Abstract
Background
Impulse control disorders (ICDs) have an increased frequency in patients with Parkinson's disease (PD), mainly because of treatment with dopamine agonists (DA). Factors related with the country of origin (culture, economy, healthcare politics) may impact phenomenology.
Objectives
To explore phenomenology of ICDs depending on the country.
Methods
A systematic review following PRISMA guidelines was performed using Pubmed database. Articles published up to 2018 in which the prevalence of ICDs was analyzed were selected.
Results
Thirty‐two studies from 22 countries worldwide were included. The highest prevalence of ICDs in each continent was found in UK (59%), USA (39.1%) and India (31.6%). Frequency of ICDs was higher in those studies with lower mean age, higher proportion of males, whenever a screening instrument was used and whenever prescription of DAs was more common. Prevalence of ICDs was higher in Western countries compared to Asian countries (20.8% vs. 12.8%, P < 0.001) as it was the proportion of patients treated with DAs (66% vs. 48.2%, P < 0.001). Hypersexuality was the most common ICD overall (up to 23.8%). The highest frequencies of compulsive buying and eating were found in Western countries. Gambling was less commonly diagnosed, but prevalence was relevant Japan (14%).
Conclusion
We observed a tendency towards a different ICD profile in different geographical areas, which may be attributable to socio‐economical, cultural or political influences in the phenomenology of these disorders. Acknowledging these differences could help their early detection, which is critical for prognosis.
Keywords: Parkinson's disease, impulse control disorders, culture, country, systematic review
Impulse control disorders (ICDs) comprehend a group of behavioral disorders defined by the inability to resist an impulse to perform an act that is harmful to the individual or to others, owing to its excessive nature, with quick unplanned responses and little assessment of the negative consequences. 1 , 2
ICDs are considered to have an increased frequency among patients with Parkinson's disease (PD), though PD alone does not increase the risk, as several studies have reported a similar frequency in non‐treated patients and the general population. 1 , 2 Certain disease‐specific changes may make these patients more prone to develop an ICD, specially when they are exposed to dopamine agonist (DA) treatment. 2 The dopamine “overdose” hypothesis 3 states that the asymmetrical neuronal degeneration taking place in PD would lead to an increased stimulation of a relatively preserved ventral stratium, causing an impaired function in the reward‐processing system. 2 , 3 Other risk factors include male sex, early onset of the disease, previous addictive behaviors, smoking, depression, a high grade of anxiety and impulsiveness at the moment of diagnosis and parkin gene related monogenic PD. 1 , 2 , 4 , 5 , 6
Different subtypes of ICDs have been described, such as pathological gambling, binge eating, compulsive buying and sexual compulsion (hypersexuality). In addition to these disorders, patients may display symptoms of impulsive behaviors that do not constitute a complete form of the disorder, recently called Impulsive Compulsive Behaviors (ICBs). These include punding, hobbyism, walkabout and dopaminergic dysregulation syndrome (DDS). 1 , 2
ICDs are being increasingly recognized as a cause of disability in the long period, worsening the quality of life and being a source of distress for patients and caregivers. 2 They are generally under‐diagnosed because they are not spontaneously reported by patients, due to embarrassment or unawareness that such symptoms may be related to PD and its treatment. Therefore, diagnosis requires a directed and systematic interview. 1 , 7 There are several instruments for the screening of ICDs, such as the Questionnaire for Impulse‐Compulsive Disorders in Parkinson's disease (QUIP) or the Minnesota Impulsive Disorders Interview (MIDI), though there is certain controversy regarding their specific diagnostic yield, as they may overestimate the presence of ICDs. 8 , 9
Several individual studies have identified possible cultural influences in the prevalence of ICDs and the frequency of specific subtypes, 1 , 5 especially among Asian and Western countries. 9 , 10 It has been suggested that economic and sociocultural factors may contribute to how ICDs manifest in different populations, which could help their early diagnosis. 1 , 10 Nevertheless, so far there are not studies specifically designed to test this hypothesis. In this systematic review, we aim to analyze differences in the frequency of the four main subtypes of ICDs in PD (gambling, binge eating, hypersexuality and compulsive buying) in different geographical regions.
Methods
A systematic review of the literature was performed using Pubmed database. The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines were followed.
Search Strategy
A search was conducted in Pubmed database with the following terms: Parkinson* AND (“impulse control disorders” OR impulse*) AND (prevalen* OR incidence). The search was limited to articles written in Spanish or English and published from January 1st, 2008 to December 31st, 2018, obtaining a total of 173 results.
This study was designed in 2018, and we established a timeframe of 10 full years for the search (2008–2017), as we considered this period was adequate to cover the most relevant publications in keeping with the current view and understanding of impulse control disorders. Last search was actually conducted in February 2019; therefore, one more year (2018) was added in order to include the latest publications.
The reference list of relevant studies was also hand‐searched for identification of further studies.
Eligibility Criteria
The following inclusion criteria were applied:
We included observational studies conducted on adult patients with diagnosis of PD, whose primary aim was to analyze the frequency of ICDs.
The frequency of each subtype of ICD (hypersexuality, pathological gambling, binge eating and compulsive buying or shopping) had to be actively investigated separately, showing the numerical data within the text, tables or figures.
The authors should specify the nationality of patients or the country from where patients were selected. Otherwise, the study could be included only if all author affiliations were from the same country, assuming that all patients were also from that same country.
The following exclusion criteria were applied:
Data from clinical trials and derived subanalysis were excluded, as well as case reports, reviews, letters to the editor and other non‐original articles.
Secondary analyses of previous observational studies were excluded.
When the same authors conducted several studies on the same cohort with different outcomes, we only included the first study and excluded the rest to avoid duplicated data.
Studies that exclusively selected patients with a previous diagnosis of an ICD were excluded.
We aimed to focus on the general population of patients with PD. Therefore, we excluded articles conducted on specific subsets of patients, such as those that had undergone surgical treatment, had specific sleep disturbances or patients with parkin‐related PD.
Selection of Studies
Three investigators (PPD, CLFE, JLCG) independently screened titles and abstracts of all the studies identified through the search and selected those studies potentially eligible. Afterwards, they independently screened full‐text publications for inclusion. Any disagreements were resolved by consulting other review authors (AAC, JCMC). A flow‐diagram of the systematic review is provided in Fig. 1.
FIG 1.
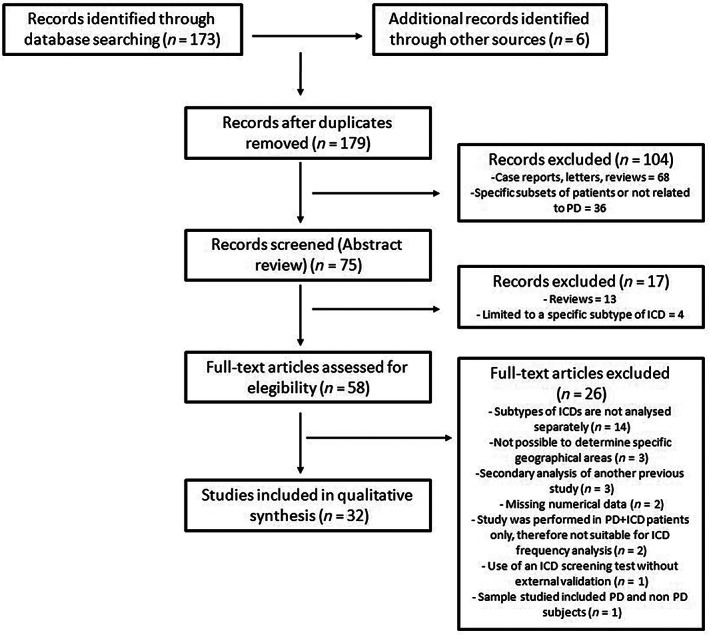
Flow‐diagram of the systematic review.
Data Collection and Synthesis
For each study, we collected the following data: country, number of patients, frequency of each subtype of ICD, mean age, proportion of male sex, proportion of patients under DA treatment and screening instruments used for the detection of ICDs. As ICBs are generally more inconsistently investigated, we chose not to include these data in our review.
In the cases of multicentre studies that included patients from different countries, we analyzed the individual data for each country separately, whenever possible. If these data were not available, these studies were analyzed attending to the wider geographical region (Europe, America, Asia).
Differences in the prevalence of ICDs can be attributed to several factors, such as DA treatment or epidemiological factors. However, in previous studies these factors have been essentially associated with the overall quantitative prevalence of any ICD. We hypothesized that country‐related factors could influence not only this overall prevalence, but also the frequency of specific subtypes of ICDs. In order to analyze this point, we focused in the most frequent subtype of ICD reported in each study from a qualitative point of view.
Data from the selected studies were extracted and double‐checked independently by three authors (PPD, CLFE, JLCG). Any disagreements were resolved by consensus or by consulting other review authors (ABC, FRJ).
Assessment of Risk of Bias
Two review authors (PPD, JLCG) assessed the risk of bias for each study. Any disagreements were resolved by discussion or by involving other investigators (ABC, FRJ). Risk of bias was assessed for the following domains:
Patient selection.
Incomplete report of baseline characteristics (age, sex, disease duration, DA treatment and other possible factors influencing the development of ICDs).
Incomplete outcome data (in our case, proportion of patients with any subtype of ICD).
Statistical Analysis
We analyzed whether there were any differences in the frequency of ICD between Asian and Western countries. The overall frequency of ICDs and utilization of DAs were calculated with the number of patients (n) diagnosed with an ICD or treated with DAs reported in each study. Significance was assessed using a chi‐square test. We set the level of significance at P < 0.01.
Results
Thirty‐two studies from 22 countries of Europe, America and Asia were selected, with a total sum of 12,911 patients (Table S1). 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 We included 15 studies conducted in Europe, 5 , 6 , 7 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 five in America 23 , 24 , 25 , 26 , 27 and 12 in Asia. 8 , 9 , 10 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 Among them, we also included three multicentric studies in which various countries of Europe, North America and South America participated. 16 , 23 , 27
We found a total prevalence of ICDs ranging between 3.53% and 59%, showing a great variability among the studies. The overall prevalence of ICDs in European and American countries was 20.8%, significantly higher than in Asian countries, with a prevalence of 12.8% (P < 0.001). The highest prevalence of ICDs in each continent was found in UK (59%), 17 India (31.6%) 34 and USA (39.1%). 25 Attending to the different subtypes of ICDs, hypersexuality was more common in Spain (23.8%) 15 and Finland (22.8%) 5 ; compulsive buying in Italy (20%) 6 and Spain (15.5%) 15 ; binge eating in USA (34.7%) 25 and UK (29%). 17 Gambling was more common in Japan (14%). 32 The presence of more than one ICD was more prevalent in Spain (34.5%) 15 and Denmark (23.3%). 13
Regarding the different continents, we found that binge eating was the most common ICD in seven of the 15 studies performed in Europe (46.6%), 11 , 16 , 17 , 19 , 20 , 21 , 22 followed by hypersexuality (40%), 5 , 7 , 13 , 14 , 15 , 18 compulsive buying (6.6%) 6 and gambling (6.6%). 12 Binge eating was also found to be the most prevalent ICD in three of the five studies conducted in America (60%), 25 , 26 , 27 followed again by hypersexuality (20%) 24 and compulsive buying (20%). 23 In Asia, seven out of 12 studies found hypersexuality as the most common ICD (58.3%), 9 , 10 , 28 , 31 , 32 , 33 , 35 followed by pathological gambling (25%), 29 , 32 , 36 binge eating (16.6%) 8 , 30 and compulsive buying (8.3%). 34
We found several studies carried out in the same country in the cases of Spain, 7 , 14 , 15 , 18 , 22 China, 9 , 10 , 29 , 36 Italy, 6 , 12 , 19 France, 11 , 21 Korea 8 , 33 and India. 34 , 35 Five studies were conducted on Spanish patients; in four of them, hypersexuality was the most common ICD 7 , 14 , 15 , 18 and binge eating in the remaining. 22 In the case of the studies conducted in China, hypersexuality was the most prevalent ICD in two of them 9 , 10 and pathological gambling in the other two. 29 , 36 In France, both studies agreed on showing binge eating as the most prevalent ICD, with hypersexuality and compulsive buying in second and third places, respectively. 11 , 21
The rest of studies from the same countries did not show coincidence; in Korea the most common ICDs were binge eating 8 and hypersexuality 33 ; in India, they were hypersexuality 35 and compulsive buying. 34 Three studies were conducted in Italy, each one reporting a different ICD as the most prevalent: compulsive buying, 6 binge eating 19 and gambling. 1
On the other hand, we observed some important differences in methods among the studies, summarized in Table S2.
Most studies were cross‐sectional studies; only three studies had a prospective design. 19 , 21 , 25 In Table S1, for the studies of Antonini et al. 19 and Corvol et al. 21 we selected the prevalence at baseline in order to make the results more comparable with the rest of studies. On the contrary, in Bastiaens et al. 25 the authors specifically selected patients without diagnosis of any ICDs. Therefore, data shown in Table S1 corresponds to the data reported by the authors in the follow‐up.
Overall, in most studies some screening tool other than clinical interview was used to detect the presence of an ICD (81.25%). The QUIP was the most common screening tool applied (59.3%). 5 , 11 , 12 , 13 , 14 , 15 , 17 , 19 , 20 , 22 , 24 , 26 , 27 , 29 , 30 , 32 , 33 , 35 , 36 Other screening tools included the MIDI (28.1%) 6 , 8 , 9 , 18 , 19 , 22 , 23 , 26 , 34 and the South Oaks Gambling Screen (SOGS; 9.4%). 5 , 6 , 10 In three studies the authors used both MIDI and QUIP 19 , 22 , 26 ; in these cases, results by QUIP are shown in Table S1 in the studies of Antonini et al. 19 and Rodríguez‐Violante et al. 26 In the study of Marín‐Lahoz et al. 22 the number of ICDs was established by clinical interview, although their severity was assessed with the QUIP. In a total of six studies the authors did not use any screening tool, making the diagnosis of ICDs based only on a systematic clinical interview. 7 , 16 , 21 , 25 , 28 , 31
There were also several differences regarding the clinical features of the patients participating in the studies, such as mean age, mean PD duration or inclusion of patients with dementia (Table S2). Vela et al. 15 selected patients with PD of early onset (not older than 44 years old at the time of diagnosis). In other three studies, the mean age was also below 60 years. 28 , 34 , 35
Regarding treatment, in four studies a stable treatment with DAs was necessary for the selection of patients; thus, all patients were being treated with these drugs. 6 , 14 , 16 , 25 This proportion differs greatly from the data reported in other studies, in which treatment with DAs could be as low as 50% of the sample. 9 , 10 , 12 , 34 , 36 Overall, 48.2% of patients were prescribed DAs in Asian countries, significantly lower than the 66% found in European and American countries (P < 0.001). Nevertheless, in most studies a specific treatment was not a condition for the selection of patients (Table S2).
In most studies, mean dopamine agonist‐ levodopa equivalent daily dose (DA‐LEDD) was recorded, with a wide range from 41 mg/d to more than 500 mg/d (Table S2). Higher doses of DAs have been associated with an increased risk of developing an ICD. 5 , 9 , 29 , 31 , 33 , 35 , 36 In most studies, there were no significant differences among specific DAs (mostly ropinirole and pramipexole), suggesting a class effect. However, some authors observed that the frequency of ICDs was significantly lower in patients taking prolonged‐release pramipexole 16 or transdermal rotigotine patches. 14 , 16 One study, however, observed and increased prevalence of ICDs in patients taking prolonged‐release pramipexole. 26
Risk of Bias of Included Studies
In most studies, patients were selected through retrospective data or in a consecutive fashion from an outpatient clinic. Some studies, however, selected patients that replied to a postal letter or an advertisement, this is, patients that actively wanted to participate in the study, 5 , 17 which constitutes a potential bias (Table S2). Patients with dementia are included in some of the studies and excluded in others, which could also influence the frequency of ICDs observed (Table S2).
Not all studies reported complete data regarding baseline characteristics or medications. The duration and doses of DA treatment were heterogeneous in our sample of studies, and some studies did not report these data (see Table S2).
Two studies did not assess the frequency of all the subtypes of ICDs, for example, binge eating was not assessed in the study of Isaias et al. 6 Also, a large multicentric study 16 did not report the prevalence of compulsive buying disorder (see Table S1).
Discussion
The variability in frequency of ICDs among different populations and cultures is an interesting, although unexplored, research area. In this systematic review, we aimed to explore these possible cultural influences in phenomenology.
First of all, we observed a great heterogeneity among the selected studies, which constitutes the main limitation of this review, as it makes difficult to compare their findings. We could not establish whether cultural factors accounted for the differences observed, as there were also big differences in samples, methods and diagnostic criteria. Nevertheless, we observed certain tendencies pointing towards this hypothesis, with some remarkable findings, especially when comparing Western and Asian countries.
Overall, prevalence of ICDs was higher in Western countries, compared with Asian countries (20.8% vs. 12.8%; P < 0.001). Previous studies have reported that PD in Asian patients is generally treated with lower dosage of DAs, for reasons that include cultural differences in perceptions of disease and treatments. 30 Accordingly, we found that the frequency of DAs utilization was lower in Taiwan, Malaysia, Japan and China than in Western countries (Table S2). Also, the lowest doses of DA‐LEDD were found in studies conducted in Asian countries (Table S2). Considering that in some studies the number of patients under DA therapy was not reflected, which constitutes a limitation, overall we observed that 66% of patients from European and American countries were taking DAs, but only 48.2% in Asian countries (P < 0.001). This finding may be explained by ethnic and cultural differences, study design or socioeconomic factors, such as insurance policies and the cost and availability of DAs. 9 , 31 Also, it has been suggested that Asian and Caucasian populations may have different distributions of dopamine receptor polymorphisms, a hypothesis that is yet to be explored. 9 , 10 If tolerance to immediate adverse events were lower in Asian populations, this would reduce the mean doses of DAs, rendering the occurrence of an ICD less likely. 30 However, pharmacokinetic studies have shown similar bioavailability of DAs in Western and Eastern individuals. 37 , 38
Furthermore, some studies have reported a higher risk of developing ICDs with oral formulations, compared to a transdermal DA (rotigotine). 14 , 16 This suggests that the route of administration may contribute to the risk of developing an ICD, which might be explained by a greater stability of its plasma levels, though the reasons are still unclear. 14 , 16 Also, the receptor affinity profile of rotigotine is somewhat different from the oral DAs, which may render its effects on impulsiveness peculiar. 14 Nevertheless, most studies in our sample did not conduct these analyses. The number of patients treated with rotigotine in our sample is probably underrepresented, as many studies may have been conducted before its implementation in the market.
On the other hand, cultural influences may not only mediate in the quantitative data observed in our results, but also in the way ICDs manifest in the different populations.
Overall, the most common subtype of ICD was hypersexuality, followed closely by binge eating, while gambling and compulsive buying were the less diagnosed ICDs. As reflected by Rodríguez‐Violante et al., 26 patients with low incomes may not be able to engage in activities such as gambling or shopping, instead directing impulsivity to other types of activities, which may explain the high prevalence of compulsive sexual behaviors (Table S1). Also, it has been suggested that sexual behavior in older patients with a degenerative disease could be regarded as a more serious problem and reported more frequently. 33 Furthermore, hypersexuality has been also associated with male sex and lower age in most studies, and its diagnostic criteria may have greater variability among different studies. 31 On the other hand, in some cases patients may be more reluctant to admit these kind of problems to a doctor, as shown by Zhang et al. 36 in Chinese patients.
Culture has been suggested to contribute significantly in the development of eating disorders, as the ideal of slim beauty promoted by the media and pursued in Western countries is different from those from the East. 39 Previous research suggests that the adoption of these cultural values by non‐Western societies tends to increase the frequency of related ICDs. 40 On the other hand, food is also considered a mean of cultural expression, and it is important in social interactions. 41 The values governing food and meals in Eastern countries are different from those in the West. 41 Some authors speculate that a greater interest in gastronomy, and the social meaning attributed to meals may also explain the increased prevalence of binge eating in Western countries (with the highest prevalence reported in USA 25 ) in comparison with Asian countries. 10
Culture also affects the way consumers approach shopping, as this is highly influenced by cultural habits, tastes, values and traditions. 42 In industrialized countries, where credit is more available, compulsive buying seems to be more frequent, up to 20% of patients. 6 In this regard, some studies have shown more hedonic shopping in these countries, while shopping values tend to be more utilitarian in Eastern countries. 42 In addition, shopping seems to be also influenced by sex, being more common in women. 23
As for pathological gambling, the availability of casinos and other facilities accounts for some of the variability, although gambling can manifest in different ways. For example, slot machines were the most preferred way of gambling in Finland, 5 casinos in USA, 23 Pachinko (pinball) in Japan 32 and “scratch and win” and lottery tickets in Mexico. 26 Gambling is illegal in Taiwan, China or Korea; therefore, the prevalence of this disorder is expected to be lower in these countries. However, it should be taken into account that lottery tickets and similar ways of gambling outside casinos may not be illegal and are not usually recognized as a gambling problem by patients. This could explain why this disorder was the most common ICD in two of the Chinese studies, because lottery tickets are not legally considered as gambling. 29 , 36 On the other hand, prevalence of gambling in Japan, Finland and USA was high compared with the rest of studies (Table S1), which likewise may be related to the easy access to Pachinko 32 and casinos. 23 Gambling is also associated with male sex and a younger age in most studies.
We expected to find similar results in studies from the same countries, as samples are more homogeneous. We observed a certain level of agreement in the cases of Spain, China and France, with hypersexuality, gambling and binge eating as the most prevalent ICDs, respectively (Table S1). 7 , 9 , 10 , 11 , 14 , 15 , 18 , 21 , 29 , 36 However, results differed in the studies performed in Italy, Korea and India. 6 , 8 , 12 , 19 , 33 , 34 , 35 Again, differences in samples and methods may account for the discrepancies found, as we will discuss below (Table S2).
It is of notice that the prevalence of ICDs varied widely (3.53%–59%) in our sample (Table S1). These variations could be due to different sample composition, methods and defining criteria. For example, the 59% prevalence is found in a study in which most patients actively chose to participate through an advertisement, which may constitute a selection bias. 17 Also, there is a prevalence of 58.3% in a study selecting patients with PD of early onset; hence much younger than patients participating in the rest of studies. 15 Younger age at onset constitutes a widely recognized risk factor for developing ICDs and the results in the study of Vela et al. 15 also showed this observation. Furthermore, there were several studies in which patients with dementia were not excluded (Table S2). Prevalence of ICDs in demented patients has been found to be significantly lower than in cognitive preserved patients, 12 and DAs may not be used in these patients according to the usual clinical practice. 16 However, the association between cognition and ICDs in PD is still debatable. 36
Large scale studies, less prone to selection bias, may render a more accurate estimation of the real prevalence of ICDs. In the DOMINION study, conducted on 3090 US and Canadian patients, the total prevalence of ICDs was 13.6%. 23 Similarly, the largest study conducted in Asia, with 1167 patients, reported a prevalence of 10.1% 8 (Table S1). In both studies MIDI was used as screening tool (Table S2). In the largest study conducted in Europe, 19 however, with a prospective design and a total sum of 1069 patients, the authors reported a higher prevalence of 28.6% at baseline in the analysis by MIDI, and 34.2% in the analysis by QUIP, finding a relatively stable frequency of ICDs during the follow‐up.
Apart from the study of Antonini et al., 19 we could only include two more studies with a prospective design. In Bastiaens et al., 25 only patients who received a predefined minimum dose of DA were included in the analysis, finding a cumulative frequency of 39.1% of ICDs after a median DA treatment duration of 21 months. Corvol et al. 21 selected patients with less than 5 years of disease duration at baseline, finding a cumulative incidence of ICDs of 46.1%, and a prevalence of 32.8% after 5 years of follow‐up.
The use of screening instruments could also account for part of the variability observed in prevalence. Overall, it was reported a higher rate of positive ICD patients in studies in which the QUIP was used. This test is known to have a high sensitivity; therefore, further diagnostic criteria should be used for a confirmative diagnosis. 9 When both MIDI and QUIP were used in the analysis, 19 , 22 , 26 prevalence by QUIP tended to be slightly higher versus the MIDI, as the QUIP assesses three additional modules (hobbyism, walkabout and DDS). Nevertheless, Antonini et al. 19 concluded that assessment using MIDI and QUIP yielded overall comparable results, suggesting that both are suitable tools for screening ICDs in patients with PD. Indeed, these observations seem also true in our results, with a total prevalence ranging from 4.14%–31.6% in studies using MIDI, to 7%–59% in studies using QUIP (or 7%–39.1% if we exclude the unusually high prevalence found in the two studies discussed before). 15 , 17
Study Limitations
Despite the fact that we excluded studies which clearly stated to be secondary analysis or performed on the same cohort as others, we ascertain the possibility of duplicated data in the cases of larger multicentric studies, as patients could have also been included in smaller single‐center studies from the same countries.
Other limitations of our review include that our search was limited to Pubmed database and to articles written either in English or Spanish, and our inclusion criteria were restrictive, which carries a potential selection bias. However, the relatively high number of individual studies included in the analysis may compensate for this. We did not find studies conducted in certain areas (for example, the continents of Australia and Africa, or large countries such as Russia), or low‐income countries. Also, in most studies ICDs were not analyzed attending to possible differences in the level of education and income of patients, except for the study of Rodríguez‐Violante et al. 26 Because of lack of full data and non‐comparable studies, a comprehensive statistical analysis could not be performed, so our hypothesis could not be properly tested. Therefore, our findings, though interesting and opening the door to new studies, should be interpreted with caution.
Conclusion
We observed a tendency towards a different ICD profile in different geographical areas, not only in terms of a quantitative measure but also in the subtypes of ICDs that manifest more frequently, which may be attributable, at least in part, to cultural, economical or social influences in the phenomenology of these disorders. These findings could help their early detection, which is critical for prognosis. Nevertheless, we observed a huge variability in methods and treatments that could also explain these results. Therefore, new prospective studies with uniform criteria, focused on investigating possible cultural influences in phenomenology of ICDs are needed to confirm these observations.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
P.P.D.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
A.B.C.: 1A, 1B, 1C, 2C, 3B
F.R.J.: 1A, 1B, 1C, 2C, 3B
J.L.C.G.: 1A, 1B, 1C, 2B, 3B
C.L.F.E.: 1A, 1B, 1C, 2A, 3B
A.A.C.: 1A, 1B, 2A, 2C, 3B
J.C.M.C.: 1A, 2C, 3B
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board and patient consent were not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. The authors declare no conflicts of interest.
Financial Disclosures for the Previous 12 Months: AAC has received honoraria as a speaker from AbbVie and Zambon. She has participated in advisory boards of AbbVie, Zambon and Bial. She has received travel grants from AbbVie and Zambon. JCMC has received honoraria as a speaker from AbbVie, Allergan, Bial, Boehringer, GSK, Krka, Merz, Ipsen, Italfarmaco, Lundbeck, Medtronic, TEVA, UCB and Zambon. He has received travel grants from AbbVie, Allergan, Bial, Italfarmaco, TEVA, UCB, Merz, Krka and Zambon. He has received research grants from AbbVie, Allergan, Merz, Italfarmaco, Lundbeck, UCB and Zambon. He has participated in advisory boards of AbbVie, Allergan, GSK, Bial, Merz, Merck, Boehringer, Ipsen, Italfarmaco, LundBeck, Orion, UCB and Zambon.
Provenance and Peer Review: Not commissioned; externally peer reviewed.
Supporting information
Table S1. Prevalence of the different subtypes of ICDs in various countries of the world. (HS: hypersexuality; BU: Compulsive buying; BE: binge eating; GA: pathological gambling). The most frequent ICD in each study is highlighted in bold.
Table S2. Differences in the baseline characteristics of patients and methods among the studies. (MIDI: Minnesota Impulsive Disorders Interview; QUIP: Questionnaire for Impulse‐Compulsive Disorders in Parkinson's disease; SOGS: South Oaks Gambling Screen; ICD: Impulse Control Disorder; DA‐LEDD: Dopamine Agonist‐Levodopa Equivalent Daily Dose; PPX: pramipexole; ROP: ropinirole).
References
- 1. Vargas AP, Costa Cardoso FE. Impulse control and related disorders in Parkinson's disease. Arq Neuropsiquiatr 2008;27(8):721–727. [DOI] [PubMed] [Google Scholar]
- 2. Weintraub D, Mamikonyan E. Impulse control disorders in Parkinson's disease. Am J Psychiatry 2019;176(1):5–11. [DOI] [PubMed] [Google Scholar]
- 3. Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R. Dopamine overdose hypothesis: Evidence and clinical implications. Mov Disord 2013;28(14):1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morgante F, Fasano A, Ginevrino M, et al. Impulsive‐compulsive behaviors in parkin ‐associated Parkinson disease. Neurology 2016;87(14):1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joutsa J, Martikainen K, Vahlberg T, Voon V, Kaasinen V. Impulse control disorders and depression in Finnish patients with Parkinson's disease. Parkinson Relat Disord 2012;18(2):155–160. 10.1016/j.parkreldis.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 6. Isaias IU, Siri C, Cilia R, De Gaspari D, Pezzoli G, Antonini A. The relationship between impulsivity and impulse control disorders in Parkinson's disease. Mov Disord 2008;23(3):411–415. [DOI] [PubMed] [Google Scholar]
- 7. Ávila A, Cardona X, Bello J, Maho P, Sastre F, Martín‐Baranera M. Trastornos del control de los impulsos y punding en la enfermedad de Parkinson: la necesidad de una entrevista estructurada. Neurologia 2011;26(3):166–172. [DOI] [PubMed] [Google Scholar]
- 8. Lee JY, Kim JM, Kim JW, Cho J, Lee WY, Kim HJ, Jeon BS. Association between the dose of dopaminergic medication and the behavioral disturbances in Parkinson disease. Parkinson Relat Disord 2010;16:202–207. 10.1016/j.parkreldis.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 9. Wang X‐P, Wei M, Xiao Q. A survey of impulse control disorders in Parkinson's disease patients in Shanghai area and literature review. Transl Neurodegen 2016;5:10–14. 10.1186/s40035-016-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan W, Ding H, Ma J, Chan P. Impulse control disorders in Parkinson's disease in a Chinese population. Neurosci Lett 2009;465(1):6–9. [DOI] [PubMed] [Google Scholar]
- 11. Pérez‐Lloret S, Rey MV, Fabre N, et al. Prevalence and pharmacological factors associated with impulse‐control disorder symptoms in patients with Parkinson disease. Clin Neuropharmacol 2012;35(6):261–265. [DOI] [PubMed] [Google Scholar]
- 12. Poletti M, Logi C, Lucetti C, et al. A single‐center, cross‐sectional prevalence study of impulse control disorders in Parkinson disease: Association with dopaminergic drugs. J Clin Psychopharmacol 2013;33(5):691–694. [DOI] [PubMed] [Google Scholar]
- 13. Callesen MB, Weintraub D, Damholdt MF, Møller A. Impulsive and compulsive behaviors among Danish patients with Parkinson's disease: Prevalence, depression, and personality. Parkinson Relat Disord 2014;20(1):22–26. [DOI] [PubMed] [Google Scholar]
- 14. García‐Ruiz PJ, Martínez‐Castrillo JC, Alonso‐Cánovas A, et al. Impulse control disorder in patients with Parkinson's disease under dopamine agonist therapy: A multicentre study. J Neurol Neurosurg Psychiatry 2014;85(8):841–845. [DOI] [PubMed] [Google Scholar]
- 15. Vela L, Martínez‐Castrillo JC, García‐Ruiz PJ, et al. The high prevalence of impulse control behaviors in patients with early‐onset Parkinson's disease: A cross‐sectional multicenter study. J Neurol Sci 2016;368:150–154. 10.1016/j.jns.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 16. Rizos A, Sauerbier A, Antonini A, EUROPAR and the IPMDS Non‐Motor‐PD‐Study Group . A European multicentre survey of impulse control behaviours in Parkinson's disease patients treated with short‐ and long‐acting dopamine agonists. Eur J Neurol 2016;23(8):1255–1261. [DOI] [PubMed] [Google Scholar]
- 17. Garlovsky JK, Simpson J, Grünewald RA, Overton PG. Impulse control disorders in Parkinson's disease: predominant role of psychological determinants. Psychol Health 2016;31(12):1391–1414. [DOI] [PubMed] [Google Scholar]
- 18. Sáez‐Francàs N, Martí Andrés G, Ramírez N, de Fàbregues O, Álvarez‐Sabín J, Casas M, Hernández‐Vara J. Factores clínicos y psicopatológicos asociados a los trastornos del control de impulsos en la enfermedad de Parkinson. Neurologia 2016;31(4):231–238. http://pesquisa.bvsalud.org/portal/resource/pt/ibc-151302. [DOI] [PubMed] [Google Scholar]
- 19. Antonini A, Barone P, Bonuccelli U, Annoni K, Asgharnejad M, Stanzione P. ICARUS study: Prevalence and clinical features of impulse control disorders in Parkinson's disease. J Neurol Neurosurg Psychiatry 2017;88(4):317–324. [DOI] [PubMed] [Google Scholar]
- 20. Erga AH, Alves G, Larsen JP, Tysnes OB, Pedersen KF. Impulsive and compulsive behaviors in Parkinson's disease: the Norwegian ParkWest study. J Parkinsons Dis 2017;7:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corvol JC, Artaud F, Cormier‐Dequaire F, et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology 2018;91(3):e189–e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marín‐Lahoz J, Pagonabarraga J, Martinez‐Horta S, et al. Parkinson's disease: Impulsivity does not cause impulse control disorders but boosts their severity. Front Psych 2018;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross‐sectional study of 3090 patients. Arch Neurol 2010;67(5):589–595. [DOI] [PubMed] [Google Scholar]
- 24. Valença GT, Glass PG, Negreiros NN, Duarte MB, Ventura LMGB, Mueller M, Oliveira‐Filho J. Past smoking and current dopamine agonist use show an independent and dose‐dependent association with impulse control disorders in Parkinson's disease. Parkinson Relat Disord 2013;19(7):698–700. 10.1016/j.parkreldis.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 25. Bastiaens J, Dorfman BJ, Christos PJ, Nirenberg MJ. Prospective cohort study of impulse control disorders in Parkinson's disease. Mov Disord 2013;28(3):327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodríguez‐Violante M, González‐Latapi P, Cervantes‐Arriaga A, Camacho‐Ordoñez A, Weintraub D. Impulse control and related disorders in Mexican Parkinson's disease patients. Parkinson Relat Disord 2014;20(8):907–910. 10.1016/j.parkreldis.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 27. Ramírez Gómez CC, Serrano Dueñas M, Bernal O, et al. A multicenter comparative study of impulse control disorder in Latin American patients with Parkinson disease. Clin Neuropharmacol 2017;40(2):51–55. [DOI] [PubMed] [Google Scholar]
- 28. Kenangil G, Özekmekçi S, Sohtaoglu M, Erginöz E. Compulsive behaviors in patients with Parkinson's disease. Neurologist 2010;16(3):192–195. [DOI] [PubMed] [Google Scholar]
- 29. Auyeung M, Tsoi TH, Tang WK, Cheung CM, Lee CN, Li R, Yeung E. Impulse control disorders in Chinese Parkinson's disease patients: the effect of ergot derived dopamine agonist. Parkinson Relat Disord 2011;17(8):635–637. [DOI] [PubMed] [Google Scholar]
- 30. Lim SY, Tan ZK, Ngam PI, et al. Impulsive‐compulsive behaviors are common in Asian Parkinson's disease patients: assessment using the QUIP. Parkinson Relat Disord 2011;17(10):761–764. 10.1016/j.parkreldis.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 31. Chiang HL, Huang YS, Chen ST, Wu YR. Are there ethnic differences in impulsive/compulsive behaviors in Parkinson's disease? Eur J Neurol 2012;19(3):494–500. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka K, Wada‐Isoe K, Nakashita S, Yamamoto M, Nakashima K. Impulsive compulsive behaviors in Japanese Parkinson's disease patients and utility of the Japanese version of the questionnaire for impulsive‐compulsive disorders in Parkinson's disease. J Neurol Sci 2013;331:76–80. 10.1016/j.jns.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 33. Kim J, Kim M, Kwon DY, Seo WK, Kim JH, Baik JS, Koh SB. Clinical characteristics of impulse control and repetitive behavior disorders in Parkinson's disease. J Neurol 2013;260:429–437. [DOI] [PubMed] [Google Scholar]
- 34. Sarathchandran P, Soman S, Sarma G, Krishman S, Kishore A. Impulse control disorders and related behaviors in Indian patients with Parkinson's disease. Mov Disord 2013;28(13):1901–1902. [DOI] [PubMed] [Google Scholar]
- 35. Sharma A, Goyal V, Behari M, Srivastva A, Shukla G, Vibha D. Impulse control disorders and related behaviours (ICD‐RBs) in Parkinson's disease patients: assessment using “questionnaire for impulsive‐compulsive disorders in Parkinson's disease” (QUIP). Ann Indian Acad Neurol 2015;18(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Qi HA, Li L, Chen W, Guo LZ. Clinical characteristics of impulse control and related disorders in Chinese Parkinson's disease patients. BMC Neurol 2017;17(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Constantinescu R. Update on the use of pramipexole in the treatment of Parkinson's disease. Neuropsychiatr Dis Treat 2008;4(2):337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cawello W, Kim SR, Braun M, Elshoff JP, Masahiro T, Ikeda J, Funaki T. Pharmacokinetics, safety, and tolerability of rotigotine transdermal system in healthy Japanese and Caucasian subjects following multiple‐dose administration. Eur J Drug Metab Pharmacokinet 2016;41(4):353–362. [DOI] [PubMed] [Google Scholar]
- 39. Sassaroli S, Veronese G, Nevonen L, Fiore F, Centorame F, Favaretto E, Ruggiero GM. Autonomy and submissiveness as cognitive and cultural factors influencing eating disorders in Italy and Sweden: An exploratory study. Eur J Psychol 2015;11(2):233–243. 10.5964/ejop.v11i2.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nasser M. Culture and weight consciousness. J Psychosom Res 1988;32(6):573–577. 10.1016/0022-3999(88)90005-0. [DOI] [PubMed] [Google Scholar]
- 41. Chang RCY, Kivela J, Mak A. Food preferences of Chinese tourists. Ann Tour Res 2010;37(4):989–1011, 2010. 10.1016/j.annals.2010.03.007. [DOI] [Google Scholar]
- 42. Sakarya S, Soyer N. Cultural differences in online shopping behavior: Turkey and the United Kingdom. Int J Electr Commerce Stud 2013;4(2):213–238. 10.7903/ijecs.1049. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Prevalence of the different subtypes of ICDs in various countries of the world. (HS: hypersexuality; BU: Compulsive buying; BE: binge eating; GA: pathological gambling). The most frequent ICD in each study is highlighted in bold.
Table S2. Differences in the baseline characteristics of patients and methods among the studies. (MIDI: Minnesota Impulsive Disorders Interview; QUIP: Questionnaire for Impulse‐Compulsive Disorders in Parkinson's disease; SOGS: South Oaks Gambling Screen; ICD: Impulse Control Disorder; DA‐LEDD: Dopamine Agonist‐Levodopa Equivalent Daily Dose; PPX: pramipexole; ROP: ropinirole).


