NAS is increasing in incidence, but there is no gold standard definition used across clinical care and public health settings.
Abstract
BACKGROUND AND OBJECTIVES:
National estimates indicate that the incidence of neonatal abstinence syndrome (NAS), a postnatal opioid withdrawal syndrome, increased more than fivefold between 2004 and 2016. There is no gold standard definition for capturing NAS across clinical, research, and public health settings. Our objective was to evaluate how different definitions of NAS modify the calculated incidence when applied to a known population of opioid-exposed infants.
METHODS:
Data for this retrospective cohort study were obtained from opioid-exposed infants born at Vanderbilt University Medical Center in 2018. Six commonly used clinical and surveillance definitions of opioid exposure and NAS were applied to the study population and evaluated for accuracy in assessing clinical withdrawal.
RESULTS:
A total of 121 opioid-exposed infants met the criteria for inclusion in our study. The proportion of infants who met criteria for NAS varied by predefined definition, ranging from 17.4% for infants who received morphine to 52.8% for infants with the diagnostic code for opioid exposure. Twenty-eight infants (23.1%) received a clinical diagnosis of NAS by a medical provider, and 38 (34.1%) received the diagnostic code for NAS at discharge.
CONCLUSIONS:
We found significant variability in the incidence of opioid exposure and NAS among a single-center population using 6 common definitions. Our findings suggest a need to develop a gold standard definition to be used across clinical, research, and public health surveillance settings.
What’s Known on This Subject:
Neonatal abstinence syndrome is a drug withdrawal syndrome that affects infants exposed to opioids during pregnancy. The rise in the opioid crisis has led to an increase in the syndrome’s incidence in the United States.
What This Study Adds:
Different definitions of neonatal abstinence syndrome and opioid exposure substantially influence estimates of incidence, highlighting the need for standardization across clinical, research, and public health settings.
Neonatal abstinence syndrome (NAS) is a postnatal drug withdrawal syndrome that occurs among opioid-exposed infants.1 The syndrome consists of a constellation of clinical signs, including irritability, hypertonia, tremors, poor feeding, and gastrointestinal disturbance. Infants with NAS have longer hospital stays, resulting in increasing burden on the health care system and caregivers.2 As the opioid crisis expanded across the United States, the incidence of NAS also grew in parallel, increasing nearly fivefold between 2004 and 2014, before plateauing at 8.8 per 1000 births in 2016.3,4
Despite the recent increasing incidence of NAS, there remains no gold standard clinical or surveillance definition of the syndrome. Semiobjective clinical tools, such as modifications of the Finnegan scoring system, are used widely across hospital systems to provide a measure of withdrawal signs and assist with determining the need for pharmacologic therapy.5 However, none of the current clinical tools to diagnose NAS have been validated, and each suffers from issues related to interrater reliability. A lack of a validated, standardized approach to diagnosing NAS may leave providers with diagnostic uncertainty and, ultimately, may lead to inconsistency in the diagnosis in clinical and research settings.6
More recently, federal and state governments created initiatives to improve public health surveillance of the syndrome and, in some cases, opioid exposure more broadly.7–10 In June 2019, the Council of State and Territorial Epidemiologists published a position statement recommending standardized surveillance definitions for use across US jurisdictions; however, uptake of the Council of State and Territorial Epidemiologists surveillance definitions is unclear. At time of this assessment, 9 states considered NAS a reportable condition,11 but each varied in their approaches. For example, some states (eg, AZ), require documented neonatal clinical signs of withdrawal, whereas others (eg, MA) rely on billing codes for surveillance. Georgia is the only state that requires reporting of all positive infant toxicology results, even in the absence of clinical signs of withdrawal.12
Although there is a strong interest in measuring opioid exposure and NAS among newborns, there remains a limited understanding of how differences in clinical and public health measurements may influence detection. To address this gap in the literature, we evaluated how the incidence of NAS differs by various clinical and surveillance definitions.
Methods
Data Sources
Data for this retrospective cohort study were obtained from Team Hope, an interdisciplinary team at Vanderbilt University Medical Center (VUMC) focused on improving the care of the opioid-exposed mother-infant dyad.13 Our study population was composed of all opioid-exposed infants born at VUMC in 2018 who were ≥35 weeks’ gestation and without critical illness (defined as a NICU stay ≥5 days). Infants were identified as opioid-exposed during clinical care by using standardized screening tools augmented by toxicology testing. Infants were cared for in the newborn nursery and/or pediatric inpatient wards and were followed by Team Hope during the course of their hospitalization. Patient demographics, clinical characteristics, and outcomes for study infants were obtained from the Team Hope database and manual chart review. Internal VUMC administrative billing data from discharge abstracts were obtained for all infants included in our study. This study was considered exempt from human subjects review by the VUMC Institutional Review Board.
Covariates and Outcomes
Data collected for all infants included sex, gestational age, maternal history of opioid use, maternal and neonatal drug screen results, hospital length of stay (LOS), modified Finnegan scores,14,15 clinical diagnosis of NAS and receipt of morphine. We defined NAS in VUMC administrative data using the International Classification of Diseases, 10th Revision, Clinical Modification, (ICD-10-CM) code P96.1 (neonatal withdrawal symptoms from maternal drugs of addiction). We defined opioid exposure using the ICD-10-CM code P04.49 (newborn affected by maternal use of other drugs of addiction). The ICD-10-CM code P04.14 (newborn affected by maternal use of opiates) was also included in the definition of opioid exposure when this code became available in October 2018.
Definitions
Informed by the literature16–19 and clinical practice, we evaluated a number of definitions of NAS. We analyzed both the clinical aspects of an infant’s hospitalization as well as surveillance measures, including state reporting and administrative billing codes. We applied the following 6 proposed definitions of opioid exposure and NAS to our study population:
infants with modified Finnegan scores of 8 twice in a row or 12 once;
infants ever having received morphine during hospitalization;
infants with a clinical diagnosis of NAS by a medical provider;
infants reported to the Tennessee Department of Health with NAS (state surveillance); Tennessee state definition is a history of maternal drug exposure as well as evidence of effect on infant (clinical signs of withdrawal or laboratory confirmatory drug tests);
infants assigned ICD-10-CM P96.1 at hospital discharge (diagnostic code for NAS); and
infants assigned ICD-10-CM P04.49 or ICD-10-CM P04.14 at hospital discharge (diagnostic codes for opioid exposure).
Analysis
We calculated descriptive statistics for all opioid-exposed infants included in our study and evaluated how different definitions of NAS influenced the incidence of the syndrome. Next, we evaluated how these definitions were affected by an infant’s LOS by separating the study population into 2 groups: infants with an LOS ≤5 days and infants with an LOS ≥6 days. An LOS of 5 days was selected as a sample cutoff because of current AAP recommendations that infants should be observed in the hospital for signs of clinical withdrawal for a minimum of 3 days after exposure to short-acting opioids or 5 to 7 days after exposure to long-acting opioids.5
Results
A total of 121 opioid-exposed Team Hope infants met the criteria for inclusion in our study. Forty-eight percent of the infants were male, and the median gestational age was 39.0 weeks (interquartile range [IQR] 37.6–39.6). Maternal toxicology testing results were positive for at least 1 substance in 104 of 110 (94.5%) of patients with available records. The remaining infants were identified on the basis of a known maternal history of opioid use documented in their medical record. The majority of infants (119 out of 121) were scored by using a modification of the Finnegan score during their hospital stay, with a median maximum score of 7.0 (IQR 5.0–10.0). A total of 28 infants were clinically diagnosed with NAS by a medical provider during their hospital stay. The baseline characteristics for infants with opioid exposure or NAS are included in Table 1.
TABLE 1.
Clinical Characteristics for Opioid-Exposed Infants With and Without a NAS Diagnosis by a Medical Provider
| Full Study Population | Without NAS Diagnosis | NAS Diagnosis | |
|---|---|---|---|
| No. infants | 121 | 93 | 28 |
| Male, n (%) | 58 (47.9) | 47 (50.5) | 11 (39.3) |
| Gestational age in wk, median (IQR) | 39.0 (37.6–39.6) | 38.7 (37.4–39.6) | 38.9 (37.8–39.6) |
| Maternal drug tests positive for 1≥ substances, % | 94.5 | 94.1 | 96.0 |
| Maximum modified Finnegan score, median (IQR) | 7.0 (5.0–10.0) | 6.9 (5.0–8.0) | 12.5 (10.0–14.3) |
| LOS in d, median (IQR) | 5.0 (5.0–7.0) | 5.0 (5.0–6.0) | 14.5 (7.8–16.0) |
Evaluation of Definitions
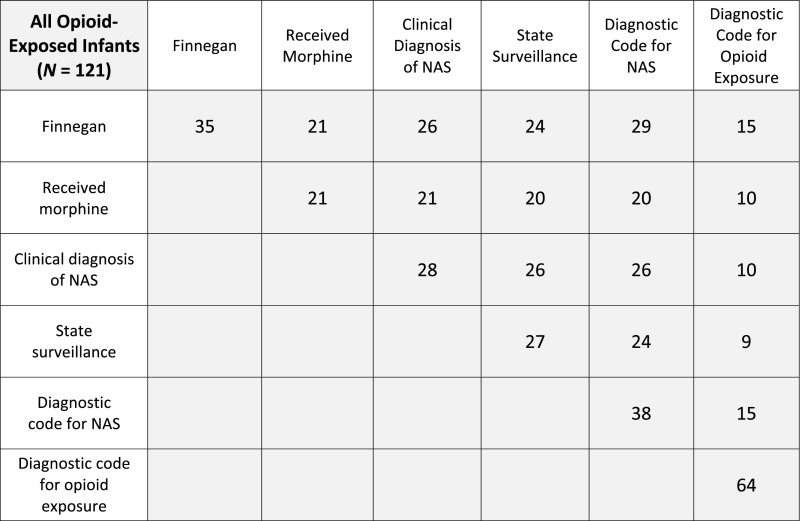
We applied the 6 predefined definitions to our study population and evaluated the clinical characteristics of infants captured by each definition. Of the 35 infants that received a Finnegan score of 8 twice consecutively or 12 once during their hospitalization, a common standard for initiating pharmacologic treatment, a total of 26 (74.3%) infants were clinically diagnosed with NAS by a medical provider, and 21 (60.0%) received morphine (Fig 1). Two additional infants received a clinical diagnosis of NAS during their hospitalization without meeting the semiobjective Finnegan score setpoint. The NAS diagnostic code (ICD-10-CM code P96.1) was assigned to a total of 38 study infants at the time of discharge, 26 (68.4%) of whom received a clinical diagnosis of NAS. Two infants were clinically diagnosed with NAS by a medical provider but did not receive the NAS diagnosis code at time of discharge: the first infant received morphine for pharmacologic management of NAS, and the other had NAS listed as a problem by the medical provider in the chart but had only nonpharmacologic management. For our entire population of opioid-exposed infants, only 64 (52.9%) infants were assigned one of the diagnostic codes for opioid exposure (ICD-10-CM code P04.49 or ICD-10-CM code P04.14). Thirty-four infants (28.1%) did not receive any of the 3 ICD-10-CM billing codes at the time of discharge.
FIGURE 1.
Matrix depicting the number of study infants that met criteria for all 6 NAS definitions, as well as the number of infants meeting criteria for combinations of definitions.
LOS
Infants were then separated into 2 categories on the basis of hospital LOS (Table 2). A total of 69 infants were observed in the hospital for ≤5 days before discharge from the hospital. Only 1 infant in this group received a clinical diagnosis of NAS by a medical provider, but 5 were assigned the diagnostic code for NAS at discharge. Fifty-two infants were hospitalized for ≥6 days. Twenty-one of these infants received morphine, and 27 were diagnosed with NAS by a medical provider. The remainder of the infants had a prolonged hospitalization because of clinical factors (which may or may not have been related to antenatal opioid exposure) or social factors (eg, foster care placement).
TABLE 2.
Number of Opioid-Exposed Infants Meeting Criteria for Our 6 NAS Definitions
| Full Study Population (n = 121) | LOS ≤5 d (n = 69) | LOS 6≥ d (n = 52) | |
|---|---|---|---|
| Finnegan score >8 twice or 12 | 35 (28.9) | 5 (7.2) | 30 (57.7) |
| Received morphine | 21 (17.4) | 0 (0) | 21 (40.4) |
| Clinical diagnosis | 28 (23.1) | 1 (1.4) | 27 (51.9) |
| State surveillance | 27 (22.3) | 1 (1.4) | 26 (50.0) |
| Diagnostic code for NAS | 38 (31.4) | 5 (7.2) | 33 (63.5) |
| Diagnostic code for opioid exposure | 64 (52.9) | 37 (53.6) | 27 (51.9) |
Data presented as n (%).
Discussion
We found substantial variation in measured NAS incidence based on common definitions used across research, clinical practice, and public health settings. There currently is no registry with clinical data for opioid-exposed infants and those diagnosed with NAS, making multisite comparisons of definitions difficult. Although limited by extrapolating data from 1 center, our results reveal that heterogeneity in definitions can substantially affect incidence estimates.
In practice, NAS is often used as a proxy of maternal opioid use; however, our findings reflect similarly published research that not all opioid-exposed infants develop clinically significant symptoms of the syndrome.20 Our 6 definitions captured anywhere from 17.4% (received morphine) to 52.9% (diagnostic codes for opioid exposure) of infants within our study. Although many hospitals use clinical tools (eg, modified Finnegan score and Eat, Sleep, Console) to assess withdrawal in opioid-exposed infants, these tools can be subjective and difficult to standardize across multiple settings and multiple institutions.21 There are a wide variety of tools for hospitals to use, and there are no empirical data that one tool is superior to another.22,23 In addition, even within our single-center study, we saw differences in individual medical provider’s assessment of signs of withdrawal, which highlights how an infant’s clinical course after opioid exposure is heterogenous. Many opioid-exposed infants may experience mild signs of withdrawal necessitating a prolonged hospitalization, but may not require pharmacologic management. In our study population, 52 infants were hospitalized for ≥6 days after birth, but only 27 of these infants were clinically diagnosed with NAS, and 33 infants were assigned the diagnostic code for NAS at discharge. Despite these clinical complexities, there are no agreed on tools that stratify infants in a scheme such as mild, moderate, and severe.
For public health surveillance, billing codes from hospital discharge abstracts are a convenient way to identify infants across multiple hospital settings and, therefore, are used widely for state surveillance and public health research.17,18,24 In our study population, the diagnostic code for NAS captured 26 of the 28 infants clinically diagnosed with NAS, similar to findings from previous work25; however, we found that 12 additional opioid-exposed infants received the diagnostic code for NAS at the time of discharge but did not demonstrate clinically significant signs of withdrawal during hospitalization to warrant a clinical diagnosis from a medical provider. The diagnostic codes for opioid exposure were applied to only 64 (52.9%) of the opioid-exposed infants in our study. Thus, our single-center study identified both underreporting of opioid exposure and overreporting of NAS in administrative billing codes. Inconsistencies in hospital coding may be secondary to incomplete documentation by the medical provider, lack of access to mother’s chart for the coders, or, simply, the lack of a clear definition of opioid exposure and NAS across settings.
If we extrapolate these findings to national data, we suspect that current estimates of the incidence of NAS and opioid exposure are likely inaccurate. National estimates suggest there were between 26 000 and 32 000 infants diagnosed with NAS in 20164,26; however there are no available national estimates of opioid exposure. In our single-center study, only 87 (71.9%) infants received any code for opioid exposure or NAS at the time of discharge, suggesting that current public surveillance efforts may not be capturing all infants. In addition, reliance on NAS as a measure for opioid exposure may substantially underestimate the true incidence of exposure.
Standardization of opioid exposure and NAS is needed for clinical practice, research, and public health surveillance, yet, currently, the data available vary in each context, and, at present, uniformity may be difficult to achieve across all settings. All infants who are antenatally exposed to opioids (on the basis of maternal history or a positive maternal drug screen result during pregnancy) should be coded with ICD-10-CM code P04.14 at the time of discharge. In clinical practice, until there is an agreed on definition for the syndrome, using a diagnosis of NAS by a medical provider, although subjective, may provide the best single measure to capture NAS and should prompt assignment of ICD-10-CM code P96.1 at discharge. Uniform criteria across all hospital settings would require consensus on the clinical measure of NAS. The variation in clinical tools available to assess opioid-exposed infants across settings may affect hospital billing data reliability and, therefore, may complicate national public health surveillance measures. For researchers using large administrative data sets or for public health surveillance, it may be possible to use a combination of objective measures to estimate NAS incidence. For example, a LOS >5 days, an ICD-10-CM diagnosis code for NAS, or indication of morphine administration (if available) would capture 47.1% of patients in our clinical sample of opioid-exposed infants, including 100% of infants that received a clinical diagnosis of NAS by a medical provider.
Our study is limited by evaluation at only a single medical center. Clinical care processes, including use of modified Finnegan scoring and pharmacologic treatment with morphine, may not be generalizable to other hospital settings. The 6 definitions in our clinical sample and administrative billing data may be prone to misclassification bias. A diagnosis of NAS in Tennessee mandates a report to the Department of Children’s Services, which may lead to a reluctance on the part of the medical provider to assign the diagnosis. In addition, assessment tools used in our study (eg, modified Finnegan scoring) can be subjective and are prone to interrater reliability challenges.
Conclusions
Detecting opioid exposure and NAS varies substantially depending on the definitions applied to infants. The lack of standardized definitions across research, clinical practice, and public health settings may substantially influence detection in each setting. Standardizing definitions across settings of opioid exposure and NAS may reduce variability in the diagnosis and improve the reliability of surveillance estimates.
Acknowledgments
We thank William Cooper, MD, and Jean Y. Ko, PhD, for their input to this article.
Glossary
- ICD-10-CM
International Classification of Diseases, 10th Revision, Clinical Modification
- IQR
interquartile range
- LOS
length of stay
- NAS
neonatal abstinence syndrome
- VUMC
Vanderbilt University Medical Center
Footnotes
Drs Doherty and Patrick conceptualized and designed the study and drafted the initial manuscript; Ms Scott designed the data collection instruments and maintained the data collection database; Ms McNeer and Ms Lovell assisted with data analysis; Drs Morad, Crook and Gay provided assistance with study design; and all authors reviewed and revised the manuscript, critically reviewed the manuscript, and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institute on Drug Abuse of the National Institutes of Health under awards K23DA038720 (Dr Patrick) and R01DA045729 (Dr Patrick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Kocherlakota Prabhakar. Neonatal abstinence syndrome. Pediatrics. 2014;134(2):e547-61. doi: 10.1542/peds.2013-3524. [DOI] [PubMed] [Google Scholar]
- 2.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–1940 [DOI] [PubMed] [Google Scholar]
- 3.Winkelman TNA, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and costs of neonatal abstinence syndrome among infants with Medicaid: 2004–2014. Pediatrics. 2018;141(4):e20173520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leech AA, Cooper WO, McNeer E, Scott TA, Patrick SW. Neonatal abstinence syndrome in the United States, 2004–16. Health Aff (Millwood). 2020;39(5):764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e540 [Google Scholar]
- 6.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–17 [DOI] [PubMed] [Google Scholar]
- 7.Protecting Our Infants Act of 2015, S 799, 114th Cong (2015-2016)
- 8.Warren MD, Miller AM, Traylor J, Bauer A, Patrick SW; Centers for Disease Control and Prevention (CDC) . Implementation of a statewide surveillance system for neonatal abstinence syndrome - Tennessee, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(5):125–128 [PMC free article] [PubMed] [Google Scholar]
- 9.Honein MA, Boyle C, Redfield RR. Public health surveillance of prenatal opioid exposure in mothers and infants. Pediatrics. 2019;143(3):e20183801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Council of State and Territorial Epidemiologists, Committee on Maternal and Child Health Neonatal abstinence syndrome standardized case definition. Available at: https://cdn.ymaws.com/www.cste.org/resource/resmgr/2019ps/final/19-MCH-01_final_7.31.19.pdf. Accessed January 3, 2020.
- 11.Chiang KV, Okoroh EM, Kasehagen LJ, Garcia-Saavedra LF, Ko JY. Standardization of state definitions for neonatal abstinence syndrome surveillance and the opioid crisis. Am J Public Health. 2019;109(9):1193–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jilani SM, Frey MT, Pepin D, et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome - six states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(1):6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crook TW, Munn EK, Scott TA, et al. Improving the discharge process for opioid-exposed neonates. Hosp Pediatr. 2019;9(8):643–648 [DOI] [PubMed] [Google Scholar]
- 14.Lipsitz PJ. A proposed narcotic withdrawal score for use with newborn infants. A pragmatic evaluation of its efficacy. Clin Pediatr (Phila). 1975;14(6):592–594 [DOI] [PubMed] [Google Scholar]
- 15.Sanlorenzo LA, Stark AR, Patrick SW. Neonatal abstinence syndrome: an update. Curr Opin Pediatr. 2018;30(2):182–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gullickson C, Kuhle S, Campbell-Yeo M. Comparison of outcomes between morphine and concomitant morphine and clonidine treatments for neonatal abstinence syndrome. Acta Paediatr. 2019;108(2):271–274 [DOI] [PubMed] [Google Scholar]
- 17.Fill M-MA, Miller AM, Wilkinson RH, et al. Educational disabilities among children born with neonatal abstinence syndrome. Pediatrics. 2018;142(3):e20180562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolia VN, Murthy K, Bennett MM, et al. Morphine vs methadone treatment for infants with neonatal abstinence syndrome. J Pediatr. 2018;203:185–189 [DOI] [PubMed] [Google Scholar]
- 19.Davis JM, Shenberger J, Terrin N, et al. Comparison of safety and efficacy of methadone vs morphine for treatment of neonatal abstinence syndrome: a randomized clinical trial. JAMA Pediatr. 2018;172(8):741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrick SW, Dudley J, Martin PR, et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135(5):842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devlin LA, Breeze JL, Terrin N, et al. Association of a simplified Finnegan neonatal abstinence scoring tool with the need for pharmacologic treatment for neonatal abstinence syndrome. JAMA Netw Open. 2020;3(4):e202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansson LM, Patrick SW. Neonatal abstinence syndrome. Pediatr Clin North Am. 2019;66(2):353–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grisham LM, Stephen MM, Coykendall MR, Kane MF, Maurer JA, Bader MY. Eat, Sleep, Console approach: a family-centered model for the treatment of neonatal abstinence syndrome. Adv Neonatal Care. 2019;19(2):138–144 [DOI] [PubMed] [Google Scholar]
- 24.Maalouf FI, Cooper WO, Slaughter JC, Dudley J, Patrick SW. Outpatient pharmacotherapy for neonatal abstinence syndrome. J Pediatr. 2018;199:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maalouf FI, Cooper WO, Stratton SM, et al. Positive predictive value of administrative data for neonatal abstinence syndrome. Pediatrics. 2019;143(1):e20174183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agency for Healthcare Research and Quality. Neonatal abstinence syndrome (NAS) among newborn hospitalizations. Available at: https://www.hcup-us.ahrq.gov/faststats/NASServlet?setting1=IP. Accessed August 3, 2020 [DOI] [PubMed]