Abstract
Rationale: Patients with severe coronavirus disease (COVID-19) have complex organ support needs that necessitate prolonged stays in the intensive care unit (ICU), likely to result in a high incidence of neuromuscular weakness and loss of well-being. Early and structured rehabilitation has been associated with improved outcomes for patients requiring prolonged periods of mechanical ventilation, but at present no data are available to describe similar interventions or outcomes in COVID-19 populations.
Objectives: To describe the demographics, clinical status, level of rehabilitation, and mobility status at ICU discharge of patients with COVID-19.
Methods: Adults admitted to the ICU with a confirmed diagnosis of COVID-19 and mechanically ventilated for >24 hours were included. Rehabilitation status was measured daily using the Manchester Mobility Score to identify the time taken to first mobilize (defined as sitting on the edge of the bed or higher) and highest level of mobility achieved at ICU discharge.
Results: A total of n = 177 patients were identified, of whom n = 110 survived to ICU discharge and were included in the subsequent analysis. While on ICU, patients required prolonged periods of mechanical ventilation (mean 19 ± 10 d), most received neuromuscular blockade (90%) and 67% were placed in the prone position on at least one occasion. The mean ± standard deviation time to first mobilize was 14 ± 7 days, with a median Manchester Mobility Score at ICU discharge of 5 (interquartile range: 4–6), which represents participants able to stand and step around to a chair with or without assistance. Time to mobilize was significantly longer in those with higher body mass index (P < 0.001), and older patients (P = 0.012) and those with more comorbidities (P = 0.017) were more likely to require further rehabilitation after discharge.
Conclusions: The early experience of the COVID-19 pandemic in the United Kingdom resembles the experience in other countries, with high acuity of illness and prolonged period of mechanical ventilation required for those patients admitted to the ICU. Although the time to commence rehabilitation was delayed owing to this severity of illness, rehabilitation was possible within the ICU and led to increased levels of mobility from waking before ICU discharge.
Clinical trial registered with ClinicalTrials.gov (NCT04396197).
Keywords: physiotherapy, coronavirus, rehabilitation, intensive care unit, mobilization
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the novel coronavirus first detected in Wuhan, China, that causes coronavirus disease (COVID-19) (1). Since initial detection of the virus, more than 17 million cases have been detected worldwide, with the most severe cases requiring admission to intensive care units (ICUs) for mechanical ventilation and organ support. These patients have high mortality and prolonged stay in the ICU and require deep sedation, neuromuscular blockade, and/or prone positioning for oxygenation (2, 3), all of which have been identified as significant risk factors for the development of ICU-acquired weakness (4). In patients with acute respiratory distress syndrome and multiple organ failure, there is substantial muscle wasting within the first week of critical illness, with losses of up to 20% by Day 7 (5). Survivors have longer-term physical, psychological, and cognitive morbidity, lasting for months to years, termed as post–intensive care syndrome (6).
Patients with COVID-19 have complex organ support needs for a prolonged period, resulting in a high incidence of neuromuscular weakness, loss of well-being, and delirium. This is predicted to create a “tsunami of rehabilitation needs” in both the short and long term (7). Early and structured rehabilitation in critical care has been shown to be safe (8) and, when implemented, is associated with significant improvements in physical and clinical outcomes (9). Our group have completed pilot feasibility studies on early mobilization in general critical care to improve these outcomes (10), but at present, no data are available to describe similar interventions and outcomes in COVID-19 populations admitted to the ICU. This prospective study is aimed to elucidate the short-term impact of early COVID-19 and associated ICU stay on physical outcomes and rehabilitation levels within the ICU. The aim of our study is to describe the demographics, clinical status, level of rehabilitation, and mobility status at ICU discharge of patients with COVID-19.
Methods
Study Design
This was a single-center, prospective, noninterventional, observational study, conducted in patients admitted to the ICU in March and April 2020 with a confirmed diagnosis of COVID-19. Participants were followed up until acute hospital discharge. This study is reported in accordance with the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines (11) and was registered with the clinical trials.gov registry (NCT04396197).
Setting
The Queen Elizabeth Hospital Birmingham is a quaternary-level acute care hospital, with one of the largest colocated ICUs in Europe. Before the COVID-19 pandemic, the standard critical care capacity for this unit was 75 beds; however, with surge planning, the overall capacity was increased to more than 200. At the peak of the COVID-19 emergency, the ICU cared for 164 patients simultaneously (COVID-19 and non–COVID-19). This capacity was increased through a variety of measures, which included caring for two patients per bed space and reduced specialist staffing ratios. Nursing ratios were one critical care nurse to four patients, supported by three non-ICU nurses, deployed from other hospital departments. Consultant intensivist staffing was at 1:35 patients, supported by doctors in training, and deployed doctors from anesthesia, medical, and surgical specialties.
Before the pandemic, physiotherapy was provided between the hours of 8 a.m. and 5 p.m., Monday to Friday, at a ratio of one physiotherapist to seven patients, with only emergency respiratory on-call provision available outside these hours. Weekend provision was delivered by a significantly reduced service as part of normal weekend working patterns in the United Kingdom. Physiotherapists within our unit assess all patients within 24 hours of admission; delivering respiratory care often termed “chest physiotherapy” and commencing rehabilitation as indicated. To meet the increasing demand expected during the pandemic, the physiotherapy service was restructured to ensure physiotherapy was available from 8 a.m. to 8 p.m., 7 days per week, with a ratio of one physiotherapist for every 10 patients. This was achieved through the redeployment of nonspecialist critical care staff from other areas of the hospital to support the critical care physiotherapy team.
Participants
Consecutive participants were included in the analysis if they met the inclusion criteria of being adults (≥18 yr of age), having a confirmed diagnosis of COVID-19, and being mechanically ventilated for at least 24 hours. This project constituted an observation of standard care delivery with no randomization and thus met the definition of a service evaluation under the National Health Service Health research authority guidelines (12). As such, ethical approval was not required, and because all outcome measures are collected as part of routine care, the need for consent was waived.
Procedure
All patients were assessed by a physiotherapist within 24 hours of admission to ICU. As there are no respiratory therapists in the United Kingdom, physiotherapists are responsible for both respiratory care and the initiation and progression of rehabilitation, where appropriate. Specifically, from a respiratory perspective, the physiotherapy team assisted with patient repositioning, including proning, and delivering chest physiotherapy to optimize secretion clearance. To support the medical and nursing teams during the pandemic, the physiotherapy team also took on increased responsibility to support management of ventilation, in accordance with lung protective ventilation guidelines. This included calculation of targets for lung protective tidal volumes, which were then displayed in the patients’ bed space, alongside twice-daily ventilation ward rounds, to ensure adherence or make the necessary adjustments to maintain these levels. As a patient’s condition stabilized, physiotherapists led and coordinated the commencement and progression of rehabilitation. Our critical care multidisciplinary team has extensive experience of delivering early and structured rehabilitation, including established safety criteria to commence mobilization, and a protocol to guide progression (10).
Outcomes
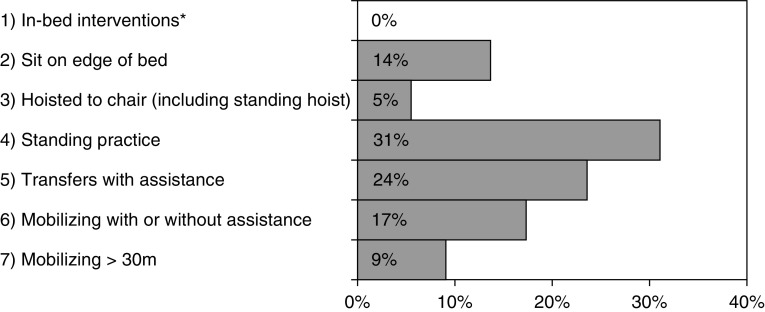
The primary outcome was the highest level of mobility achieved at the point of ICU discharge, as measured by the Manchester Mobility Score (MMS). The MMS is a simple seven-point mobility scale (see Figure 1) used and validated for assessing mobility levels within critical care (13). Secondary outcomes included the number of days taken to first mobilize (defined as an MMS of 2 or higher, i.e., sitting on the edge of the bed or higher) and the location of hospital discharge, which was treated as an ordinal variable with categories of Home (No Rehabilitation), Home (With Rehabilitation), or Inpatient Rehabilitation.
Figure 1.
Manchester Mobility Score at ICU discharge. *Includes passive movements, active exercise, chair position in bed. ICU = intensive care unit.
Data Collection
Data were collected prospectively throughout the evaluation period using patient noting and electronic databases. Baseline data, including demographics, ventilation days, sedation days, renal replacement therapy using continuous venovenous hemofiltration at any point during ICU admission, tracheostomy insertion, length of stay for both ICU and the ward, and mortality, were obtained from electronic databases administered by dedicated data scientists. Other factors that may have contributed to the development of ICU-acquired weakness and therefore delays in mobilization were also collected retrospectively from patient noting. Specifically, this included data regarding aspects of critical care management, including the use of neuromuscular blocking agents, proning, and the presence of delirium, defined by a positive result on the Confusion Assessment Method ICU (CAM-ICU) at any point during the ICU stay. The presence of ICU-acquired weakness during awakening was defined as a Medical Research Council sum score of <48 (4). Rehabilitation outcomes were collected immediately after physiotherapy sessions and recorded using the MMS. Frailty scores were collected routinely as part of admission assessment using the Clinical Frailty Score (14).
Statistical Analysis
Initially, the characteristics of the cohort were summarized, with continuous variables reported as means ± standard deviations where normally distributed, and medians and interquartile ranges (IQRs) reported otherwise. Comparisons between those patients who died in the ICU and those who survived to ICU discharge were then performed, using Mann-Whitney U tests for ordinal or continuous variables and Fisher’s exact tests for nominal variables. Associations between patient characteristics and physical outcomes were then assessed. MMS at ICU discharge was treated as a continuous variable in this analysis but was reported as the proportion of patients with a score of five or more points in each group, to simplify interpretation. Comparisons across nominal variables were performed using Mann-Whitney U tests or Kruskal-Wallis tests for variables with two or more than two categories, respectively. To assess associations with ordinal and continuous variables, P values were derived from Spearman’s correlation coefficients. All analyses were performed using IBM SPSS 22 (IBM Corp.), with P < 0.05 deemed to be indicative of statistical significance throughout.
Results
Baseline Patient Characteristics
During the observation period, N = 177 patients were admitted to the ICU with confirmed COVID-19 infection. Of these, 110 (62%) patients survived to ICU discharge and were included in subsequent analysis. Patients who died within the ICU were significantly older, with a higher incidence of comorbidities and higher frailty scores (Table 1).
Table 1.
Patient demographics at ICU admission
| Factor | Died in ICU |
||
|---|---|---|---|
| No (n = 110) | Yes (n = 67) | P Value | |
| Demographics | |||
| Age, yr | 53 ± 12 | 62 ± 13 | <0.001 |
| Sex, % M | 83 (75) | 44 (66) | 0.172 |
| BMI, kg/m2 | 0.194* | ||
| <20 | 0 (0) | 0 (0) | — |
| 20–24 | 14 (13) | 12 (18) | — |
| 25–29 | 42 (38) | 27 (40) | — |
| 30–39 | 39 (35) | 23 (34) | — |
| 40+ | 15 (14) | 5 (7) | — |
| Ethnicity | 0.152 | ||
| White | 53 (48) | 38 (57) | — |
| Asian | 38 (35) | 23 (34) | — |
| Black | 8 (7) | 5 (7) | — |
| Mixed/other | 11 (10) | 1 (1) | — |
| Clinical frailty score | <0.001* | ||
| 1 | 23 (21) | 3 (4) | — |
| 2 | 32 (29) | 12 (18) | — |
| 3 | 35 (32) | 29 (43) | — |
| >3 | 20 (18) | 23 (34) | — |
| APACHE II risk [N = 85] | 16 (13–25) | 16 (13–18) | 0.174 |
| Comorbidities | |||
| Hypertension | 50 (45) | 25 (37) | 0.347 |
| Diabetes mellitus | 34 (31) | 30 (45) | 0.076 |
| Cardiovascular disease | 10 (9) | 10 (15) | 0.327 |
| Cerebrovascular disease | 7 (6) | 8 (12) | 0.266 |
| COPD | 5 (5) | 9 (13) | 0.045 |
| Asthma | 17 (15) | 6 (9) | 0.255 |
| Chronic kidney disease | 7 (6) | 8 (12) | 0.266 |
| Malignancy | 2 (2) | 11 (16) | <0.001 |
| Charlson comorbidity index | <0.001* | ||
| 0–1 | 41 (37) | 14 (21) | — |
| 2–3 | 49 (45) | 23 (34) | — |
| 4–5 | 17 (15) | 21 (31) | — |
| >5 | 3 (3) | 9 (13) | — |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; BMI = body mass index; COPD = chronic obstructive pulmonary disease; ICU = intensive care unit.
Continuous variables are reported as mean ± standard deviation or median (interquartile range), as applicable, with P values from Mann-Whitney U tests. Categorical variables are reported as n (%), with P values from Fisher’s exact tests, unless stated otherwise. All statistics are based on N = 177, unless stated otherwise. Bold typeface P values are significant at P < 0.05.
P value from a Mann-Whitney U test, as the factor is ordinal.
The mean age of patients surviving to ICU discharge was 53 ± 12 years, 75% were male, and the majority were of White (48%) or Asian (35%) ethnic backgrounds (Table 1). Although there was a low incidence of frailty, the majority of the cohort was classified as overweight or obese (body mass index [BMI]: 25+ kg/m2, 87%). Chronic medical conditions were common in this critically ill population, with 45% having hypertension and 31% having diabetes mellitus; the median Charlson Comorbidity Index was 2 (IQR: 1–3).
ICU-Level Therapies and Outcomes
ICU-level therapies and outcomes for the 110 ICU survivors are presented in Table 2. All patients required mechanical ventilation, with a mean duration of 19 ± 10 days (range: 2–59). A tracheostomy was inserted in 77% of patients, and 67% of patients were placed in the prone position on one or more occasion. All patients were sedated, for a mean duration of 13 ± 6 days, and 90% received neuromuscular blockade, for a median of 7 (IQR: 4–11) days. Renal failure requiring continuous venovenous hemofiltration developed in 34% of patients. A high prevalence of delirium was observed, with 69% of patients scoring positive on CAM-ICU assessment during their ICU stay. ICU-acquired weakness was present on awakening for all patients.
Table 2.
ICU therapy and outcomes
| Factor | Statistic |
|---|---|
| ICU therapy | |
| Mechanical ventilation | 110 (100) |
| Duration of ventilation, d | 19 ± 10 |
| Tracheostomy | 85 (77) |
| Prone position | 74 (67) |
| Renal failure requiring CVVH | 37 (34) |
| Sedated | 110 (100) |
| Duration of sedation, d | 13 ± 6 |
| Neuromuscular blockade | 99 (90) |
| Duration of blockade, d, N = 81* | 7 (4–11) |
| | |
| ICU outcomes | |
| ICU-acquired weakness on awakening† | 110 (100) |
| Delirium in ICU | 76 (69) |
| Mobilized in ICU | 110 (100) |
| Time to first mobilize, d | 14 ± 7 |
| ICU LOS, d | 22 ± 11 |
| MMS at ICU discharge | |
| 1 | 0 (0) |
| 2 | 15 (14) |
| 3 | 6 (5) |
| 4 | 34 (31) |
| 5 | 26 (24) |
| 6 | 19 (17) |
| 7 | 10 (9) |
| | |
| Hospital outcomes | |
| Mortality after ICU discharge | 1 (1) |
| Post-ICU LOS, d, N = 109‡ | 11 (6–18) |
| Readmitted to ICU, N = 109‡ | 2 (2) |
| MMS at hospital discharge, N = 109‡ | |
| 1 | 0 (0) |
| 2 | 0 (0) |
| 3 | 2 (2) |
| 4 | 1 (1) |
| 5 | 1 (1) |
| 6 | 14 (13) |
| 7 | 91 (83) |
| Discharge destination, N = 109* | |
| Home, no rehab | 55 (50) |
| Home, with rehab | 46 (42) |
| Inpatient rehab | 8 (7) |
Definition of abbreviations: CVVH = continuous venovenous hemofiltration; ICU = intensive care unit; LOS = length of stay; MMS = Manchester Mobility Score.
Data are reported as N (%), mean ± standard deviation, or median (interquartile range), as applicable, and are based on N = 110, unless stated otherwise.
In patients with neuromuscular blockade, where the duration was recorded.
ICU-acquired weakness was diagnosed with a Medical Research Council sum score of <48/60.
In patients discharged from hospital.
In total, the mean length of stay in the ICU was 22 ± 11 days. All patients were mobilized in the ICU, with a mean time to mobilization of 14 ± 7 days. At the time of ICU discharge, the median MMS was 5 (IQR: 4–6), with 50% able to step transfer or walk (MMS of 5+, Figure 1)
Hospital Outcomes
A single patient (1%) died in the hospital following ICU discharge, following a cardiac arrest on the ward. Two (2%) patients were readmitted to the ICU before discharge, both as a result of respiratory deterioration secondary to newly diagnosed hospital-acquired pneumonia. Patients were discharged from the hospital a median of 11 days (IQR: 6–18) after being discharged from the ICU (Table 2). Fifty-five (50%) patients were discharged home without requiring further rehabilitation, whereas 46 (42%) required further rehabilitation at home, and 8 (7%) required ongoing inpatient rehabilitation. At the time of hospital discharge, the majority of patients were able to step transfer or walk (MMS of 5+), with 83% scoring 7 points on the MMS and therefore able to walk >30 m independently. The four (4%) patients with MMS scores of less than five were all discharged for ongoing inpatient rehabilitation.
Associations With Physical Outcomes
Associations between patient characteristics, namely, the time to mobilize, MMS at ICU discharge, and hospital discharge destination, were then assessed. Analysis of the latter was treated “Home (No Rehab),” “Home (With Rehab),” and “Inpatient Rehab” as an ordinal scale. The results of these analyses are reported in Tables 3 and 4.
Table 3.
Associations with physical outcomes (part 1)
| Factor | Days to First Mobilize | MMS 5+ at ICU Discharge | Hospital Discharge Destination* |
||||
|---|---|---|---|---|---|---|---|
| N | N | Home (No Rehab) | Home (With Rehab) | Inpatient Rehab | |||
| Age, yr† | P = 0.638‡ | P = 0.094‡ |
P = 0.012‡ |
||||
| <45 | 22 | 14 ± 7 | 12 (55) | 22 | 16 (73) | 5 (23) | 1 (5) |
| 45–54 | 35 | 14 ± 6 | 20 (57) | 34 | 18 (53) | 13 (38) | 3 (9) |
| 55–64 | 35 | 15 ± 7 | 16 (46) | 35 | 16 (46) | 16 (46) | 3 (9) |
| 65+ | 18 | 11 ± 6 | 7 (39) | 18 | 5 (28) | 12 (67) | 1 (6) |
| Sex | P = 0.235 | P = 0.225 |
P = 0.161 |
||||
| F | 27 | 13 ± 7 | 12 (44) | 27 | 10 (37) | 15 (56) | 2 (7) |
| M | 83 | 14 ± 6 | 43 (52) | 82 | 45 (55) | 31 (38) | 6 (7) |
| BMI, kg/m2 | P < 0.001‡ | P = 0.262‡ |
P = 0.768‡ |
||||
| 20–24 | 14 | 10 ± 5 | 9 (64) | 14 | 7 (50) | 6 (43) | 1 (7) |
| 25–29 | 42 | 13 ± 7 | 20 (48) | 41 | 23 (56) | 12 (29) | 6 (15) |
| 30–39 | 39 | 14 ± 6 | 19 (49) | 39 | 19 (49) | 19 (49) | 1 (3) |
| 40+ | 15 | 18 ± 6 | 7 (47) | 15 | 6 (40) | 9 (60) | 0 (0) |
| Ethnicity | P = 0.256 | P = 0.970 |
P = 0.242 |
||||
| White | 53 | 13 ± 7 | 25 (47) | 52 | 28 (54) | 20 (38) | 4 (8) |
| Asian | 38 | 15 ± 6 | 20 (53) | 38 | 16 (42) | 22 (58) | 0 (0) |
| Black | 8 | 18 ± 8 | 5 (63) | 8 | 3 (38) | 2 (25) | 3 (38) |
| Other | 11 | 11 ± 6 | 5 (45) | 11 | 8 (73) | 2 (18) | 1 (9) |
| Clinical frailty score† | P = 0.317‡ | P = 0.033‡ |
P = 0.031‡ |
||||
| 1 | 23 | 12 ± 6 | 14 (61) | 22 | 16 (73) | 5 (23) | 1 (5) |
| 2 | 32 | 14 ± 7 | 18 (56) | 32 | 15 (47) | 15 (47) | 2 (6) |
| 3 | 35 | 14 ± 6 | 17 (49) | 35 | 17 (49) | 15 (43) | 3 (9) |
| 4–5 | 20 | 14 ± 6 | 6 (30) | 20 | 7 (35) | 11 (55) | 2 (10) |
| ICNARC risk† | P = 0.814‡ | P = 0.688‡ |
P = 0.087‡ |
||||
| <10 | 19 | 12 ± 5 | 8 (42) | 19 | 14 (74) | 4 (21) | 1 (5) |
| 10–19 | 35 | 14 ± 6 | 17 (49) | 35 | 16 (46) | 16 (46) | 3 (9) |
| 20–29 | 17 | 14 ± 6 | 6 (35) | 17 | 10 (59) | 7 (41) | 0 (0) |
| 30+ | 28 | 13 ± 7 | 16 (57) | 27 | 9 (33) | 16 (59) | 2 (7) |
| APACHE II† | P = 0.108‡ | P = 0.420‡ |
P = 0.019‡ |
||||
| <12 | 7 | 10 ± 5 | 3 (43) | 7 | 7 (100) | 0 (0) | 0 (0) |
| 12–15 | 8 | 11 ± 5 | 5 (63) | 8 | 6 (75) | 1 (13) | 1 (13) |
| 16–23 | 12 | 16 ± 5 | 6 (50) | 12 | 5 (42) | 7 (58) | 0 (0) |
| 24+ | 10 | 13 ± 3 | 5 (50) | 9 | 4 (44) | 5 (56) | 0 (0) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; BMI = body mass index; ICNARC = Intensive Care National Audit and Research Centre; ICU = intensive care unit; MMS = Manchester Mobility Score.
Data are reported as n (%) or mean ± standard deviation, as applicable, and P values are from Mann-Whitney U/Kruskal-Wallis tests, unless stated otherwise. Bold typeface P values are significant at P < 0.05.
Analysis of discharge destination includes only those that were discharged from hospital (N = 109) and treats the three destinations as an ordinal variable.
The continuous variable was used to generate P values, before it was divided into categories for reporting in the table.
P value from Spearman’s correlation coefficient, as the factor is ordinal/continuous.
Table 4.
Associations with physical outcomes (part 2)
| Factor | Days to First Mobilize | MMS 5+ at ICU Discharge | Hospital Discharge Destination* |
||||
|---|---|---|---|---|---|---|---|
| N | N | Home (No Rehab) | Home (With Rehab) | Inpatient Rehab | |||
| Hypertension | P = 0.108 | P = 0.307 |
P = 0.436 |
||||
| No | 60 | 13 ± 6 | 32 (53) | 59 | 33 (56) | 20 (34) | 6 (10) |
| Yes | 50 | 15 ± 7 | 23 (46) | 50 | 22 (44) | 26 (52) | 2 (4) |
| Diabetes mellitus | P = 0.392 | P = 0.967 |
P = 0.952 |
||||
| No | 76 | 13 ± 6 | 38 (50) | 76 | 39 (51) | 30 (39) | 7 (9) |
| Yes | 34 | 15 ± 7 | 17 (50) | 33 | 16 (48) | 16 (48) | 1 (3) |
| Cardiovascular disease | P = 0.792 | P = 0.019 |
P = 0.760 |
||||
| No | 100 | 14 ± 7 | 53 (53) | 99 | 51 (52) | 40 (40) | 8 (8) |
| Yes | 10 | 14 ± 6 | 2 (20) | 10 | 4 (40) | 6 (60) | 0 (0) |
| Cerebrovascular disease | P = 0.425 | P = 0.490 |
P = 0.570 |
||||
| No | 103 | 14 ± 7 | 52 (50) | 102 | 52 (51) | 43 (42) | 7 (7) |
| Yes | 7 | 12 ± 6 | 3 (43) | 7 | 3 (43) | 3 (43) | 1 (14) |
| COPD | P = 0.891 | P = 0.653 |
P = 0.718 |
||||
| No | 105 | 14 ± 7 | 53 (50) | 104 | 52 (50) | 44 (42) | 8 (8) |
| Yes | 5 | 14 ± 6 | 2 (40) | 5 | 3 (60) | 2 (40) | 0 (0) |
| Asthma | P = 0.499 | P = 0.733 |
P = 0.697 |
||||
| No | 93 | 14 ± 7 | 48 (52) | 92 | 47 (51) | 39 (42) | 6 (7) |
| Yes | 17 | 14 ± 5 | 7 (41) | 17 | 8 (47) | 7 (41) | 2 (12) |
| Chronic kidney disease | P = 0.845 | P = 0.232 |
P = 0.905 |
||||
| No | 103 | 14 ± 7 | 53 (51) | 102 | 52 (51) | 42 (41) | 8 (8) |
| Yes | 7 | 14 ± 2 | 2 (29) | 7 | 3 (43) | 4 (57) | 0 (0) |
| Malignancy | P = 0.866 | P = 0.751 |
P = 0.495 |
||||
| No | 108 | 14 ± 7 | 54 (50) | 107 | 55 (51) | 44 (41) | 8 (7) |
| Yes | 2 | 13 ± 1 | 1 (50) | 2 | 0 (0) | 2 (100) | 0 (0) |
| CCI† | P = 0.990‡ | P = 0.061‡ |
P = 0.017‡ |
||||
| 0–1 | 41 | 14 ± 7 | 24 (59) | 40 | 25 (63) | 13 (33) | 2 (5) |
| 2–3 | 49 | 14 ± 7 | 23 (47) | 49 | 24 (49) | 19 (39) | 6 (12) |
| 4–5 | 17 | 13 ± 5 | 8 (47) | 17 | 4 (24) | 13 (76) | 0 (0) |
| >5 | 3 | 16 ± 5 | 0 (0) | 3 | 2 (67) | 1 (33) | 0 (0) |
| Delirium in ICU | P = 0.947 | P = 0.428 |
P = 0.305 |
||||
| No | 34 | 14 ± 6 | 18 (53) | 34 | 14 (41) | 18 (53) | 2 (6) |
| Yes | 76 | 14 ± 7 | 37 (49) | 75 | 41 (55) | 28 (37) | 6 (8) |
Definition of abbreviations: CCI = Charlson Comorbidity Index; COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; MMS = Manchester Mobility Score.
Data are reported as n (%) or mean ± standard deviation, as applicable, and P values are from Mann-Whitney U/Kruskal-Wallis tests, unless stated otherwise. Bold typeface P values are significant at P < 0.05.
Analysis of discharge destination includes only those that were discharged from hospital (N = 109) and treats the three destinations as an ordinal variable.
The continuous variable was used to generate P values before it was divided into categories for reporting in the table.
P value from Spearman’s correlation coefficient, as the factor is ordinal/continuous.
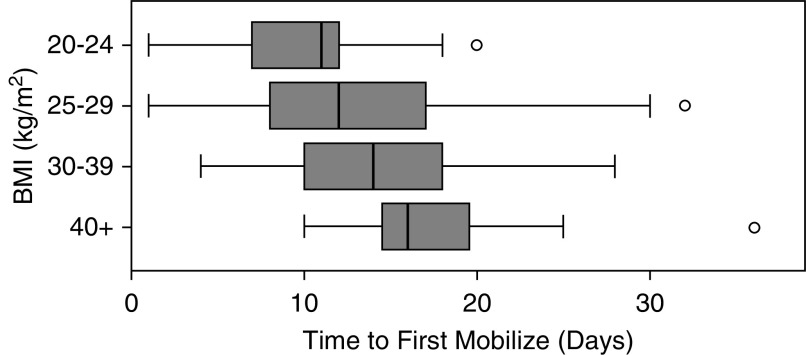
The time taken to first mobilize was found to increase significantly with BMI (Figure 2), from a mean of 10 days to 18 days (P < 0.001) for those with BMI of 20–24 versus 40+ kg/m2. At the time of ICU discharge, MMS scores were found be significantly lower in patients with a higher frailty score at admission (P = 0.033) and in those with preexisting cardiovascular disease (P = 0.019). In those that were discharged from hospital, older patients (P = 0.012), as well as those who were frail (P = 0.031) or had a higher Charlson Comorbidity Index (P = 0.017), were significantly more likely to require further rehabilitation.
Figure 2.
Time to first mobilize by BMI. BMI = body mass index.
Discussion
This single-center study describes the rehabilitation within the ICU for 110 critically ill, mechanically ventilated patients who presented with acute hypoxic respiratory failure and laboratory-confirmed COVID-19 infection and survived to ICU discharge. The patients admitted to our ICU showed a similar course to those described in China (2), requiring prolonged periods of mechanical ventilation with a high use of neuromuscular blockade and prone positioning. The incidence of delirium was high, and all patients presented with ICU-acquired weakness at first awakening. Despite this, rehabilitation was feasible, with all patients mobilized at least once before ICU discharge and half of the patients having regained the ability to stand and step transfer to a chair before discharge from the ICU to the ward.
The patients in our observational cohort demonstrated the significant impact of COVID-19 and necessity for prolonged periods of mechanical ventilation and ICU support. Despite initial concerns regarding the insertion of tracheostomies owing to the potential risk to healthcare workers, a large proportion (77%) of patients included in the analysis had a tracheostomy performed to support weaning and rehabilitation. Our standard unit approach is to consider tracheostomy after 10 days of mechanical ventilation; hence, this high proportion is representative of the long periods of mechanical ventilation required in this cohort. Almost all patients required neuromuscular blockade to optimize ventilation, and two-thirds were placed into the prone position.
Because of the severity of illness and related organ support, it took a mean of 14 days for patients to mobilize for the first time. It is helpful to compare these findings with previous published trials of early mobilization within ICU populations. In our own previously published randomized controlled trial of earlier mobilization, the time to first mobilize in the intervention group was reported as 8 days, although the median duration for mechanical ventilation in this trial was only 10 days (15). Although the patients with COVID-19 were delayed in starting mobilization, this closely matched the median duration of sedation and therefore suggested patients were mobilized within 24 hours of stopping sedation. Additionally, it is worthy of note that the first mobilization took place 5 days before patients had been fully weaned from mechanical ventilation. Although all patients initially presented with ICU-acquired weakness, commencing mobilization earlier meant that they had started to regain strength and mobility while in the ICU, meaning that half of patients were discharged from the ICU either stepping to a chair or walking.
Within our secondary analysis, a number of factors were found to affect physical outcomes. Importantly, we found that BMI had a significant impact on time to first mobilize, with those patients with a BMI of 40+ kg/m2 taking an average of 8 days longer to sit on the edge of the bed for the first time, compared with those with a BMI of <25 kg/m2. Obese patients present an additional challenge for rehabilitation within the ICU, often requiring multiple members of staff for rehabilitation and repositioning. As a result, some of this delay may have been related to a lack of staff availability or time constraints. Given the high proportion of patients with a raised BMI (87% were 25+ kg/m2), workforce planning for any future surges and ongoing rehabilitation would need to factor this increased dependency. Additionally, patients who were older and had higher Charlson comorbidity scores and higher frailty scores achieved lower levels of mobility at the point of ICU discharge and were more likely to be discharged from the hospital with ongoing rehabilitation requirements. This may provide a framework for identifying high-risk patients who would require more robust pathways of rehabilitation following discharge from ICU.
Because of the nature of the pandemic, there is a danger that rehabilitation, particularly in the ICU, is not seen as a priority. The constant need to free up capacity to meet an ever-increasing demand of new admissions can mean that the primary focus is placed on stability, survival, and early discharge from both the ICU and hospital (16). The consequence of this is patients being discharged home at lower functional levels seen with a lack of community resource available to support recovery. We know from our past experiences that patients with prolonged ICU stays and profound ICU-acquired weakness are often left with significant physical, cognitive, and mental health impairments. Optimizing the survivorship of patients with COVID-19 demands the implementation of early and structured rehabilitation programs that commence in the ICU and continue after discharge (17).
It is important to note that for patients within the ICU during the COVID-19 pandemic, a number of additional barriers to rehabilitation were present. First, there were significant challenges due to the physical layout of the unit and a lack of space, with two patients in each bed space limiting the use of rehabilitation equipment and chairs. Because of the increased workload and altered staff models, there was limited capacity in the already stretched nursing workforce to assist with rehabilitation. A significant proportion of our patients were identified to have delirium, with at least one positive CAM-ICU result during their ICU stay. This is in keeping with other previous research, which has suggested delirium rates of up to 80%, particularly in those requiring prolonged periods of mechanical ventilation (18). However, there are specific additional risks for the development or exacerbation of delirium and psychological distress within the ICU for patients with COVID-19. These include the need for staff to wear personal protective equipment, relatives being unable to visit patients, and the use of shared bed spaces, potentially exposing the patient to scenes that may be distressing. The effects of the COVID-19 pandemic in exacerbating patients’ delirium is subsequently expected to increase the risk for long-term cognitive impairment (17), making this an area in need of urgent evaluation and investigation.
Our study has a number of notable limitations. First, this is a single-center observational study with only a small sample size and thus may not be representative of other populations. Second, because of the urgent focus and elevated critical care numbers, only limited information was collected regarding the rehabilitation provided. Although this was useful to identify time to mobilize and the overall level of mobility, the lack of ongoing strength assessment limits any conclusions regarding overall physical recovery. Third, because those patients that died in the ICU were excluded from the analysis, the findings of the study are only applicable to patients that survived to ICU discharge, which likely represents a biased sample of those admitted to the ICU.
Conclusions
The early experience of the COVID-19 pandemic in the United Kingdom resembles the experience in other countries, with high acuity of illness and prolonged period of mechanical ventilation required for those patients admitted to the ICU. Although time to commence rehabilitation was delayed owing to this severity of illness, rehabilitation was possible within the ICU and led to increasing levels of mobility before ICU discharge. Despite the significant strain on the service, we were still able to deliver a high level of rehabilitation, even during the peak of the surge in admissions. Ongoing planning for future surges needs to consider this important aspect of care to ensure rehabilitation can still be prioritized for this patient group. Given the high degree of ICU-acquired weakness and high levels of delirium, there is likely to be a significant need for ongoing rehabilitation, both in hospital and following hospital discharge.
Footnotes
Authors Contributions: D.M. helped design the study, conduct the study, analyze and interpret the data, and draft and critically revise the manuscript. J.W. helped design the study, conduct the study, and critically revise the manuscript. J.H. helped interpret the data and draft and critically revise the manuscript. T.V. helped design the study, interpret the data, and critically revise the manuscript. T.W. helped analyze and interpret the data and critically revise the manuscript. C.S. helped design the study, interpret the data, and draft and critically revise the manuscript. All authors read and approved the final version of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the Queen Elizabeth Hospital Birmingham COVID-19 Research Team
References
- 1.World Health Organization Coronavirus disease (COVID-19) Situation Report–119. 2020 [accessed 2020 Nov 11]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200518-covid-19-sitrep-119.pdf?sfvrsn=4bd9de25_4.
- 2.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 4.Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274. doi: 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 6.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 7.Thornton J. Covid-19: the challenge of patient rehabilitation after intensive care. BMJ. 2020;369:m1787. doi: 10.1136/bmj.m1787. [DOI] [PubMed] [Google Scholar]
- 8.Nydahl P, Sricharoenchai T, Chandra S, Kundt FS, Huang M, Fischill M, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14:766–777. doi: 10.1513/AnnalsATS.201611-843SR. [DOI] [PubMed] [Google Scholar]
- 9.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41:1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 10.McWilliams D, Weblin J, Atkins G, Bion J, Williams J, Elliott C, et al. Enhancing rehabilitation of mechanically ventilated patients in the intensive care unit: a quality improvement project. J Crit Care. 2015;30:13–18. doi: 10.1016/j.jcrc.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE) explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 12.NHS Health Research Authority. Is my study research? 2020 [accessed 2020 May 5]. Available from: http://www.hra-decisiontools.org.uk/research/
- 13.McWilliams D, Atkins G, Hodson J, Boyers M, Lea T, Snelson C. Feasibility and reliability of the Manchester Mobility Score as a measure of physical function within the Intensive Care Unit. ACPRC J. 2015;48:26–33. [Google Scholar]
- 14.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McWilliams D, Jones C, Atkins G, Hodson J, Whitehouse T, Veenith T, et al. Earlier and enhanced rehabilitation of mechanically ventilated patients in critical care: a feasibility randomised controlled trial. J Crit Care. 2018;44:407–412. doi: 10.1016/j.jcrc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Escalon MX, Herrera J. Adapting to the coronavirus disease 2019 pandemic in New York City. Am J Phys Med Rehabil. 2020;99:453–458. doi: 10.1097/PHM.0000000000001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6:60. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]