on physical examination; other clinical evidence of CNS disease; or greater than 1 seizure prior to admission. Additional exclusion criteria for CC included the following: illness requiring medical care within the previous 4 weeks or major medical or neurologic abnormalities on screening physical examination.
Ethical approval for the study was obtained from Makerere University School of Medicine Research and Ethics Committee, the Institutional Review Board at the University of Minnesota, and the Uganda National Council for Science and Technology. Written informed consent was obtained from parents or guardians of study participants.
Clinical and Demographic Assessment
All children underwent a medical history, physical examination, and laboratory testing. Emotional stimulation in the home was measured using age-appropriate versions of the Home Observation for the Measurement of the Environment (32). Socioeconomic status was measured as described (33).
Cognitive Assessment and Follow-Up
Cognitive assessments were conducted at enrollment (CC) or a week after discharge (CM and SMA) and at 6, 12, and 24 months follow-up by trained neuropsychology testers with a Bachelor’s degree in psychology. Testing was conducted in quiet testing rooms at Mulago National Referral Hospital. The Mullen Scales of Early Learning was used to measure cognitive ability in children less than 5 years (34). Scores from fine motor, visual reception, receptive language, and expressive language scales were summed to generate the early learning composite score as a measure of overall cognitive ability. In children greater than or equal to 5 years, the Kaufman Assessment Battery for Children, Second Edition was used to measure overall cognitive ability (35). Neuropsychology testers were blinded to study group. Age-adjusted z scores were created using the CC as described (4).
Laboratory Testing
After obtaining consent from parents or guardians for participation in the study, a blood draw was performed for study specific procedures. Within 2 hours of sample collection, blood samples were sent to the performing laboratory for processing and storage. If a sample was collected after hours, the sample was stored at 4–8°C until the following morning when it was processed and stored in aliquots at −80°C until testing. Giemsa stained peripheral blood smears were assessed for Plasmodium species and quantified using standard protocols. Biochemistries were performed for lactate, glucose, lactate dehydrogenase (LDH), creatinine, blood urea nitrogen (BUN), and albumin by the Advanced Research & Diagnostic Laboratory at the University of Minnesota. Sickle cell genotype was determined as described (36). To assess for bacteremia, approximately 1–3 mL of whole blood was inoculated into pediatric blood culture bottles (BD BACTEC, Peds Plus/F, Franklin Lakes, NJ), which were incubated in an automatic BACTEC 9050 Blood culture system (Becton, Dickinson and Company, Franklin Lakes, NJ) for up to 5 days. Positive samples were Gram-stained and subcultured on blood agar, chocolate agar, or MacConkey agar plates.
Immunoassays
EDTA or lithium heparin anti-coagulated plasma samples were stored at −80°C until testing. Samples with sufficient volume were tested using commercial assays with 10% of samples tested in duplicate. There were 191 samples unavailable for testing. Comparisons of participants included versus not included are shown in Table S1 (Supplemental Digital Content 1, http://links.lww.com/CCM/F598). vWF antigen was measured using the REAADS vWF:Antigen enzyme-linked immunosorbent assay (ELISA) (Corgenix, Broomfield, CO) and expressed as a relative percent of reference plasma. Angiopoietin-1 and angiopoietin-2 were measured using a DuoSet ELISA (R&D Systems, Minneapolis, MN). Soluble adhesion molecules sICAM-1, sVCAM-1, P-Selectin, and sE-Selectin were measured by cytometric bead assay (R&D Systems) with a Bio-Plex-200 system (Bio-Rad, Hercules, CA). Plasma P. falciparum histidine-rich protein-2 quantification was performed using the Malaria Antigen CELISA (Cellabs, Brookvale, NSW, Australia), and parasite biomass was calculated as described (37). Cytokines were measured in plasma and CSF using by magnetic cytometric bead assay (EMD Millipore, Billerica, MA) (8). Kynurenic acid was measured as described (9). CSF tau was measured using the Luminex-based human tau (total) Singleplex Bead Kit (Invitrogen, Carlsbad, CA). Asymmetric dimethylarginine (ADMA) was measured by ELISA (DLD Diagnostika GmbH, Hamburg, Germany). CSF albumin was quantified using the Bromocresol Purple Albumin Assay (Sigma-Aldrich, St. Louis, MO).
Assessment of Kidney Function
AKI was defined using a single admission creatinine using the Kidney Disease: Improving Global Outcomes criteria where a 1.5× increase in creatinine from baseline constituted AKI (38). AKI was further staged based on the fold increase in creatinine: stage 1, 1.5–1.9×; stage 2, 2.0–2.9×; stage 3, greater than or equal to 3.0× or an estimated glomerular filtration rate (eGFR) of less than 35 mL/min/1.73 m2 using the Bedside Schwartz equation (eGFR = 0.413 × creatinine/height, where height is measured in cm) (39). Baseline creatinine was estimated using the CC to generate a creatinine-for-height curve as described (30).
Statistical Analysis
Demographic and clinical characteristics were compared using Student t test or Wilcoxon rank-sum test for continuous measures, and Pearson chi-square test or Fisher exact test for categorical variables. A nonparametric test for trend was used to assess median differences in biomarker levels across AKI stages. To assess correlations between continuous variables, Spearman correlation was used. Multiple linear regression models were used to evaluate the relationship between severe malaria criteria and endothelial activation. Longitudinal mixed-effects modeling was used to assess the association of endothelial markers on cognition in children with severe malaria where observations within subject were correlated using a subject-specific intercept, and time points were treated as categorical variables as described (10). We adjusted for disease severity (presence of coma, number of seizures during hospitalization, AKI) and sociodemographic variables associated with child development (age, sex, height-for-age and weight-for-age z scores, socioeconomic status, home environment, parental education, and preschool education of the child). Variables were selected for inclusion into multivariable models based on a documented relationship between the variables and outcome, or an a priori hypothesized relationship. To adjust for multiple comparisons, the Benjamini-Hochberg false discovery rate was applied at a threshold of 0.05 as indicated in figure and table legends.
RESULTS
Demographic Characteristics of the Study Population
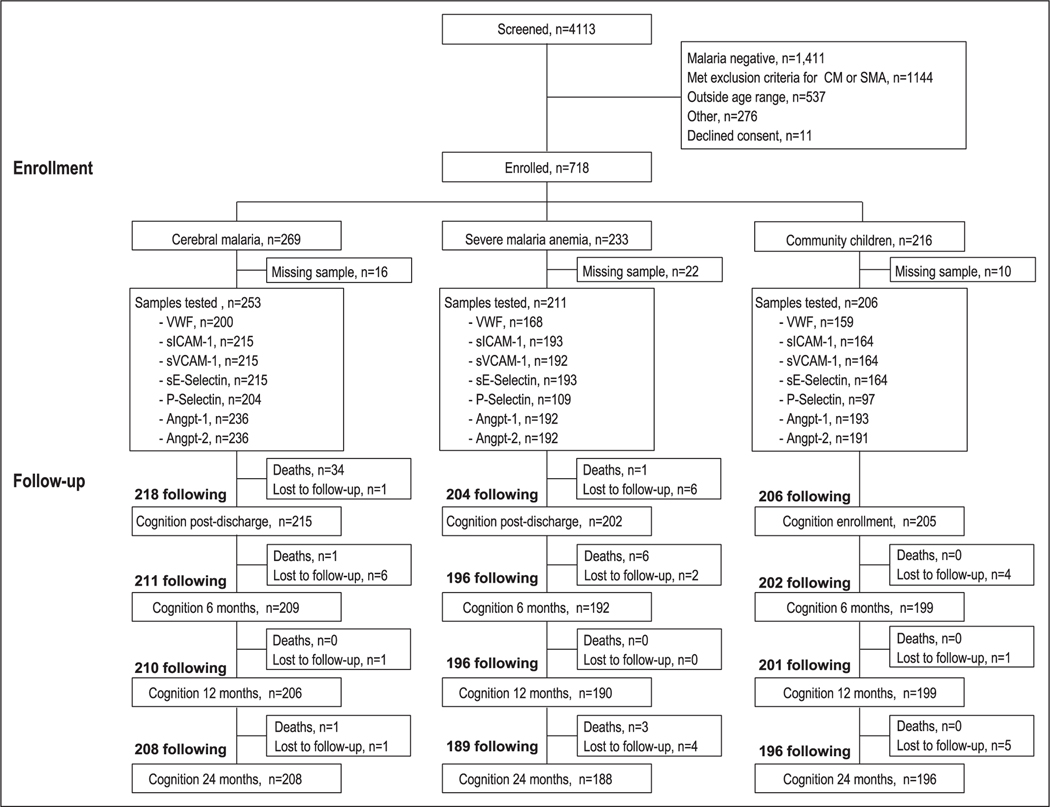
A description of the samples available for testing is shown in Figure 1. A total of 3,881 children with severe malaria were screened for the study. There were 191 samples unavailable for testing. Comparisons of participant participants included versus not included are shown in Table S1 (Supplemental Digital Content 1, http://links.lww.com/CCM/F598). The median age of children enrolled was 3.8 years old and 44.0% of children were female. Children with SMA were younger than children with CM or CC and were more likely to have sickle cell anemia than the CC or children with CM (Table 1). Among CC, the prevalence of asymptomatic parasitemia was 15.4% by microscopy and 35.3% by polymerase chain reaction (PCR). Asymptomatic malaria in CC defined by microscopy or PCR was associated with elevations in vWF, sICAM-1, sVCAM-1, and P-Selectin compared with CC without malaria (Table S2, Supplemental Digital Content 1, http://links.lww.com/CCM/F598). There were no differences in the angiopoietin-2-Tie-2 system or sE-Selectin in CC with asymptomatic malaria.
Figure 1.
Study flow chart. Flow chart of study population. Angpt-1 = angiopoietin-1, Angpt-2 = angiopoietin-2, CM = cerebral malaria, sE-Selectin = soluble E-Selectin, sICAM-1 = soluble intercellular adhesion molecule-1, SMA = severe malaria anemia, sVCAM-1 = soluble vascular cell adhesion molecule-1, vWF = von Willebrand factor.
TABLE 1.
Demographic Characteristics of Children With Cerebral Malaria, Severe Malarial Anemia, and Community Children
| Severe Malaria | P | |||||
|---|---|---|---|---|---|---|
| Characteristic | CC (n = 2 06) | CM (n = 253) | SMA (n = 211) | Combined (n = 464) | CC vs SM | CM vs SMA |
| Demographics | ||||||
| Age (yr) | 3.7 (2.7–4.8) | 3.5 (2.5–4.9) | 2.8 (2.1–4.5) | 3.3 (2.2–4.6) | 0.004 | 0.0003 |
| Sex (female) | 107 (51.9) | 105 (41.5) | 83 (39.3) | 188 (40.5) | 0.006 | 0.636 |
| Weight-for-age z score | −0.9 (−1.5 to −0.2) | −1.2 (−1.9 to −0.5) | −1.5 (−2.2 to −0.7) | −1.3 (−2.0 to −0.6) | < 0.0001 | 0.024 |
| Height-for-age z score | −1.1 (−1.7 to −0.3) | −0.8 (−1.5 to 0.2) | −1.0 (−1.9 to −0.3) | −0.9 (−1.7 to −0.1) | 0.153 | 0.004 |
| HIV infected | 2 (1.0) | 5 (2.2) | 5 (2.4) | 10 (2.3) | 0.255 | 0.856 |
| Sickle cell genotype | < 0.0001 | < 0.0001 | ||||
| HbAA | 168 (81.6) | 248 (98.8) | 188 (89.5) | 436 (94.6) | ||
| HbAS | 38 (18.5) | 2 (0.8) | 2 (1.0) | 4 (0.9) | ||
| HbSS | 0 (0.0) | 1 (0.4) | 20 (9.5) | 21 (4.6) | ||
| Laboratory findings | ||||||
| WBC | 8.9 (7.3–10.7) | 9.4 (7.2–14.0) | 11.2 (8.3–16.0) | 10.4 (7.6–15.0) | < 0.0001 | 0.0002 |
| Platelet | 376 (282–448) | 61 (34–109) | 149 (93–224) | 96 (49–169) | < 0.0001 | < 0.0001 |
| Lactate | Not measured | 3.7 (2.0–6.6) | 4.7 (2.8–77) | 4.1 (2.4–7.1) | Not measured | 0.010 |
| Glucose | Not measured | 6.4 (4.9–8.9) | 6.3 (4.7–8.0) | 6.4 (4.8–8.5) | Not measured | 0.148 |
| Hemoglobin | 11.9 (11.0–12.5) | 6.6 (5.1–8.6) | 3.9 (3.2–4.5) | 4.8 (3.7–7.0) | < 0.0001 | < 0.0001 |
| Positive blood culture | Not done | 18 (7.5) | 12 (6.4) | 30 (7.0) | Not done | 0.679 |
| Severe malaria complications on admission | ||||||
| Prostration | Not applicable | 253 (100.00) | 91 (43.1) | 344 (74.1) | Not applicable | < 0.0001 |
| Coma | Not applicable | 253 (100.00 | 0 (0.0) | 253 (54.5) | Not applicable | < 0.0001 |
| Repeated convulsions | Not applicable | 159 (62.9) | 1 (0.5) | 160 (34.5) | Not applicable | < 0.0001 |
| Deep breathing | Not applicable | 22 (8.7) | 12 (5.7) | 34 (7.3) | Not applicable | 0.216 |
| Acute kidney injury | Not applicable | 109 (44.5) | 53 (26.9) | 162 (36.7) | Not applicable | < 0.0001 |
| Jaundice | Not applicable | 130 (51.4) | 157 (74.4) | 287 (61.9) | Not applicable | < 0.0001 |
| Shock | Not applicable | 1 (0.4) | 0 (0.0) | 1 (0.2) | Not applicable | 1.000 |
| Severe anemia | Not applicable | 57 (22.5) | 211 (100.0) | 268 (57.8) | Not applicable | < 0.0001 |
| Lactic acidosis | Not applicable | 82 (35.0) | 86 (44.1) | 168 (39.2) | Not applicable | 0.056 |
| Hypoglycemia | Not applicable | 18 (7.2) | 9 (4.5) | 27 (6.0) | Not applicable | 0.230 |
| Abnormal bleeding | Not applicable | 8 (3.2) | 1 (0.5) | 9 (1.9) | Not applicable | 0.037 |
| Hyperparasitemia | Not applicable | 38 (15.1) | 15 (71) | 53 (11.5) | Not applicable | 0.007 |
| Number of severe malaria criteria | Not applicable | 4 (3–5) | 3 (2–4) | 4 (3–5) | Not applicable | < 0.0001 |
| Outcomes | ||||||
| In-hospital deaths | Not applicable | 34 (13.4) | 1 (0.5) | 35 (7.6) | Not applicable | < 0.0001 |
| Post-discharge deaths | 0 (0.0) | 2 (0.9) | 9 (4.3) | 11 (2.6) | 0.020 | 0.033 |
| Lost to follow-up | 10 (4.9) | 9 (3.6) | 12 (5.7) | 21 (4.5) | 0.852 | 0.272 |
| Endothelial activation markers | ||||||
| von Willebrand factor | 47.6 (23.0–79.2) | 173.3 (99.6–281.7) | 128.9 (65.3–233.9) | 152.9 (87.0–261.1) | < 0.0001 | 0.002a |
| Soluble intercellular adhesion molecule-1 | 169.4 (74.6–658.2) | 729.2 (242.8–1,557) | 665.1 (266.6–1,357) | 696.5 (253.3–1,437) | < 0.0001 | 0.51 |
| Soluble vascular cell adhesion molecule-1 | 1,088 (796–1,529) | 3,826 (2,715–6,315) | 3,255 (2,529–4,821) | 3,519 (2,574–5,384) | < 0.0001 | 0.006a |
| P-Selectin | 41.2 (30.0–52.1) | 54.2 (38.3–78.0) | 47.9 (35.7–66.6) | 51.6 (37.0–73.3) | < 0.0001 | 0.08 |
| Soluble E-Selectin | 86.1 (65.1–109.9) | 186.9 (142.2–249.2) | 189.9 (132.6–256.9) | 188.3 (135.9–250.9) | < 0.0001 | 0.86 |
| Angiopoietin-1 | 11.4 (5.9–15.9) | 2.9 (1.5–5.6) | 4.3 (2.0–77) | 3.5 (1.7–6.5) | < 0.0001 | 0.0007a |
| Angiopoietin-2 | 0.3 (0.1–0.6) | 1.9 (1.0–3.3) | 1.8 (1.0–3.2) | 1.80 (0.99–3.29) | < 0.0001 | 0.80 |
| Angiopoietin-2:angiopoietin-1 | 0.03 (0.01–0.07) | 0.61 (0.28–1.62) | 0.39 (0.17–1.29) | 0.51 (0.23–1.48) | < 0.0001 | 0.009a |
CC = community children, CM = cerebral malaria, SMA = severe malaria anemia.
Relationships significant following adjustment for multiple comparisons.
Data presented as median (interquartile range) or n (%).
Data analyzed using Wilcoxon rank-sum test for continuous measures or Pearson χ2 test or Fisher exact test, as appropriate, for categorical variables.
Children with severe anemia and CM were classified as having CM.
Severe malaria refers to children with CM or SMA.
Association of Endothelial Activation With Severe Malaria and Disease Severity
Consistent with previous reports, there was evidence of endothelial activation in CM and SMA compared with CC (adjusted p < 0.05 for all) (Table S1, Supplemental Digital Content 1, http://links.lww.com/CCM/F598). Further, vWF and sVCAM-1 levels were higher and angiopoietin-1 levels were lower in CM compared with SMA (Table 1). There were no differences in markers of endothelial activation in children with bacteremia compared with children without bacteremia (Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/F598)
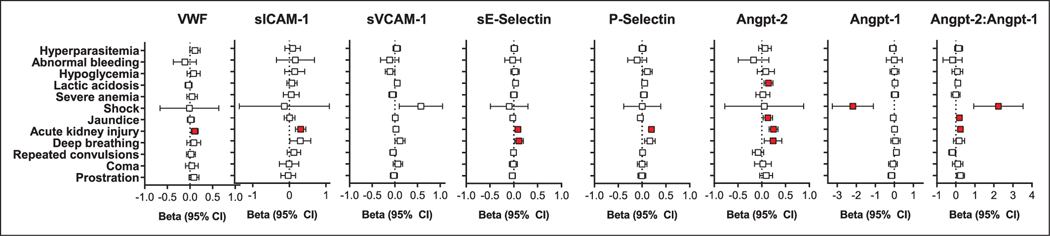
As there is considerable phenotypic heterogeneity in endothelium, and the extracellular environment may drive specific changes in protein expression, we evaluated the relationship between severe malaria complications and endothelial activation (Fig. 2). For subsequent analyses, children with CM and SMA were grouped to evaluate the relationships between endothelial activation and disease severity as the combined severe malaria group is more representative of severe malaria (Table 1). Severe malaria complications associated with endothelial activation were as follows: AKI (vWF, sICAM-1, P-Selectin, sE-Selectin, angiopoietin-2, angiopoietin-2:angiopoietin-1); deep breathing (sE-Selectin, angiopoietin-2); lactic acidosis (angiopoietin-2); jaundice (angiopoietin-2, angiopoietin-2:angiopoietin-1); and shock (angiopoietin-1, angiopoietin-2:angiopoietin-1) (adjusted p < 0.05 for all; Fig. 2), using multiple linear regression models (Table 1 for severe malaria frequency by group).
Figure 2.
Association between endothelial activation and severe malaria criteria. Plots showing the regression coefficient (95% CI) for each severe malaria complication and its association with a log10 change in the endothelial marker. Analyses were conducted using linear regression models, with false discovery rate applied at a threshold of 0.05 adjusting for eight regression models. Relationships significant following adjustments for multiple testing are indicated in red. Angpt-1 = angiopoietin-1, Angpt-2 = angiopoietin-2, sE-Selectin = soluble E-Selectin, sICAM-1 = soluble intercellular adhesion molecule-1, sVCAM-1 = soluble vascular cell adhesion molecule-1, vWF = von Willebrand factor.
Endothelial activation in malaria-associated AKI has not been well described. We evaluated the relationship between endothelial activation and AKI severity and observed increases in vWF, sICAM-1, P-Selectin, sE-Selectin, and angiopoietin-2 across AKI stage (adjusted ptrend < 0.05; Table 2).
TABLE 2.
Trend in Endothelial Activation Across Stages of Acute Kidney Injury in Children With Severe Malaria
| Biomarkers | No AKI (n = 280) | AKI Stage 1 (n = 93) | AKI Stage 2 (n = 46) | AKI Stage 3 (n = 23) | ptrend |
|---|---|---|---|---|---|
| von Willebrand factor | 126.0 (70.7–242.9) | 164.7 (99.8–277.1) | 210.6 (147.9–318.5) | 247.4 (152.0–412.9) | < 0.001a |
| Soluble intercellular adhesion molecule-1 | 406.0 (207.1–1,073.9 | 1,236.5 (314.9–1,858.0) | 1,444.2 (632.9–1,872.1) | 1,541.0 (935.4–2,181.6) | < 0.001a |
| Soluble vascular cell adhesion molecule-1 | 3,519 (2,550–5,519) | 3,822 (2,692–6,278) | 3,417 (2,606–4,861) | 4,034 (2,795–5,851) | 0.266 |
| P-Selectin | 42.9 (32.9–61.0) | 70.4 (47.4–86.3) | 68.0 (53.1–86.4) | 83.5 (62.1–150.8) | < 0.001a |
| Soluble E-Selectin | 173.3 (124.7–241.2) | 211.8 (164.8–275.6) | 216.3 (165.9–277.4) | 250.1 (155.4–289.7) | < 0.001a |
| Angiopoietin-1 | 3.5 (1.6–6.9) | 3.0 (1.8–6.2) | 4.3 (2.1–7.3) | 3.7 (2.4–5.4) | 0.864 |
| Angiopoietin-2 | 1.5 (0.8–2.5) | 2.1 (1.5–3.7) | 2.9 (1.8–4.4) | 6.2 (2.7–9.1) | < 0.001a |
| Angiopoietin-2:angiopoietin-1 | 0.41 (0.18–1.11) | 0.73 (0.29–1.50) | 0.80 (0.22–2.55) | 2.07 (0.94–2.36) | < 0.001a |
AKI = acute kidney injury.
Significant tests for trend following adjustment for multiple comparisons.
Data presented as median (interquartile range). AKI defined and staged using Kidney Disease: Improving Global Outcomes criteria. Data analyzed using a nonparametric trend across AKI stage. False discovery rate was applied at a threshold of 0.05 adjusting for eight comparisons.
Association Between Endothelial Activation and Mortality
We assessed the association between endothelial activation and mortality (Table S4, Supplemental Digital Content 1, http://links.lww.com/CCM/F598). Consistent with previous studies, there were increases in median angiopoietin-2 (p = 0.038), angiopoietin-2:angiopoietin-1 (p = 0.013), and P-Selectin (p = 0.016) levels in children who died compared with survivors. When the analysis was restricted to CM (accounting for 97.1% of deaths), angiopoietin-2 (p = 0.016), and angiopoietin-2:angiopoietin-1 (p = 0.023) remained associated with mortality.
Relationship Between Endothelial Activation and Cognition in Survivors
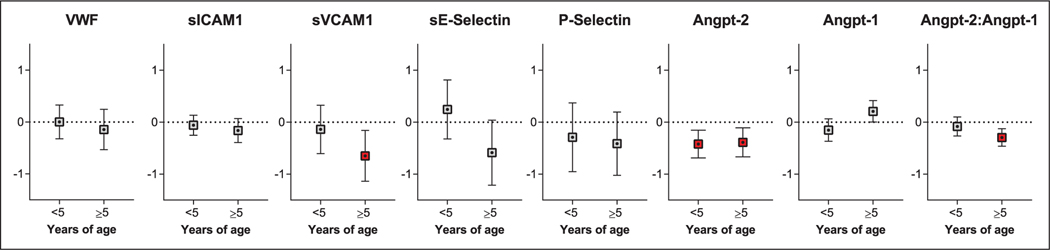
To determine whether endothelial activation is related to cognition, we evaluated whether admission protein levels were associated with age-adjusted cognitive z scores over time (Fig. 3; Table S5, Supplemental Digital Content 1, http://links.lww.com/CCM/F598) adjusting for disease severity (presence of coma, number of seizures during hospitalization, AKI) and socioeconomic and demographic factors associated with child development (age, sex, weight-for-age and height-for-age z scores, socioeconomic status, home environment, parental education, and preschool education). A log10 increase in angiopoietin-2 was associated with lower cognitive z scores across age groups (children < 5, β −0.42, 95% CI, −0.69 to −0.15; children ≥ 5, beta −0.39, 95% CI, −0.67 to −0.11) (Fig. 3; Table S5, Supplemental Digital Content 1, http://links.lww.com/CCM/F598). In children greater than 5 years old a log10 increase in sVCAM-1 (β −0.65, 95% CI, −1.14 to –0.16) and the angiopoietin-2:angiopoietin-1 ratio (β −0.29, 95% CI, −0.46 to −0.12) were associated with worse cognition (Fig. 3; Table S5, Supplemental Digital Content 1, http://links.lww.com/CCM/F598).
Figure 3.
Estimates from linear mixed-effects modeling evaluating endothelial activation and longitudinal age-adjusted z scores in cognition by age group. Estimates (95% CI) from linear mixed-effects models evaluating longitudinal changes in age-adjusted z scores in children with severe malaria based on the log10 concentrations in endothelial markers at admission. All models adjusted for disease severity at presentation (presence of coma, number of seizures during hospitalization, acute kidney injury) and sociodemographic factors (age, sex, weight-for-age and height-for-age z scores, socioeconomic status, home environment, parental education, and preschool attendance). The false discovery rate was applied at a threshold of 0.05 adjusting for eight comparisons in each age group. Relationships significant following adjustment for multiple testing are indicated in red. Angpt-1 = angiopoietin-1, Angpt-2 = angiopoietin-2, sE-Selectin = soluble E-Selectin, sICAM-1 = soluble intercellular adhesion molecule-1, sVCAM-1 = soluble vascular cell adhesion molecule-1, vWF = von Willebrand factor.
Relationship Between Endothelial Activation, Parasite Burden and Host Response
We explored the relationship between endothelial activation and severe malaria pathogenesis by correlating endothelial markers with parasite biomass, biochemical markers of disease severity (lactate, LDH, total bilirubin, creatinine, BUN), inflammation (tumor necrosis factor [TNF]-α, interleukin [IL]-10, IL-4), nitric oxide bioavailability (ADMA), and blood-brain barrier function (CSF-to-plasma albumin ratio) (Table S6, Supplemental Digital Content 1, http://links.lww.com/CCM/F598). We present results significant after adjusting for multiple comparisons (adjusted p < 0.05).
Consistent with regression analyses, angiopoietin-1 was negatively associated with both sequestered (rho, −0.223) and circulating parasite biomass (rho, −0.137). Endothelial mediators associated with deep breathing also correlated with lactate (sICAM-1: rho, 0.166; sVCAM-1: rho, 0.110; P-Selectin: rho, 0.259; sE-Selectin: rho, 0.202; and angiopoietin-2: rho, 0.339). Further, we observed a positive correlation between all markers and LDH except angiopoietin-1, where angiopoietin-1 was negatively correlated with LDH. All markers were positively correlated with TNF-α and IL-10, except for angiopoietin-1, which was negatively correlated. IL-4 was negatively correlated with vWF, sICAM-1, P-Selectin, sE-Selectin, and angiopoietin-2:angiopoietin-1 and positively correlated with sVCAM-1 and angiopoietin-1, consistent with IL-4 mediated expression of sVCAM-1 (40). All proteins except for vWF and sVCAM-1 were positively correlated with ADMA. Only angiopoietin-2 (rho, 0.217) and the angiopoietin-2:angiopoietin-1 ratio (rho, 270) were associated with an increase in the CSF-to-plasma albumin index.
To explore possible mechanisms between endothelial activation and worse cognition, we correlated markers of endothelial activation with selected CSF markers (TNF-α, kynurenic acid, and tau) associated with worse neurocognitive outcomes in CM (8–10) (Table S6, Supplemental Digital Content 1, http://links.lww.com/CCM/F598). We observed positive correlations between plasma sVCAM-1 (rho, 0.331) and angiopoietin-2 (rho, 0.254) and CSF TNF-α, and correlations between a number of endothelial markers with CSF kynurenic acid (sICAM-1: rho, 0.283; sVCAM-1: rho, 0.310; P-Selectin: rho, 0.493; sE-Selectin: rho, 0.301; angiopoietin-2: rho, 0.531; and angiopoietin-2:angiopoietin-1: rho, 0.425) and CSF tau (P-Selectin: rho, 0.226; angiopoietin-2: rho, 0.413; and angiopoietin-2:angiopoietin-1: rho, 0.231).
DISCUSSION
In this report, we show endothelial activation in severe malaria is associated with parasite burden, inflammation and hemolysis, and reduced nitric oxide bioavailability. Further, angiopoietin-2 and the angiopoietin-2:angiopoietin-1 ratio were associated with blood-brain barrier dysfunction, elevated neuroactive metabolites in the CSF, and worse cognition. Collectively, the data in this study support the hypothesis that endothelial activation is central to severe malaria pathophysiology and related to long-term morbidity in survivors.
Angiopoietin-2 is the first plasma marker to show a relationship with worse cognitive function (Fig. 3). Angiopoietin-2 is also the first marker associated with cognitive injury in both younger (< 5 yr old) and older children (≥ 5 yr old). In this cohort, the angiopoietin-2-Tie-2 pathway and sVCAM-1 were associated with worse cognition, strengthening the argument that endothelial activation and loss of endothelial integrity is a critical process in neurodevelopmental injury in severe malaria. Consistent with this, the correlations observed between angiopoietin-2 and TNF-α (8), kynurenic acid (9, 41), and tau (10, 13, 42) in the CSF are consistent with our current knowledge of neurodevelopmental injury in severe malaria survivors. Together, these results and preclinical data suggest strategies to promote endothelial stability through Tie-2 receptor may be neuroprotective in severe malaria (43).
Angiopoietin-2 and P-Selectin are both released from Weibel-Palade bodies and showed similar trends for many outcomes. They were both associated with the presence and severity of AKI, elevated in children who died, and showed similar correlations with lactate, LDH, TNF-α, IL-10, and nitric oxide bioavailability (ADMA). However, a few key differences between angiopoietin-2 and P-Selectin were noted. Angiopoietin-2 was associated with sequestered parasite biomass, while P-Selectin was not. Further, angiopoietin-2 was associated with increases in the CSF-to-plasma albumin index and an independent risk Factor for worse cognition. These differences may be explained by differential trafficking of proteins in Weibel-Palade bodies (44), or the cellular source. P-Selectin is expressed by both endothelium and platelets, so differences between angiopoietin-2 and P-Selectin may reflect differential contributions of platelets and endothelium to P-Selectin release.
The present study also highlights an association between endothelial activation and AKI (30). The kidney is highly vascularized and sensitive to reductions in blood flow (45). Although kidneys represent less than 0.08% of body mass, they receive 20% of cardiac output. The majority of children in this study (93.3%) had a BUN-to-creatinine ratio suggestive of reduced renal blood flow. Our results are consistent with a study of adults with Plasmodium knowlesi malaria where angiopoietin-2 was identified as an independent predictor of AKI (46). P. knowlesi shares pathophysiologic features with P. falciparum including reduced nitric oxide bioavailability, increased intravascular hemolysis, endothelial activation, and inflammation. In mice, administration of exogenous angiopoietin-1 (cartilage oligomeric matrix protein-Ang-1) has been shown to decrease lipopolysaccharide-induced AKI (47), suggesting strategies to promote endothelial stability may have broad clinical impact.
This study had several strengths including its prospective design and longitudinal assessment of cognitive outcomes over two years follow-up. The CC were used to generate age-adjusted z scores for cognition. As the study was designed to assess cognition, we measured sociodemographic factors known to impact child development and used cognitive assessment tools validated in Ugandan children.
Study limitations include enrollment of children with coma and severe anemia that may affect generalizability. The children in this cohort presented with multiple severe malaria criteria, but the utility of these biomarkers to predict neurocognitive impairment or mortality in other manifestations of severe malaria needs to be confirmed. Urine was not collected, so we cannot comment on whether changes in AKI reflect increased production and/or altered renal clearance of analytes. However, previous studies have demonstrated angiopoietin-2 is not detectable in the urine of healthy subjects or cleared by dialysis (48), suggesting reduced glomerular filtration in AKI is not the only explanation for increases in protein levels.
In summary, angiopoietin-2 is a novel risk factor for worse cognition in children surviving severe malaria. This is the first report of a plasma marker associated with worse cognition and adds to a growing body of evidence supporting the angiopoietin-Tie-2 system in the pathogenesis of critical illness. Together, these results show an association between endothelial activation, blood-brain barrier dysfunction, and markers of brain parenchymal injury suggesting that strategies to promote endothelial integrity warrant further investigation to see if they promote survival and reduce long-term neurocognitive injury in malaria.
Supplementary Material
ACKNOWLEDGMENTS
We thank the children and their parents who participated in the study, and the study team for their dedicated effort in treating the children and collecting the data.
This work was supported by the National Institute of Neurological Disorders and Stroke and the Fogarty International Center (grants R01NS055349 and D43 NS078280).
Drs. Ouma, Ssenkusu, Shabani, Datta, Opoka, Idro, Bangirana, Park, John, and Conroy received support for article research from the National Institutes of Health. Dr. Kain’s institution received funding from Canadian Institutes of Health Research (CIHR), and he received support for article research from the CIHR. Dr. John’s institution received funding from National Institute of Neurological Disorders and Stroke and Fogarty International Center. Drs. Kain and Conroy are listed as inventors on patents (held by University Health Network) involving the use of angiopoietin-1 and angiopoietin-2 as prognostic biomarkers in critical illness. Dr. Joloba has disclosed that he does not have any potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.World Health Organization: World Malaria Report 2018. Geneva, Switzerland, World Health Organization, 2018 [Google Scholar]
- 2.Boivin MJ, Bangirana P, Byarugaba J, et al. : Cognitive impairment after cerebral malaria in children: A prospective study. Pediatrics 2007; 119:e360–e366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John CC, Bangirana P, Byarugaba J, et al. : Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 2008; 122:e92–e99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangirana P, Opoka RO, Boivin MJ, et al. : Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis 2014; 59:336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Idro R, Carter JA, Fegan G, et al. : Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child 2006; 91:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holding PA, Stevenson J, Peshu N, et al. : Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg 1999; 93:529–534 [DOI] [PubMed] [Google Scholar]
- 7.John CC, Panoskaltsis-Mortari A, Opoka RO, et al. : Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am J Trop Med Hyg 2008; 78:198–205 [PMC free article] [PubMed] [Google Scholar]
- 8.Shabani E, Ouma BJ, Idro R, et al. : Elevated cerebrospinal fluid tumour necrosis factor is associated with acute and long-term neurocognitive impairment in cerebral malaria. Parasite Immunol 2017; 39: doi: 10.1111/pim.12438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmberg D, Franzén-Röhl E, Idro R, et al. : Cerebrospinal fluid kynurenine and kynurenic acid concentrations are associated with coma duration and long-term neurocognitive impairment in Ugandan children with cerebral malaria. Malar J 2017; 16:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta D, Conroy AL, Castelluccio PF, et al. : Elevated cerebrospinal fluid tau protein concentrations on admission are associated with long-term neurologic and cognitive impairment in Ugandan children with cerebral malaria. Clin Infect Dis 2020; 70:1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce RW, Giuliano JS Jr, Pober JS: Endothelial cell function and dysfunction in critically ill children. Pediatrics 2017; 140:e20170355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beare NA, Harding SP, Taylor TE, et al. : Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis 2009; 199:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medana IM, Lindert RB, Wurster U, et al. : Cerebrospinal fluid levels of markers of brain parenchymal damage in Vietnamese adults with severe malaria. Trans R Soc Trop Med Hyg 2005; 99: 610–617 [DOI] [PubMed] [Google Scholar]
- 14.Turner GD, Ly VC, Nguyen TH, et al. : Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol 1998; 152:1477–1487 [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin D, de Laat B, Jenkins PV, et al. : Severe Plasmodium falciparum malaria is associated with circulating ultra-large von Willebrand multimers and ADAMTS13 inhibition. PLoS Pathog 2009; 5:e1000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollestelle MJ, Donkor C, Mantey EA, et al. : von Willebrand factor propeptide in malaria: Evidence of acute endothelial cell activation. Br J Haematol 2006; 133:562–569 [DOI] [PubMed] [Google Scholar]
- 17.Graham SM, Chen J, Chung DW, et al. : Endothelial activation, haemostasis and thrombosis biomarkers in Ugandan children with severe malaria participating in a clinical trial. Malar J 2016; 15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridges DJ, Bunn J, van Mourik JA, et al. : Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood 2010; 115:1472–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdman LK, Dhabangi A, Musoke C, et al. : Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: A retrospective case-control study. PLoS One 2011; 6:e17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combes V, Rosenkranz AR, Redard M, et al. : Pathogenic role of P-selectin in experimental cerebral malaria: Importance of the endothelial compartment. Am J Pathol 2004; 164:781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang WL, Li J, Sun G, et al. : P-selectin contributes to severe experimental malaria but is not required for leukocyte adhesion to brain microvasculature. Infect Immun 2003; 71:1911–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiedler U, Reiss Y, Scharpfenecker M, et al. : Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 2006; 12:235–239 [DOI] [PubMed] [Google Scholar]
- 23.Thurston G, Rudge JS, Ioffe E, et al. : Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000; 6:460–463 [DOI] [PubMed] [Google Scholar]
- 24.Yeo TW, Lampah DA, Gitawati R, et al. : Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 2008; 105:17097–17102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovegrove FE, Tangpukdee N, Opoka RO, et al. : Serum angiopoietin-1 and −2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 2009; 4:e4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conroy AL, Phiri H, Hawkes M, et al. : Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: A retrospective case-control study. PLoS One 2010; 5:e15291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conroy AL, Glover SJ, Hawkes M, et al. : Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: A retrospective case-control study*. Crit Care Med 2012; 40:952–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conroy AL, Hawkes M, Elphinstone RE, et al. : Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis 2016; 3:ofw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sypniewska P, Duda JF, Locatelli I, et al. : Clinical and laboratory predictors of death in African children with features of severe malaria: A systematic review and meta-analysis. BMC Med 2017; 15:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conroy AL, Opoka RO, Bangirana P, et al. : Acute kidney injury is associated with impaired cognition and chronic kidney disease in a prospective cohort of children with severe malaria. BMC Med 2019; 17:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickson MR, Conroy AL, Bangirana P, et al. : Acute kidney injury in Ugandan children with severe malaria is associated with long-term behavioral problems. PLoS One 2019; 14:e0226405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caldwell BM, Bradley RH: Home Inventory Administration Manual. Third Edition Little Rock, AR, University of Arkansas, 2001 [Google Scholar]
- 33.Bangirana P, John CC, Idro R, et al. : Socioeconomic predictors of cognition in Ugandan children: Implications for community interventions. PLoS One 2009; 4:e7898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullen E: Mullen Scales of Early Learning. Circle Pines, MN, American Guidance Services, 1995 [Google Scholar]
- 35.Kaufman AS, Kaufman NL: Kaufman Assessment Battery for Children Manual. Second Edition Circle Pines, MN, American Guidance Service, 2004 [Google Scholar]
- 36.Opoka RO, Bangirana P, Idro R, et al. : Lack of mortality in 22 children with sickle cell anemia and severe malarial anemia. Pediatr Blood Cancer 2018; 65: 10.1002/pbc.26745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendriksen IC, Mwanga-Amumpaire J, von Seidlein L, et al. : Diagnosing severe falciparum malaria in parasitaemic African children: A prospective evaluation of plasma PfHRP2 measurement. PLoS Med 2012; 9:e1001297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 1: doi: 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 39.Schwartz GJ, Muñoz A, Schneider MF, et al. : New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iademarco MF, Barks JL, Dean DC: Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest 1995; 95:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medana IM, Day NP, Salahifar-Sabet H, et al. : Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis 2003; 188:844–849 [DOI] [PubMed] [Google Scholar]
- 42.Medana IM, Idro R, Newton CR: Axonal and astrocyte injury markers in the cerebrospinal fluid of Kenyan children with severe malaria. J Neurol Sci 2007; 258:93–98 [DOI] [PubMed] [Google Scholar]
- 43.Higgins SJ, Purcell LA, Silver KL, et al. : Dysregulation of angiopoietin-1 plays a mechanistic role in the pathogenesis of cerebral malaria. Sci Transl Med 2016; 8:358ra128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiedler U, Scharpfenecker M, Koidl S, et al. : The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004; 103: 4150–4156 [DOI] [PubMed] [Google Scholar]
- 45.Verma SK, Molitoris BA: Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol 2015; 35: 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barber BE, Grigg MJ, Piera KA, et al. : Intravascular haemolysis in severe Plasmodium knowlesi malaria: Association with endothelial activation, microvascular dysfunction, and acute kidney injury. Emerg Microbes Infect 2018; 7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim DH, Jung YJ, Lee AS, et al. : COMP-angiopoietin-1 decreases lipopolysaccharide-induced acute kidney injury. Kidney Int 2009; 76:1180–1191 [DOI] [PubMed] [Google Scholar]
- 48.David S, Kümpers P, Lukasz A, et al. : Circulating angiopoietin-2 levels increase with progress of chronic kidney disease. Nephrol Dial Transplant 2010; 25:2571–2576 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.