Substantial site-to-site variation exists in the characteristics and care of infants with NOWS; consideration of this is essential to optimize care for this vulnerable population.
Abstract
BACKGROUND AND OBJECTIVES:
Variation in pediatric medical care is common and contributes to differences in patient outcomes. Site-to-site variation in the characteristics and care of infants with neonatal opioid withdrawal syndrome (NOWS) has yet to be quantified. Our objective was to describe site-to-site variation in maternal-infant characteristics, infant management, and outcomes for infants with NOWS.
METHODS:
Cross-sectional study of 1377 infants born between July 1, 2016, and June 30, 2017, who were ≥36 weeks’ gestation, with NOWS (evidence of opioid exposure and NOWS scoring within the first 120 hours of life) born at or transferred to 1 of 30 participating hospitals nationwide. Site-to-site variation for each parameter within the 3 domains was measured as the range of individual site-level means, medians, or proportions.
RESULTS:
Sites varied widely in the proportion of infants whose mothers received adequate prenatal care (31.3%–100%), medication-assisted treatment (5.9%–100%), and prenatal counseling (1.9%–75.5%). Sites varied in the proportion of infants with toxicology screening (50%–100%) and proportion of infants receiving pharmacologic therapy (6.7%–100%), secondary medications (1.1%–69.2%), and nonpharmacologic interventions including fortified feeds (2.9%–90%) and maternal breast milk (22.2%–83.3%). The mean length of stay varied across sites (2–28.8 days), as did the proportion of infants discharged with their parents (33.3%–91.1%).
CONCLUSIONS:
Considerable site-to-site variation exists in all 3 domains. The magnitude of the observed variation makes it unlikely that all infants are receiving efficient and effective care for NOWS. This variation should be considered in future clinical trial development, practice implementation, and policy development.
What’s Known on This Subject:
Variation in health care contributes to discrepancies in outcomes. Understanding this variation is critical. Multiple factors contribute to the potential for site-level variation to exist for neonates with opioid withdrawal, but supportive literature is limited.
What This Study Adds:
We observed substantial site-to-site variation in maternal-infant characteristics, infant management, and outcomes for infants with neonatal opiod withdrawal syndrome. Rates of maternal medication-assisted treatment, infant pharmacologic treatment, and infant length of stay varied widely across sites.
Neonatal opioid withdrawal syndrome (NOWS) is part of a national public health crisis, with an increasing number of infants requiring prolonged hospitalization at a high cost to families, communities, and health care systems.1–4 NOWS affects all regions in the United States, with care being provided for these infants in various hospital settings and varying levels of newborn care.1,5,6 This variation in care setting, in combination with regional differences in the epidemic and lack of a solid evidence base to inform a standard of care for the management of these infants,7–11 has led to the potential for variation across sites in the characteristics, provision of care, and outcomes of infants with NOWS. Although variation in care results in discrepancies in health care outcomes in many areas of medicine,12 the ramifications of this variation for infants with NOWS, if present, would likely be magnified in this already vulnerable population. Thus, quantifying this variation is critical to optimizing care. Despite the importance of this issue, reports of between-site variation in NOWS care are limited to small cohort studies13–15 and analyses of variation in protocols and self-reported practices rather than infant-level data on actual care provided.16–18 In addition, site variation in the characteristics of mothers of opioid-exposed infants, although potentially warranted and unmodifiable,19 may contribute to site-to-site variation in both the management and outcomes of these infants and has not been described. The lack of literature specific to site variation in the characteristics and care of infants with NOWS represents a fundamental gap in the understanding of the opioid crisis. Understanding variation across sites will be critical to improving outcomes for infants with NOWS through the identification of targets for the informed development of clinical trials, practice implementation, and policy development.
To quantify the magnitude of site-to-site variation, a large multicenter, cross-regional study is needed to allow for a diverse sample of sites and generalizability of results. Additionally, infant-level data are essential to assess the degree of variation in maternal-infant characteristics and to assess the care actually provided by sites. The objective of the Advancing Clinical Trials in Neonatal Opioid Withdrawal (ACT NOW) Current Experience Study was to quantify site-to-site variation in (1) maternal-infant characteristics (maternal care during pregnancy and in utero exposures), (2) infant management, and (3) outcomes for infants with NOWS.
Methods
Study Design
This observational cross-sectional study included infants born at or transferred to 1 of 30 hospitals in the ACT NOW Collaborative between July 1, 2016, and June 30, 2017 (Supplemental Fig 5). The ACT NOW Collaborative is a partnership between the Environmental influences on Child Health Outcomes (ECHO) Institutional Development Award States Pediatric Clinical Trials Network (ISPCTN)20 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD) Neonatal Research Network (NRN)21 and is part of the National Institutes of Health’s trans-agency Helping to End Addiction Long-term Initiative.22 This collaborative is a diverse network of sites, including both academic and community centers18 highly impacted by the opioid crisis. All participating sites had either local or central institutional review board approval to perform this study. The University of Arkansas for Medical Sciences provided central institutional review board approval.
Data Source
Data were abstracted by trained research personnel, from maternal and infant medical records for participants who met study criteria. The Medical Record Abstraction Quality Assurance and Control framework was used to improve accuracy of data abstraction.23,24 The data collection form is provided in Supplemental Fig 6.
Case Identification
Broad inclusion criteria were selected at the outset to enhance identification of the final population of interest for the study and included gestational age ≥36 weeks and any 1 of the following: (1) NOWS scoring within the first 120 hours of life, (2) history of maternal opioid use during pregnancy, (3) record of a positive maternal screen result for opioids (during the second or third trimester of gestation), or (4) infant toxicology screen positive for opioids. Site investigators identified infants using search terms in electronic medical record (EMR) systems whenever possible. However, hospital EMR capabilities differed across the collaborative; thus, sites were allowed to use search terms, International Classification of Diseases, 10th Revision codes, or both to identify cases. A total of 2786 mother-infant dyads were identified following this process. Identified medical records were then reviewed by research personnel for the inclusion and exclusion criteria to identify eligible infants. Exclusion criteria included major birth defects (Supplemental Fig 6), neonatal encephalopathy, seizure disorder, receipt of respiratory support after 72 hours of life, major surgical interventions, and exposure to opioids unrelated to the treatment of NOWS during the newborn hospitalization. In total, 1808 mother-infant dyads met the eligibility criteria and represent the ACT NOW Current Experience Study population for whom complete data were collected.
As planned prospectively for the current analysis, the study population was further refined to include only infants with both NOWS scoring within the first 120 hours of life and documented opioid exposure, thus identifying the population of infants with NOWS as defined for this study. As such, 431 infants were excluded (n = 140 because of a lack of NOWS scoring within the first 120 hours of life and n = 291 because of a lack of documented opioid exposure), resulting in an analytic sample of 1377 mother-infant dyads (Supplemental Fig 7).
Analysis-Specific Case Identification
For site-level analysis of the proportion of infants receiving pharmacologic treatment, infants who were transferred to a participating hospital for care were excluded (n = 132) to avoid including infants transferred explicitly for pharmacologic treatment, which would artificially inflate the proportion of infants receiving pharmacologic treatment at these sites. Infants discharged from the hospital or transferred to another institution while receiving pharmacologic therapy, either an opioid (n = 57) or secondary medication (n = 96), were excluded from the length of treatment (LOT) analyses for the respective medication because inclusion of these infants would bias results toward a shorter LOT. Infants who were discharged from the hospital on opioid therapy or transferred to another institution while still receiving opioid therapy (n = 57) were excluded from the length of stay (LOS) analysis, as well as those infants whose LOS was determined to be prolonged because of non-NOWS issues (infection, hyperbilirubinemia, respiratory illness, or other) (n = 197). These infants were excluded to avoid bias toward a shorter or longer LOS, respectively.
Statistical Analysis
Overall, key maternal, infant, and hospital characteristics were summarized by using basic descriptive statistics. Means and SD or medians and interquartile ranges (IQRs) were used for continuous variables as appropriate, whereas counts and proportions were used for categorical variables. Additionally, site-level variation for selected parameters was described by using summary statistics and represented graphically by using box plots.
Results
Hospital Characteristics
The incidence of NOWS across the 30 participating hospitals ranged from 4 to 423 cases (mean 31.8 ± 75.9) per 1000 birth admissions, and the absolute number of NOWS cases per site ranged from 4 to 161 (mean 45.9 ± 39.8) during the study period. Participating hospitals cared for infants with NOWS in various locations,25 including in level 1: well-newborn nurseries (n = 28 hospitals, 93.3%); level 2: special care nurseries (n = 11, 36.7%); level 3: NICU (n = 18, 60%); and level 4: regional NICUs (n = 9, 30%), general pediatric units (n = 7, 23.3%), and other locations (n = 1, 3.3%). NOWS care occurred in >1 location at 27 of the 30 hospitals (90%), with the number of locations ranging from 1 to 5 (mean 2.5 ± 1). During the study period, all participating hospitals used the Finnegan Neonatal Abstinence Scoring Tool (FNAST)26 or modification thereof27 for the assessment of withdrawal severity. Twenty-eight hospitals (93.3%) initiated pharmacologic therapy as part of their NOWS management for at least some infants. The remaining 2 hospitals transferred infants to another hospital if they were assessed as requiring pharmacologic therapy.
Maternal and Infant Characteristics
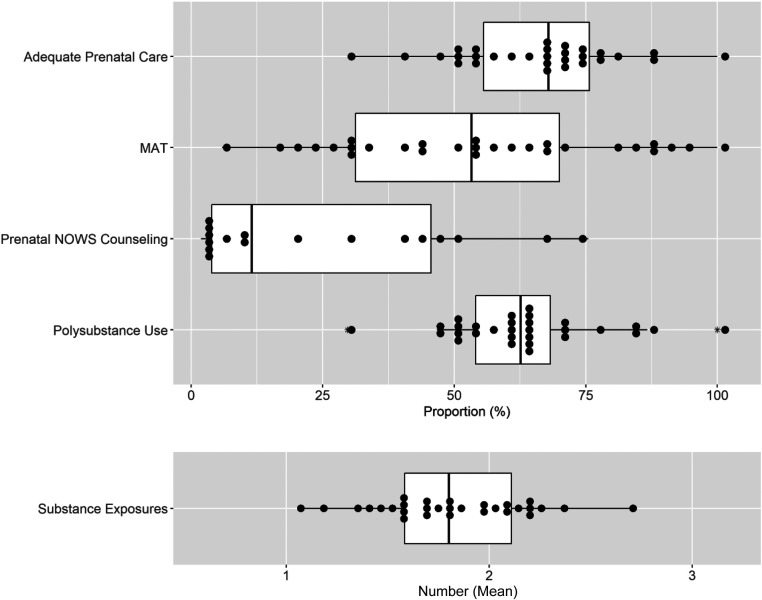
Across the study population, 68.2% (95% confidence interval [CI]: 65.7–70.7) of mothers received adequate prenatal care, defined as ≥3 visits during pregnancy with the first visit occurring before the third trimester,28 62.4% (95% CI: 59.8–64.9) received medication-assisted treatment (MAT) during pregnancy, and 18.4% (95% CI: 16.3–20.4) had anticipatory prenatal counseling specific to the care their infant would receive after delivery (Table 1). Across sites, the proportion of mothers receiving adequate prenatal care and MAT varied widely, ranging from 31.3% to 100% (mean 66.1%; 95% CI: 60.5–71.7) and 5.9% to 100% (mean 54.2%; 95% CI: 44.3–64.0), respectively (Fig 1). The proportion of mothers receiving prenatal counseling also varied widely across sites, from 1.9% to 75.5% (mean 24.9%; 95% CI: 12.3–37.6).
TABLE 1.
Selected Maternal and Infant Characteristics
| Descriptive Statistics (N = 1377) | |
|---|---|
| Infant demographics | |
| Gestational age at birth, wk, mean (SD) [95% CI] | 38.8 (1.4) [38.7–38.8] |
| Birth, wt (kg), mean (SD) [95% CI] | 3.0 (0.5) [3.0–3.1] |
| Birth head circumference, cm, mean (SD) [95% CI] | 33.6 (2.0) [33.5–33.7] |
| Male sex, n (%) [95% CI] | 704 (51.1) [48.5–53.8] |
| Maternal measures | |
| Gravidity, median (Q1, Q3) [95% CI] | 3 (2, 5) [3–3] |
| Parity, median (Q1, Q3) [95% CI] | 2 (1, 3) [2–2] |
| Race37 n (%) [95% CI] | |
| Non-Hispanic white | 940 (68.3) [65.8–70.7] |
| Non-Hispanic Black | 99 (7.2) [5.8–8.6] |
| Hispanic | 90 (6.5) [5.2–7.8] |
| Other | 248 (18.0) [16.0–20.0] |
| Adequate prenatal care,28 n (%) [95% CI] | 939 (68.2) [65.7–70.7] |
| MAT, n (%) [95% CI] | 859 (62.4) [59.8–64.9] |
| Prenatal NOWS counseling, n (%) [95% CI] | 253 (18.4) [16.3–20.4] |
| Polysubstance use, n (%) [95% CI] | 814 (59.1) [56.5–61.7] |
| RUCA,29 n (%) [95% CI] | |
| Metropolitan | 1001 (72.7) [70.3–75.1] |
| Micropolitan | 179 (13.0) [11.2–14.8] |
| Small town | 125 (9.1) [7.6–10.6] |
| Rural | 72 (5.2) [4.1–6.4] |
Q1, first quartile; Q3, third quartile; RUCA, rural-urban commuting area.
FIGURE 1.
Site-level variation in maternal and infant characteristics. The box plots consist of the 25th (quartile 1), 50th (median), and 75th (quartile 3) percentiles. The length of the whiskers represents the minimum (quartile 1 – 1.5 × IQR) and maximum (quartile 3 + 1.5 × IQR), where IQR = quartile 3 – quartile 1 (IQR). Each dot represents a single site proportion or mean, as appropriate, and the asterisk denotes outliers.
Overall, the mean gestational age of infants in this study population was 38.8 ± 1.4 weeks and the mean birth weight was 3.0 ± 0.5 kg. Other growth parameters are provided in Table 1. Overall, infants were born primarily to mothers who lived in metropolitan areas (72.9%; 95% CI: 70.6–75.3) based on rural-urban commuting area codes29 (Table 1). Across the study population, in utero polysubstance exposure, defined as exposure to an opioid and an additional psychotropic substance (exclusive of nicotine), identified by maternal history or maternal or infant toxicology screens, was documented in 63.5% (95% CI: 60.9–66.0) of infants, and the number of exposures ranged from 1 to 7 (mean 1.8 ± 1.1) with 117 unique exposure combinations. Across sites, the proportion of infants with in utero polysubstance exposure ranged from 29.7% to 100% (mean 62.9%; 95% CI: 57.7–68.1) and the mean number of substances infants were exposed to in utero ranged across sites from 1.1 to 2.7 (mean 1.8 ± 0.4) (Fig 1).
Management and Outcomes
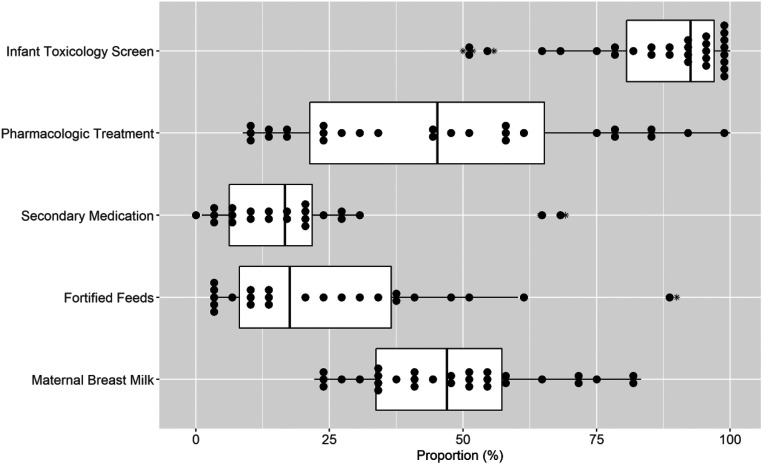
Across the study population, infant toxicology screens were obtained for 85.2% (95% CI: 83.3–87.1) of infants. Use of infant toxicology screens varied across sites, with 50% to 100% of infants being screened (mean 86.4%; 95% CI: 80.9–92.0) (Fig 2). Infants with NOWS received care in various hospital locations. Across sites with both a level 1 nursery and a level ≥2 care setting (n = 26), the proportion of infants cared for in the level 1 care setting at some point after delivery ranged from 11.8% to 100% (mean 74.1%; 95% CI: 64.2–83.9) and the proportion of infants cared for in the level ≥2 care setting ranged from 19.1% to 100% (mean 65.8%; 95% CI: 54.8–76.8). Of those infants who received pharmacologic therapy at these sites, the proportion of infants cared for in the level ≥2 care setting at some point during their admission ranged from 7.9% to 87.8% (mean 42.2%; 95% CI: 32.9–51.5).
FIGURE 2.
Site-level variation in infant management. The box plots consist of the 25th (quartile 1), 50th (median), and 75th (quartile 3) percentiles. The length of the whiskers represents the minimum (quartile 1 – 1.5 × IQR) and maximum (quartile 3 + 1.5 × IQR), where IQR = quartile 3 – quartile 1 (IQR). Each dot represents a single site proportion and the asterisk denotes outliers.
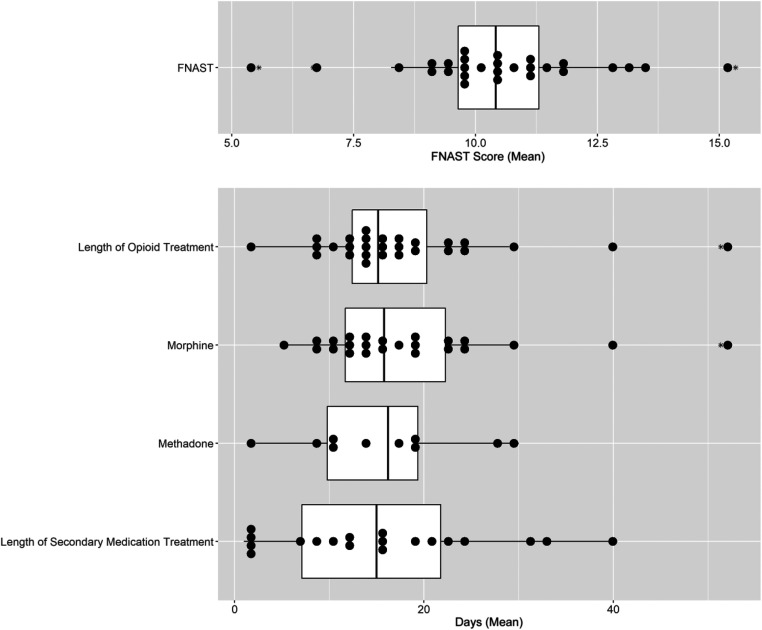
All infants were assessed for withdrawal severity by using the FNAST (or modification thereof). For infants who received pharmacologic therapy, the mean of the 3 scores before the initiation of pharmacologic therapy was determined for each site. Sites were pooled for this analysis because thresholds for these tools are comparable. Site means ranged from 5.6 to 15.3 (mean 10.5 ± 2.0; Fig 3).
FIGURE 3.
Site-level variation in the initiation and length of pharmacologic therapy. The box plots consist of the 25th (quartile 1), 50th (median), and 75th (quartile 3) percentiles. The length of the whiskers represents the minimum (quartile 1 – 1.5 × IQR) and maximum (quartile 3 + 1.5 × IQR), where IQR = quartile 3 – quartile 1 (IQR). Each dot represents a single site mean and the asterisk denotes outliers.
Across the study population, 48.3% (95% CI: 45.7–50.9; n = 665) of infants received pharmacologic therapy for NOWS. Of these infants, 85.9% (95% CI: 83.2–88.5) received morphine as the primary pharmacologic treatment, whereas 13.4% (95% CI: 10.8–16.0) received methadone, 0.3% (95% CI: 0.0–0.7) received buprenorphine, and 0.3% (95% CI: 0.0–0.7) received phenobarbital as their primary medication. Of the pharmacologically treated infants, 32.2% (95% CI: 28.6–35.7) received a secondary medication. Across sites, the proportion of infants receiving pharmacologic therapy ranged from 6.7% to 100% (mean 40.2%; 95% CI: 29.7–50.7) (Fig 2). The use of secondary medications also varied across sites (Fig 2). Of the 28 sites that provided pharmacologic therapy for NOWS, 5 sites never used secondary medications. Among the remaining sites, the proportion of pharmacologically treated infants who received a secondary medication ranged from 1.1% to 69.2%. Across the study population, clonidine was the most frequently used secondary medication (54.2%; 95% CI: 47.5–60.9), followed by phenobarbital (37.4%; 95% CI: 30.9–43.9), morphine (4.7%; 95% CI: 1.9–7.5), and methadone (3.7%; 95% CI: 1.2–6.3).
In addition to the site variation seen in the initiation of pharmacologic therapy, there was also variation observed in the use of these medications. For example, for infants receiving morphine, site-to-site variation was observed in the mean maximum morphine dose administered, with a range of 0.038 to 0.129 mg/kg (mean 0.069 ± 0.020 mg/kg), and in the mean final morphine dose given before discontinuation, with a range of 0.004 to 0.053 mg/kg (mean 0.022 ± 0.014 mg/kg).
Of the infants receiving pharmacologic therapy, 57 of the 662 infants (8.6%; 95% CI: 6.5–10.8) who received an opioid as their primary medication while inpatient were discharged from the hospital or transferred to another institution while still receiving opioid therapy. Of the 214 infants who received a secondary medication while inpatient, 96 infants (44.9%; 95% CI: 38.2–51.5) were discharged or transferred on this medication. Of those infants who completed pharmacologic therapy while inpatient, the mean opioid LOT varied across sites from 2.3 to 51.3 days (mean 17.7 ± 9.8 days) and the mean secondary medication LOT varied from 1 to 39.5 days (mean 15.4 ± 11.4 days) (Fig 3).
All sites used multiple nonpharmacologic measures as part of the first-line therapy for NOWS. However, variation was seen across sites in the use of individual practices. For example, the proportion of infants receiving fortified feeds ranged from 2.9% to 90.0% (mean 24.6%; 95% CI: 15.3–33.9), and the proportion of infants receiving maternal breast milk during the birth admission ranged from 22.2% to 83.3% (mean 47.9%; 95% CI: 41.4–54.4) (Fig 2).
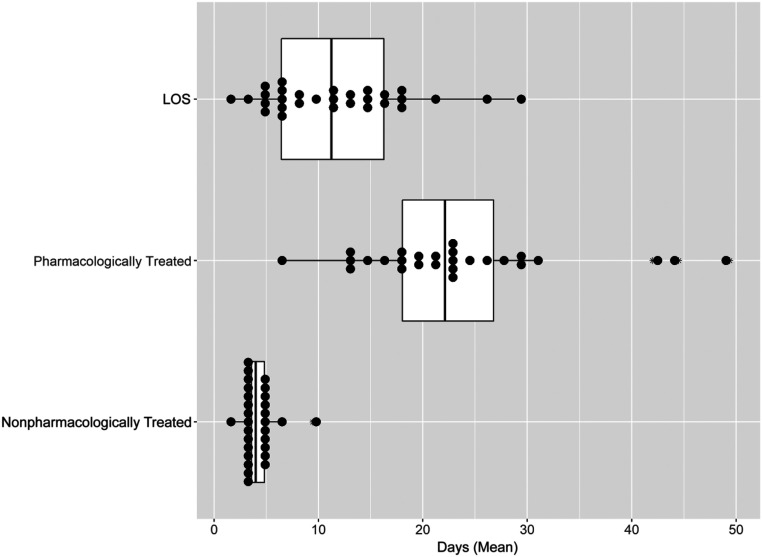
Mean LOS varied across sites from 2 to 28.8 days (mean 11.6 ± 6.7 days). This range consisted of 2 nearly distinct distributions, with site-level variation seen in LOS for both pharmacologically treated infants (across sites, the mean LOS ranged from 6.5 to 49.4 days [mean 23.5 ± 9.7 days]) and nonpharmacologically treated infants (across sites, the mean LOS ranged from 2 to 9.5 days [mean 4.3 ± 1.4 days]) (Fig 4). Across the study population, most infants were discharged from the hospital with a parent (75.7%; 95% CI: 73.4–77.9), whereas 9.0% (95% CI: 7.5–10.5) were discharged with a relative and 12.3% (95% CI: 10.5–14.0) with adoptive parents or into foster care. Across sites, the proportion of infants discharged from the hospital with a parent ranged from 33.3% to 91.1% (mean 70.7%; 95% CI: 66.0–75.4).
FIGURE 4.
Site-level variation in infant LOS. The box plots consist of the 25th (quartile 1), 50th (median), and 75th (quartile 3) percentiles. The length of the whiskers represents the minimum (quartile 1 – 1.5 × IQR) and maximum (quartile 3 + 1.5 × IQR), where IQR = quartile 3 – quartile 1 (IQR). Each dot represents a single site mean and the asterisk denotes outliers.
Discussion
Marked site-level variation was identified in this multicenter, geographically diverse observational study of infants with NOWS. Substantial variation was identified in maternal-infant characteristics and in nearly every aspect of infant management and outcomes explored. There was remarkable variation in the receipt of pharmacologic therapy, which likely represents a driver for the variation observed in LOS. In this study, we build on the existing literature by not only demonstrating an association between LOS and pharmacologic therapy,30 but by also describing variations in care that underlie this observation. Specifically, wide variation was seen across sites in many key parameters: FNAST (or modification thereof) score thresholds used to prompt initiation of pharmacologic therapy, the maximum dose of morphine administered; the final morphine dose administered before discontinuation, LOT, and the use of secondary medications. Wide variation was also seen across sites in MAT use during pregnancy, use of prenatal care, and custodial care at discharge. Although this variation is arguably less modifiable by clinicians caring for infants with NOWS, it serves to illustrate relevant variations in population context. These population-based variations, although they may lie beyond the direct care of the infant, likely influence infant outcomes and may be modifiable by clinical care elsewhere in the health care system or through policy changes.
This work is an important contribution to the literature surrounding NOWS because of the breadth of geographic regions from which the population was drawn and the number of hospitals engaged and serves to provide a foundation for future work in the field. Understanding site-to-site variation in NOWS care is a critical first step to appropriately minimizing this variation. Although, as noted, some of the observed site-level variation may be due to population differences or warranted variation, which is unmodifiable and anticipated,19 it is also likely that a significant portion of the variation seen is unwarranted.19 Although unwarranted variation can have several causes,31 in this case, it appears likely that it results from gaps in the evidence base to support standard care practices for infants with NOWS, resulting in a lack of consensus for management guidelines.19,31,32 Potentially modifiable, unwarranted variation can and should be minimized to improve outcomes. Local efforts to decrease unwarranted variation through the protocolization of care for infants with NOWS have shown promise in the ability to achieve this goal.32–35 Nationally, the NOWS landscape is shifting quickly as clinicians and researchers look to improve care and develop an evidence base to support a standard practice. Although critically important, it is likely that these efforts have further contributed to the site-level variation described. In the current study, we highlight the need for collaborative, high-quality research to provide the generalizable evidence needed for the development of standard practice guidelines. Fortunately, such research is currently being undertaken.36 However, these research efforts take time; thus, acknowledging the variation described, whether warranted, is critical. This variation may significantly hinder the generalizability of results from single-center clinical trials and quality improvement work to a broader population.
This observational study is limited by potential selection bias in the use of sites from the ACT NOW Collaborative, which may not be wholly representative of the opioid epidemic, challenges associated with identification of the desired population that were due in part to limitations of the individual site EMRs, and limitations inherent to documentation in the medical record and medical record abstraction accuracy. However, extensive procedures were implemented to maximize the quality of abstraction. Lastly, in this study, we did not explore the degree to which warranted variation contributed to the variation observed because it was outside of the scope of this work. Given the wide variation in the volume of infants with NOWS cared for at each site, we did consider the possibility that the variation observed in this study may be due in part to the variation in site volume. We observed no statistical differences by site volume of NOWS in the variables presented (Supplemental Table 2, Supplemental Figs 8–11) with the exception of the proportion of mothers receiving MAT. Across sites, the mean proportion of mothers receiving MAT was larger among high-volume hospitals compared to low- and medium-volume hospitals.
Conclusions
Understanding the current landscape of NOWS is critical to inform decisions made regarding future research and the development of programs, policies, and practices to improve care for these infants. The degree of variation in the characteristics, management, and outcomes of infants with NOWS observed in this study is substantial and likely greater than clinicians working in their own local settings would have expected. Although the variation described likely reflects a lack of optimal care for many infants with NOWS, it also identifies a significant opportunity to improve the care provided to these infants.
Acknowledgments
The following investigators, in addition to those listed as authors, participated in this study:
ISCPTN Steering Committee Chair: Jill G. Joseph, MD PhD.
NRN Steering Committee Chair: Richard A. Polin, MD (Division of Neonatology, College of Physicians and Surgeons, Columbia University; 2011–present).
Alaska Native Medical Center (Alaska Native Tribal Health Consortium and Southcentral Foundation) (ISCPTN: UG1OD024944): Rosalyn Singleton, MD; Matthew Hirschfeld, MD PhD; Jennifer Shaw, PhD; Amy Swango-Wilson, RN PhD; Mary Herrick, MD; and Christine Hallas, PNP.
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (NRN: U10 HD27904; ISCPTN: UG1 OD024951): Abbot Laptook, MD; Martin Keszler, MD; Thomas Chun, MD; Phyllis Dennery, MD; Angelita M. Hensman, PhD, MS, RNC-NIC; and Elizabeth Trailburns, RN.
Arkansas Children’s Research Institute, University of Arkansas for Medical Sciences, and Arkansas Children’s Hospital (ISCPTN: UG1OD024945): Clare Nesmith, MD; Sherry Courtney, MD; Frederick Barr, MD; Laura James, MD; Denise Pearson, RN, CPN; Jana McConnell, RN; and Melanie Mason, RN.
Case Western Reserve University, University Hospital Cincinnati's Cleveland Medical Center, and Rainbow Babies & Children’s Hospital (NRN: UG1 HD21364): Michele Walsh, MD; Moira Crowley, MD; Anna Marie Hibbs, MD; Nancy S. Newman, BA, RN; and Leslie Clarke, RN, BSN, MBA.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (NRN: UG1 HD27853): Cathy Grisby, BSN, CCRC; Traci Beiersdorfer, BSN; and Greg Muthig, BS.
Dartmouth College, Dartmouth-Hitchcock Medical Center, and Children’s Hospital at Dartmouth-Hitchcock (ISCPTN: UG1OD024946): Paul Palumbo, MD; J. Dean Jarvis, BSN, MBA, RN; and Mary McNally, BSRT, RRT.
Duke University ECHO Coordinating Center (1U2COD023375-01): P. Brian Smith, MD.
ECHO Program, Office of the Director, National Institutes of Health: Carol J. Blaisdell, MD, MEd; Mary Roary, PhD; and Divya Kalaria, MD.
NICHD: Andrew Bremer, MD; and Stephanie Wilson Archer, MA.
The Robert Larner, M.D. College of Medicine at The University of Vermont, The University of Vermont Children’s Hospital, and The University of Vermont Medical Center (ISCPTN: UG1OD024955): Adrienne Pahl, MD; Kelly Cowan, MD; Jerilyn Matayer, RN; Ethan Jones, MPH; and Laurie Chassereau, RN.
Louisiana State University, Pennington Biomedical Research Center, Tulane University School of Medicine, and Tulane Lakeside Hospital for Women and Children (ISCPTN: UG1OD024959): Stacy Drury, MD, PhD; Elizabeth Lindsay, MD; Daniel S. Hsia, MD; Kelsey Confreda, MPH; Cade Herman, BS; and Tegan Clarke, BA.
Nationwide Children’s Hospital, The Abigail Wexner Research Institute at Nationwide Children’s Hospital, The Ohio State University Wexner Medical Center, The Ohio State College of Medicine, and Center for Perinatal Research (NRN: UG1 HD68278): Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Nathalie L. Maitre, MD, PhD; Kristina M. Reber, MD; Erin L. Keel, DNP, APRN, NNP-BC; Patricia Luzader, RN; Margaret K. Burns, RN BSN, MS; Jacqueline McCool; Teri McCarty, PharmD; and Pavel Prusakov, PharmD.
Nemours/Alfred I. duPont Hospital for Children and ChristianaCare (ISCPTN: UG1OD024958): Judith Ross, MD; Kelly Gray, RN; Amy Mackley, CNS, MSN; and Karen Kowal, PAC.
Research Triangle Institute International (U10 HD36790): Carla M. Bann, PhD; Jamie E. Newman, PhD, MPH; Jeanette O’Donnell Auman, BS; Marie G. Gantz, PhD; and Kristin M. Zaterka-Baxter, RN BSN CCRP.
University of Arkansas for Medical Sciences Data Coordination and Operations Center and Department of Biostatistics (ISPCTN: U24OD024957): Jeannette Lee, PhD; Anita Walden, MS; Kimberly Harris, PhD; Amy Doville, MBA, CCRP; Irene Chedjieu, BDS, MPH; Lora Lawrence, RN, CCRP; Emil Seker, MSIQ; Vaishali Thombre, MS; and Sunitha Kenchey, MS.
University of Hawaii at Manoa and the Kapiolani Medical Center for Women and Children (ISCPTN: UG1OD024948): Akshatha, MD; Charles Neal, MD; Bruce Shiramizu, MD; Moara Palma, PhD; and Annette Amiotte, RN, BSN.
University of Kansas Medical Center, the University of Kansas Health System, Children’s Mercy Kansas City, and Pittsburg State University Irene Ransom Bradley School of Nursing (ISCPTN: UG1OD024943): Krishna Dummula, MD; Barbara Pahud, MD; Kristi Frisbee, DNP; Melissa Lopez, BSN; Barbara McClaskey, MN, PhD; and Megan Bledsoe, PhDc, MSc.
University of Louisville, Norton Hospital Newborn Nursery, and Norton Children's Hospital (ISCPTN: UG1OD024954): Sara Watson, MS, MD; Janice Sullivan, MD; Jennifer Nason, RN, BSN; Laura Thomas, RN, BSN; Stephanie Houston, BSN, RNC-NIC; and Jackie Perry Boyd, RN, BSN.
University of Mississippi Medical Center (ISCPTN: UG10D024942): Lauren Tucker, MD; J. Marc Majure, MD; Dana Lindsay, RN, BSN; Takila Keys, MHS, RHIA; and Lacy Malloch, BS.
University of Montana, Community Medical Center, St. Vincent Healthcare, and Billings Clinic (ISCPTN: UG1OD024952): Paul Smith, DO; Lauren Parks, PharmD; Helen Rusette, MPH; Sara Cox-McClure, RN, BSN; Susan McAtee, RN, BSN; and Dawn Hedstrom, RN-NIC.
University of Nebraska Medical Center (ISCPTN: UG1OD024953): Ann Anderson Berry, MD, PhD; Russell McCulloh, MD; Kelly Erickson, MPH; and Rachel Wellman, RN, BSN, MS.
University of New Mexico Health Sciences Center and University of New Mexico School of Medicine (NRN: U10 HD53089; ISCPTN: UG1OD024947; National Center for Advancing Translational Sciences: UL1 TR41): Jessie Maxwell, MD; Heather Pratt-Chavez, MD; Kristi L. Watterberg, MD; Alberta Kong, MD; Hengameh Raissy, PharmD; Grace McCauley, MPH; Sandra Beauman, MSN, RNC-NIC; Sara Sanders, RN, MS; Mary Hanson, RN; and Olivia Nunez, BS.
University of Oklahoma Health Sciences Center, Comanche County Memorial Hospital, and University of Oklahoma Children’s Hospital (ISCPTN: UG1OD024950): Kimberly Ernst, MD; Abhishek Makkar, MD; Edgardo Szyld, MD; Paul Darden, MD; Christi Madden, MPA; Michael McCoy, MS, APRN; Ashley Anderson, RN, BSN; Erin Bohon, LPN; Erica Doefler, NNP; LaDale Johnson, NNP; Lindsay Cobianchi, BS; and Shannon Wilson, CMA.
University of South Carolina at Columbia, Spartanburg Medical Center, Medical University of South Carolina, McLeod Regional Medical Center, and Pediatrix Medical Group of South Carolina (ISCPTN: UG1OD024956): Jaime Brown, MD; Efrain Sanchez-Rivera, MD; Laura Valleni, MD; Julie Ross, MD; Brian Wood, MD; Lisa Knight, MD; Hanna Sahhar, MD; Devonne Gerstenacker, BS, RN, MSN; Sarah Roland, RN, MSN; Veronia Oakes, BSN, RNC-NIC; Heather Heape, RN; Barbara Thompson, LPN; Sarah Newman-Norland, MA; Mary Freeman, BS; and Carolyn Emeneker, BA.
West Virginia University (ISCPTN: UG1OD024949): Lesley Cottrell, PhD; Lee Pyles, MD; Phillip Saul, MD; Michelle Schaffer, RN; and Kaitlyn Earle, BSN.
The National Institutes of Health, the NICHD, and the National Center for Advancing Translational Sciences provided support for the NRN, and the ECHO Program (of the National Institutes of Health Office of the Director) provided support for the ISPCTN. Although the NICHD and NIH ECHO staff had input into the study design, conduct, analysis, and article drafting, the comments and views of the authors do not necessarily represent the views of NICHD or the ECHO Program, the National Institutes of Health, the Department of Health and Human Services, or the US government.
Data were collected at participating sites of the NICHD NRN and participating sites of the ECHO ISPCTN and transmitted to the Data Coordination and Operations Center of the University of Arkansas for Medical Sciences for this study. Drs Jeannette Lee and Jessica Snowden (Data Coordination and Operations Center principal investigators) had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We thank our medical and nursing colleagues who agreed to take part in this study.
Glossary
- ACT NOW
Advancing Clinical Trials in Neonatal Opioid Withdrawal
- CI
confidence interval
- ECHO
Environmental influences on Child Health Outcomes
- EMR
electronic medical record
- FNAST
Finnegan Neonatal Abstinence Scoring Tool
- IQR
interquartile range
- ISPCTN
Institutional Development Award States Pediatric Clinical Trials Network
- LOS
length of stay
- LOT
length of treatment
- MAT
medication-assisted treatment
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NOWS
neonatal opioid withdrawal syndrome
- NRN
Neonatal Research Network
Footnotes
Drs Young, Devlin, Das, Higgins, Merhar, Simon, P. Smith, Poindexter, and Snowden made substantial contributions to the conception and design of the study, interpretation of the data, and drafting of the manuscript; Drs Fuller, Paul, Sánchez, and Whalen and Ms Crawford contributed to the design of the data collection forms and data acquisition; Dr Ounpraseuth and Mr Hu conducted statistical analysis, provided interpretation of the data, substantially contributed to the Methods and Results sections of the manuscript, and created graphics; Drs Annett, Lester, Atz, Cottrell, Czynski, Newman, Semmens, M. Smith, and Turley helped with acquisition of data and provided input on data interpretation; and all authors have reviewed the manuscript for important intellectual content, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: All phases of this study were supported by the following grants: U10 HD27904, U10 HD36790, U10 HD53089, U24OD024957, UG1 HD21364, UG1 HD27853, UG1 HD68278, UG1OD024942, UG1OD024943, UG1OD024944, UG1OD024945, UG1OD024946, UG1OD024947, UG1OD024948, UG1OD024949, UG1OD024950, UG1OD024951, UG1OD024952, UG1OD024953, UG1OD024954, UG1OD024955, UG1OD024956, UG1OD024958, UG1OD024959, UL1 TR41, 1U2COD023375-01. This article is the product of work from the National Institute of Child Health and Human Development Neonatal Research Network and the Environmental influences on Child Health Outcomes Institutional Development Award States Pediatric Clinical Trials Network. Both networks are cooperative agreements with the National Institutes of Health. Only National Institutes of Health staff listed as authors have contributed to this article. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012 [published correction appears in J Perinatol. 2015;35(8):667]. J Perinatol. 2015;35(8):650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934–1940 [DOI] [PubMed] [Google Scholar]
- 3.Clark ME, Cummings BM, Kuhlthau K, Frassica N, Noviski N. Impact of pediatric intensive care unit admission on family financial status and productivity: a pilot study. J Intensive Care Med. 2019;34(11–12):973–977 [DOI] [PubMed] [Google Scholar]
- 4.Lyu H, Xu T, Brotman D, et al. Overtreatment in the United States. PLoS One. 2017;12(9):e0181970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118–2126 [DOI] [PubMed] [Google Scholar]
- 6.Holmes AV, Atwood EC, Whalen B, et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics. 2016;137(6):e20152929. [DOI] [PubMed] [Google Scholar]
- 7.Wachman EM, Schiff DM, Silverstein M. Neonatal abstinence syndrome: advances in diagnosis and treatment. JAMA. 2018;319(13):1362–1374 [DOI] [PubMed] [Google Scholar]
- 8.Reddy UM, Davis JM, Ren Z, Greene MF; Opioid Use in Pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes Workshop Invited Speakers . Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol. 2017;130(1):10–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Substance Abuse and Mental Health Services Administration Clinical Guidance for Treating Pregnant and Parenting Women With Opioid Use Disorder and Their Infants. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018. Available at: https://store.samhsa.gov/product/Clinical-Guidance-for-Treating-Pregnant-and-Parenting-Women-With-Opioid-Use-Disorder-and-Their-Infants/SMA18-5054. Accessed April 9, 2019
- 10.US Government Accountability Office Newborn Health: Federal Action Needed to Address Neonatal Abstinence Syndrome. Washington, DC: US Government Accountability Office; 2017. Available at: https://www.gao.gov/assets/690/687580.pdf. Accessed November 1, 2017
- 11.Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e540 [Google Scholar]
- 12.Goodman DC, Little GA, Harrison WN, Moen A, Mowitz ME, Ganduglia-Cazaban C. The Dartmouth Atlas of Neonatal Intensive Care. Lebanon, NH: The Dartmouth Institute of Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth; 2019 [PubMed] [Google Scholar]
- 13.Hall ES, Wexelblatt SL, Crowley M, et al. ; OCHNAS Consortium. A multicenter cohort study of treatments and hospital outcomes in neonatal abstinence syndrome. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/134/2/e527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA. Variation in treatment of neonatal abstinence syndrome in US children’s hospitals, 2004-2011. J Perinatol. 2014;34(11):867–872 [DOI] [PubMed] [Google Scholar]
- 15.Friedman H, Parkinson G, Tighiouart H, et al. Pharmacologic treatment of infants with neonatal abstinence syndrome in community hospitals compared to academic medical centers. J Perinatol. 2018;38(12):1651–1656 [DOI] [PubMed] [Google Scholar]
- 16.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–17 [DOI] [PubMed] [Google Scholar]
- 17.Bogen DL, Whalen BL, Kair LR, Vining M, King BA. Wide variation found in care of opioid-exposed newborns. Acad Pediatr. 2017;17(4):374–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snowden JN, Akshatha A, Annett RD, et al. The ACT NOW clinical practice survey: gaps in the care of infants with neonatal opioid withdrawal syndrome. Hosp Pediatr. 2019;9(8):585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dartmouth Atlas Project. The Dartmouth Atlas of Health Care. Available at: https://www.dartmouthatlas.org/. Accessed August 29, 2019
- 20.Environmental influences on Child Health Outcomes. Clinical trials (ECHO IDeA States Pediatric Clinical Trials Network). Available at: https://echochildren.org/idea-states-pediatric-clinical-trials-network/. Accessed October 1, 2019
- 21.Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Who we are. Available at: https://neonatal.rti.org/. Accessed October 1, 2019
- 22.National Institutes of Health Helping to end addiction long-term initiative. Available at https://heal.nih.gov/. Accessed October 1, 2019
- 23.Zozus MN, Pieper C, Johnson CM, et al. Factors affecting accuracy of data abstracted from medical records. PLoS One. 2015;10(10):e0138649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zozus MN, Young LW, Simon AE, et al. Training as an intervention to decrease medical record abstraction errors multicenter studies. Stud Health Technol Inform. 2019;257:526–539 [PMC free article] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics Committee on Fetus And Newborn Levels of neonatal care. Pediatrics. 2012;130(3):587–597 [DOI] [PubMed] [Google Scholar]
- 26.Finnegan LP, Connaughton JF Jr., Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2(1–2):141–158 [PubMed] [Google Scholar]
- 27.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Survey of morbidity and mortality among high risk preterm infants (GDB) manual of operations. Available at: https://neonatal.rti.org/pdf/GDBPublic/Public_GDB_Manual.pdf. Accessed October 12, 2018
- 29.US Department of Agriculture Rural-urban commuting area (RUCA) codes. Available at: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/. Accessed October 12, 2018
- 30.Milliren CE, Gupta M, Graham DA, Melvin P, Jorina M, Ozonoff A. Hospital variation in neonatal abstinence syndrome incidence, treatment modalities, resource use, and costs across pediatric hospitals in the United States, 2013 to 2016. Hosp Pediatr. 2018;8(1):15–20 [DOI] [PubMed] [Google Scholar]
- 31.Wennberg JE. Tracking Medicine: A Researcher’s Quest to Understand Health Care. New York, NY: Oxford University Press; 2010 [Google Scholar]
- 32.Goodman DC. Unwarranted variation in pediatric medical care. Pediatr Clin North Am. 2009;56(4):745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patrick SW, Schumacher RE, Horbar JD, et al. Improving care for neonatal abstinence syndrome. Pediatrics. 2016;137(5):e20153835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohio Children’s Hospital Neonatal Research Consortium. Enteral morphine or methadone protocols for neonatal abstinence syndrome (NAS) from maternal exposure. Available at: https://opqc.net/sites/bmidrupalpopqc.chmcres.cchmc.org/files/NAS/Ohio%20Childrens%20NAS%20Treatment%20Protocol%200822%202013%20%20FINALrev2.pdf. Accessed August 22, 2013
- 35.Asti L, Magers JS, Keels E, Wispe J, McClead RE Jr. A quality improvement project to reduce length of stay for neonatal abstinence syndrome. Pediatrics. 2015;135(6). Available at: www.pediatrics.org/cgi/content/full/135/6/e1494 [DOI] [PubMed] [Google Scholar]
- 36.National Institutes of Health ; Helping to End Addiction Long-term Initiative. NIH HEAL Initiative research plan. Available at: https://heal.nih.gov/about/research-plan#references. Accessed October 1, 2019
- 37.National Institutes of Health ; National Center for Complementary and Integrative Health. Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. Available at: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html. Accessed October 12, 2018