Abstract
A workshop “Electronic Health Records and Pulmonary Function Data: Developing an Interoperability Roadmap” was held at the American Thoracic Society 2019 International Conference. “Interoperability” is defined as is the ability of different information-technology systems and software applications to directly communicate, exchange data, and use the information that has been exchanged. At present, pulmonary function test (PFT) equipment is not required to be interoperable with other clinical data systems, including electronic health records (EHRs). For this workshop, we assembled a diverse group of experts and stakeholders, including representatives from patient-advocacy groups, adult and pediatric general and pulmonary medicine, informatics, government and healthcare organizations, pulmonary function laboratories, and EHR and PFT equipment and software companies. The participants were tasked with two overarching Aobjectives: 1) identifying the key obstacles to achieving interoperability of PFT systems and the EHR and 2) recommending solutions to the identified obstacles. Successful interoperability of PFT data with the EHR impacts the full scope of individual patient health and clinical care, population health, and research. The existing EHR–PFT device platforms lack sufficient data standardization to promote interoperability. Cost is a major obstacle to PFT–EHR interoperability, and incentives are insufficient to justify the needed investment. The current vendor–EHR system lacks sufficient flexibility, thereby impeding interoperability. To advance the goal of achieving interoperability, next steps include identifying and standardizing priority PFT data elements. To increase the motivation of stakeholders to invest in this effort, it is necessary to demonstrate the benefits of PFT interoperability across patient care and population health.
Keywords: interoperability, pulmonary function testing, electronic health record
Contents
Overview
Introduction
Methods
Preworkshop
Workshop
Document Development
An Overview of Key Terminology
Results
The Value of Successful PFT–EHR Interoperability
Real-World Case Studies in Successful PFT–EHR Interoperability
Priority Obstacle 1: Lack of Infrastructure Standardization
Priority Obstacle 2: Lack of Financial and Regulatory Incentives.
Priority Obstacle 3: Lack of Flexible Implementation
Conclusions
Overview
The American Thoracic Society (ATS) and National Heart, Lung, and Blood Institute (NHLBI) cofunded a workshop entitled “Electronic Health Records and Pulmonary Function Data: Developing an Interoperability Roadmap” at the ATS 2019 International Conference. “Interoperability” is defined as the ability of different information-technology systems and software applications to directly communicate, exchange data, and use the information that has been exchanged (1, 2). At present, pulmonary function test (PFT) equipment is not required to be interoperable with other clinical data systems used at the point of care, including electronic health records (EHRs). For this workshop, we assembled a diverse group of experts and stakeholders, including representatives from patient-advocacy groups, adult and pediatric general and pulmonary medicine, informatics, government and healthcare organizations, pulmonary function laboratories, and EHR and PFT equipment and software companies. The participants were tasked with two overarching objectives: 1) identifying the key obstacles to achieving interoperability of PFT systems and the EHR and 2) recommending solutions to the identified obstacles. This report summarizes findings of the workshop.
The key conclusions are as follows:
-
•
Successful interoperability of PFT data with the EHR impacts the full scope of individual patient health and clinical care, population health, and research. At the individual patient level, the ability to track lung measurements seamlessly over time and across healthcare platforms would permit earlier detection of disease onset and monitoring progression of chronic lung diseases. In clinical care, interoperability would facilitate efforts to improve the quality of care by providing real-time access to information necessary to diagnose and treat lung disease. Within health systems, interoperability will enable measuring variability in the quality of care, including the frequency of overdiagnosis and underdiagnosis, and support the expansion of value-based care. Successful PFT interoperability would permit robust population assessments, such as assessments of growth and aging patterns over the life span and of the burden of respiratory disease associated with a range of demographic and environmental factors.
In addition, the development of virtual life-span cohorts would accelerate research in chronic lung diseases, including evaluation of exposures and interventions as these change over time.
-
•
The existing EHR–PFT device platforms lack sufficient data standardization to promote interoperability. A key component of interoperability is the ability of data traveling from the many PFT systems to be recognized by different EHRs. There is currently no consensus on interface standards between different PFT devices and EHRs. Necessary EHR standardization includes definition of common and prioritized PFT data elements, data outputs, labels, data structure, and indicator variables for quality metrics and other metadata.
-
•
Cost is a major obstacle to PFT–EHR interoperability, and incentives are insufficient to justify the needed investment. Institutions and individual practices are not able to prioritize interoperability because of limited financial resources and the lack of a clear return on investment. Motivators to drive stakeholders to divert financial and person-hour resources to interoperability efforts are needed. PFT equipment and EHR companies may lack motivation because of the perceived benefits of maintaining proprietary product uniqueness and profit opportunities. Potential incentives to enhance motivation include PFT interoperability as a meaningful use metric, professional-society standards defining components needed to achieve interoperability, and demonstration projects measuring the effects of interoperability on clinical care, efficiency, value-based care, and cost-effectiveness.
-
•
The current vendor–EHR system lacks sufficient flexibility, thereby impeding interoperability. The PFT–EHR workflow must serve various stakeholders, from the individual healthcare professional to the large healthcare and research organization. There is a need to develop approaches to support flexible implementation across this range of users. In addition, there is currently no approach to retrofit existing systems with data archived in different formats. To move forward with interoperability, approaches will need to be developed to address variability between systems to retain some degree of flexibility while applying standards. Resources to address different needs, including those for existing and future equipment, will be considered in developing a timeline and approach focused on the process of implementation.
Introduction
The EHR is a powerful tool that has the potential to facilitate communication across healthcare systems. As of 2017, 96% of nonfederal acute-care hospitals have adopted some form of EHR. PFTs are critical to assessment and management in lung health (3). PFTs can be conducted in early childhood and are used to track lung growth over time. PFTs are necessary to diagnose lung diseases and to monitor disease progression and response to therapy. As such, PFT data play a key role in patient care, clinical research, and efforts to promote high-value health care. The ability to access PFT data within and between healthcare systems holds great promise for improving our understanding of lung diseases and the respiratory health of patients and the population. This would not only allow monitoring of trends in forced expiratory volume in 1 second in the same way one can typically monitor trends in other clinical laboratory values (e.g., white-blood-cell count or hemoglobin A1c) within the EHR but would also allow visualization of flow-volume loops and indicators of testing quality (e.g., acceptable and reproducible spirometric effort). To date, efforts to interface PFTs with the EHR have been siloed and have not benefited from the unified engagement of the healthcare and pulmonary community. The ultimate goal is to have standards for a core set of PFT data to be transferred to the EHR, both as discrete data elements and for creation of graphical displays. Standardization across the healthcare setting will facilitate early detection and monitoring of lung disease in patients and assessment of lung health in populations to improve healthcare delivery and discovery through research.
Methods
Preworkshop
Initial discussions of the topic of PFT–EHR interoperability occurred at the annual meeting of the Pulmonary Collaborative Research Group, funded by the Patient-Centered Outcomes Research Institute/Patient-Centered Outcomes Research Network. Funding for the workshop on this topic was provided by the ATS. The NHLBI provided additional funds to support the meeting. After receipt of funding, the two co-chairs (Drs. M.C.M. and M.B.D.) identified key stakeholders in the area of PFT–EHR interoperability. The working group included representatives from patient-advocacy groups, adult and pediatric general and pulmonary medicine, informatics, government and healthcare organizations, pulmonary function laboratories, and EHR and PFT equipment and software companies (see online supplement for complete list of participants and affiliations). These stakeholders were asked to provide case studies relevant to their experiences and background. Before the in-person workshop, all participants completed an open-ended survey identifying the top three obstacles and solutions in PFT–EHR interoperability. These results were used to inform the structure of the workshop.
Workshop
The workshop was broadly structured around two themes: 1) review of case studies in PFT–EHR interoperability and 2) identification of the top three obstacles and solutions in PFT–EHR interoperability (see online supplement for detailed agenda). The workshop began with an afternoon session comprising four presentations on the value of and obstacles to PFT–EHR interoperability. This was followed by a general discussion and a breakout session to prioritize obstacles that needed to be tackled to achieve interoperability. The full group was reconvened, the breakout groups’ conclusions were presented to all participants, and a full group discussion occurred to reach agreement on the priority obstacles. The second day included presentations focused on processes for successful PFT–EHR interoperability, followed by a general discussion and breakout sessions on proposed solutions to interoperability challenges. The full group then reconvened with reports from breakout sessions and the final prioritization of solutions to PFT–EHR interoperability challenges.
Document Development
After the workshop, individual presenters provided written summaries of their talks to the co-chairs for synthesis into one manuscript. Additional information was included from written notes of the meeting, and relevant references were incorporated. The draft manuscript was circulated to all workshop participants for comments. Multiple revisions were conducted by the co-chairs, with review and final approval by the full committee. Authors with conflicts of interest related to employment did not write, review, or edit content related to their employers’ commercial interests.
An Overview of Key Terminology
Any discussion of the topic of PFTs and the EHR requires a familiarity with the key terminology of this field (Table 1). Interoperability is the ability of different information-technology systems and software applications to communicate, exchange data, and use the information that has been exchanged (1, 2). “Integration” refers to the connection of systems so that data from one can be accessed by the other, but this often necessitates the use of other software called “middleware.” Interoperability differs from integration in that interoperability achieves direct, real-time data exchange, a concept that is critical in health care. Conceptually, this can be likened to the difference between having a conversation in a common language (interoperability) and having a conversation through an interpreter (integration) (4, 5). To achieve interoperability, and specifically to achieve interoperability with respect to PFT data exchange, a critical step is designating the common language and standardizing the elements of this language (e.g., data outputs, labels/terms, data structure). Another key step is to ensure that all of those involved in the conversation agree to the common language and adopt its elements (e.g., PFT equipment standardizes output, EHR inputs align, healthcare systems require standard formats).
Table 1.
Glossary of terms relevant to PFT–EHR interoperability
| APIs (47) | An API is an interface that allows unrelated software programs to communicate with one another. They act as bridges between two applications, allowing data to flow regardless of how each application was originally designed. |
| Connectivity (48) | The ability to connect to or communicate with another computer or computer system. |
| EHR (49) | An electronic record of health-related information on an individual that conforms to nationally recognized interoperability standards and that can be created, managed, and consulted by authorized clinicians and staff across more than one healthcare organization. |
| FHIR (50, 51) | FHIR (pronounced “fire”) is a standard for exchanging healthcare information electronically, describing data formats and elements (known as "resources"). The standard was created by the HL7 healthcare standards organization. |
| HEDIS (52) | HEDIS is one of health care’s most widely used performance-improvement tools. HEDIS includes more than 90 measures across 6 domains of care. Health plans collect data about their performance on certain services and types of care. They report the data to the NCQA, which rates health plans. Health plans use HEDIS to see where they are performing well and where they need to improve. Employers and consumers can also use HEDIS measures when deciding what health plan to choose. |
| HL7 International (53) | The HL7 standards are produced by HL7 International, an international standards organization. HL7 and its members provide a framework (and related standards) for the exchange, integration, sharing, and retrieval of electronic health information. These standards define how information is packaged and communicated from one party to another, setting the language, structure, and data types required for seamless integration between systems. HL7 standards support clinical practice and the management, delivery, and evaluation of health services and are recognized as the most commonly used in the world. |
| Interoperability (1, 2, 4, 5, 28, 30, 47, 48, 54–58) | In health care, interoperability is the ability of different information-technology systems and software applications to communicate; to exchange data accurately, effectively, and consistently; and to use the information that has been exchanged. |
| Integration (1, 5, 57) | Integration is the process of combining multiple different, often disparate, subsystems to function together as a unified whole. This often includes building of customized architecture or structure of applications (“middleware”). Conceptually, the difference between interoperability and integration can be likened to the difference between having a conversation in a common language (interoperability) and having a conversation through an interpreter (integration). |
| LOINC (38) | LOINC were developed to provide a definitive standard for identifying clinical information in electronic reports. The LOINC database provides a set of universal names and ID codes for identifying laboratory and clinical test results. LOINC are intended to identify the test result or clinical observation. |
| NCQA (8) | The NCQA is an independent U.S. nonprofit organization that works to improve the quality of health care through standardized measures and accreditation. |
| SNOMED (9, 30, 59) | SNOMED is a not-for-profit organization with a mission to produce and enhance the vocabulary that enables the clear exchange of health information for all. SNOMED owns and operates SNOMED CT, a systematically organized collection of medical terms standardized for use in the electronic exchange of medical information. SNOMED CT provides codes, terms, synonyms, and definitions used in clinical documentation and reporting. |
| SQL (60) | SQL (pronounced “sequel”) is a standardized programming language that is used to operate databases. SQL is a standard language that facilitates management of relational databases and performance of various operations (deletion, fetching, modifying) on the data in databases. |
Definition of abbreviations: API = application-programming interface; CT = Clinical Terms; EHR = electronic health record; FHIR = Fast Healthcare Interoperability Resources; HEDIS = Healthcare Effectiveness Data and Information Set; HL7 = Health Level 7; ID = identifier; LOINC = Logical Observation Identifiers Names and Codes; NCQA = National Committee for Quality Assurance; PFT = pulmonary function test; SNOMED = Systematized Nomenclature of Medicine; SQL = Structured Query Language.
Results
The Value of Successful PFT–EHR Interoperability
Spirometry was developed in the 1840s. The attributes that made it appealing were that it was simple, noninvasive, reproducible, and relatively inexpensive. Newer technologies have reduced the equipment size and increased its portability, which further broadens the sites at which these measurements can be taken. Evaluating, managing, and prognosticating for individual patients with respiratory diseases all require spirometry. This is true for common airway diseases, including asthma and chronic obstructive pulmonary disease (COPD), as well as for rarer conditions, such as cystic fibrosis and interstitial lung diseases. There are now well-established international standards for test performance and international consensus statements for disease management (6–10). International efforts have also focused on refining reference standards to understand expected normal values of lung function throughout the life span (11, 12). When performed according to standards with attention to quality control, the test is highly reproducible within a healthy individual. Pulmonary function is thus a benchmark value, akin to height, which can be tracked for a person’s lifetime with individual-specific inferences being made. These measurements become particularly useful in disease states (e.g., cystic fibrosis, after lung transplantation, stratification for asthma severity, determination of COPD progression). Prognostic scores, such as the Gender, Age, and Physiology variables (GAP) score for idiopathic pulmonary fibrosis (13) and the body mass index, airflow obstruction, dyspnea, and exercise capacity index (14) for COPD, use spirometry as a key variable.
Development of consistent interoperability standards for inclusion of PFTs in EHRs would benefit many populations served by a healthcare system. Current information about objective measures of population respiratory health is primarily based on large population-based surveys that have included PFTs, like the Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey (14). These use strict protocols to ensure that PFTs are conducted and data are collected in a standardized manner (e.g., adhering to ATS standards [15, 16], using the same spirometry equipment, using a small pool of trained technicians, etc.). Cohort studies that have assessed lung function over time have provided valuable insights about modifiable risk factors that affect lung development and risk of chronic lung disease (17–22). Studies of this scientific rigor are costly and take years to design and carry out but do not represent the entire population. Having the ability to access and aggregate pulmonary function data from EHRs across multiple healthcare-delivery systems would facilitate population-level research that provides more timely information at a lower cost. In addition to providing objective information about the population burden of respiratory impairment, linking pulmonary function data with other data elements in EHRs would provide an avenue for identifying modifiable risk factors, such as behavioral habits or occupational exposures, that could be targets for interventions to improve respiratory health. It could also be used by payers and providers to track adherence to practice guidelines and for quality improvement. Thus, there are many ways that standardized, interoperable inclusion of pulmonary function data in EHRs would benefit the design and implementation of high-value models of health care for people at risk of lung disease (e.g., smokers) and for those who already have developed lung disease (e.g., asthma, COPD).
Real-World Case Studies in Successful PFT–EHR Interoperability
The National Institute for Occupational Safety and Health (NIOSH) Coal Workers’ Health Surveillance Program is a national program that provides respiratory health screening to U.S. coal miners. Regulations established in 2014 enabled spirometry to be added to existing screening tests (23, 24). To ensure high-quality spirometry across a national network of approved testing facilities, specific requirements are outlined in regulations for spirometric technician training, spirometric equipment, and spirometric procedures (15, 24). Standardization of electronic data output from spirometers is an important aspect of the program. This allows facilities to electronically submit spirometric results, including raw flow-time spirometric data points from each maneuver and other respiratory and miner information to NIOSH. Standardization allows NIOSH to rapidly import data from each maneuver into a software program to perform spirometric-test quality review, distribute data for interpretation, and generate and send reports about the testing back to the miner. Because the Coal Workers’ Health Surveillance Program needed standardized spirometric data-output and electronic data-transfer capabilities to support its spirometric surveillance network, NIOSH began working directly with interested spirometer manufacturers in 2014 to provide these capabilities in specific spirometer models approved for use in the program (listed on the NIOSH web page) (25). The development of Spirometry Longitudinal Data Analysis (SPIROLA) software represents another NIOSH effort involving interoperability (26). SPIROLA software provides a computerized approach to assist with monitoring longitudinal spirometric data. SPIROLA software is freely available for download on the CDC’s website and has been used in occupational settings to monitor worker longitudinal lung function and spirometric quality (27). These NIOSH efforts demonstrate PFT interoperability initiatives that have been successful in occupational health settings.
Rady Children’s Hospital serves as the referral center for respiratory conditions for over 95% of the children who live in San Diego County. In the original EHR system, the pediatric pulmonary physicians provided interpretations for all testing that occurred outside of allergy outpatient visits, requiring multiple workflows and often resulting in delays in patient care. To implement the program, Rady Children’s Hospital first aligned the machines to one manufacturer to minimize technical build. Infrastructure such as cloud/network storage was established, with assessment of internet bandwidth to determine whether wireless or wired data connections were best to send data from the PFT machine to the network and EHR. At the time of implementation approximately 7 years ago, few if any hospitals were integrating PFT results into their EHR, requiring Rady Children’s Hospital to work closely with both PFT vendors and EHR developers to create the mapping for successful implementation. Physicians, respiratory therapists, and staff worked together to optimize workflows by creating order protocols and result-routing schemas. PFT reports and synopsis views were built to display long-term trends within a patient’s chart across encounters. Discrete result values were transferred to the EHR, and the approach was designed so that all PFT interpretation was performed within the EHR. The benefit of interpreting testing within the EHR is that test interpretations exist within the ecosystem of the EHR and can be mined, just as a complete blood count (CBC) result can be mined. The implementation of this interoperability project has been extremely helpful in terms of ensuring no input-result errors, cost savings on effort for data entry, and improved turn-around time for PFT results in patient care, registries, and research.
Kaiser Permanente is an integrated managed-care consortium with a mission of providing high-quality patient-centered care. Circa 2010, Kaiser Permanente Northwest had only two workarounds for PFT viewing in their EHR: 1) manual data entry of key data that could later be pulled into a clinician note through an automated phrase or 2) scanned portable document–format files attached to a clinical note that were often not discoverable by any subsequent EHR search. The Kaiser Permanente HealthConnect team in the region worked with a national build of master files to map data to the Logical Observation Identifiers Names and Codes (28) and Health Level 7 (HL7) fields (29) (where these fields were lacking, data were added to concepts in the Systematized Nomenclature of Medicine [SNOMED] system) (30) to build a point-to-point interface using vendor software as an intermediary of the spirometer database. As many fields did not have the HL7 interface, discrete data tables and variables were created. After implementation in 2012, the system seamlessly accommodated discrete comprehensive spirometric, diffusion, plethysmographic, and exercise data in a unique results tab in the EHR. Users of Kaiser Permanente HealthConnect can now view flowsheets of detailed data, view results-tab formatting with temporal trends, or open a portable document–format file with flow-volume loops and spirometer-generated values. The discrete data fields are also fed from the EHR in a weekly data transfer to the Kaiser Northwest Center for Health Research to incorporate these results into the comprehensive research data warehouse for clinical and health-service studies performed in the region.
Internationally, there are examples of successful interoperability. During the last decade, several initiatives have been developed in Catalonia (Spain) to implement the digitalization of spirometry throughout the territory, as a first step to include all lung function tests. The main objective of the Catalonian initiative is to “Implement high quality forced spirometry for diagnosis and management of chronic respiratory conditions in primary care.” (31). Importantly, concurrent work promoted by the Office for Standards and Interoperability of the Catalonian Department of Health and by the Plan for the Digitalization of Medical Images involved creation of data standards for spirometry. These standards have been based on version 3 of HL7, Clinical Document Architecture (CDAR) release 2, to facilitate interoperability (32). The CDAR for spirometry includes the data of the patient, the information about the context of the test, the resulting clinical parameters, and the flow-volume and volume-time graphs, as well as the original signal (raw data) captured by the spirometer. The benefits of the implementation of the spirometric program are 1) enhanced automatic quality assessment, 2) accessibility of standardized (and quality-labeled) testing across healthcare tiers, 3) generation of individual standardized reports including historical information of lung function, 4) data analytics allowing the longitudinal assessment of lung function changes with relevant implications in future personalized patient management, and 5) accessibility of the report and access to off-line remote support by specialized professionals.
Highlighting that lessons from one region can be transferred to another, during the period of 2010–2013, another Spanish health administration in Basque Country (33, 34), decided to incorporate the model of quality analysis of spirometry developed in Catalonia, which is now implemented in 100% of that territory. This implementation required an expert to review all spirometry in the region, which resulted in several studies that developed and evaluated an automatic quality algorithm. The model operationalizes a comprehensive application of the ATS/European Respiratory Society recommendations for automatic quality control of spirometric testing. Furthermore, this model represents the key elements facilitating the design and future deployment of a high-quality spirometric program based on remote automatic evaluation of the testing. Burgos and colleagues (1) have reported the effectiveness of a web-based application for remote off-line expert support to enhance the quality of spirometry in primary care (35). High-quality testing improved in a sustainable manner with remote support. These results demonstrate the potential positive impact on quality-assurance programs of spirometry performed by nonexperts.
These case studies demonstrate that PFT–EHR interoperability is feasible and valuable across different settings. In addition, each case outlines some of the obstacles to and solutions for achieving interoperability. Other obstacles identified by participants in the preworkshop survey are summarized in Table 2. The next section of this workshop report focuses on the top three obstacles and proposed solutions related to PFT–EHR interoperability identified during the in-person workshop, as summarized in Table 3.
Table 2.
Key obstacles to PFT–EHR interoperability by stakeholder group
| Stakeholder Group | Identified Obstacles |
|---|---|
| The patient | • Evolving technologies require evolving integration |
| • Limited approaches to address home data collection and desire for an app | |
| • Lack of seamless information connectivity across time and geography | |
| The clinician or researcher | • Complex steps to extract data from EHR into clinical notes |
| • Heterogeneous PFT data types and sites of data storage preclude monitoring trends over time for an individual patient from childhood through adult life | |
| • Limitations of EHRs in primary care and limited information exchange between primary care providers and specialists impairs continuity of care | |
| • PFT data are often sequestered. For example, PFT data obtained on a person as part of their workplace screening is not incorporated into their health record and is therefore unavailable when they develop a health problem later in life | |
| • Inability to integrate images into the EHR limits ability to assess flow-volume loops and other quality metrics | |
| • Lack of metadata to indicate quality of testing | |
| • Interfacing costs and lack of clear value to reimbursement and healthcare outcomes | |
| • Clinically generated PFT data lacks uniform, discrete, structured data elements | |
| • Inability to retrofit existing PFT data because of cost and person-hour limitations | |
| • Inability to integrate PFT data with data regarding underlying disease type and severity | |
| • Raw data underlying flow-volume curves needed to assess technical errors with greater precision are generally not provided | |
| The device manufacturers | • Lack of IT resources from purchasers |
| • Lack of open application-programming interfaces for clinical data | |
| • Lack of consensus on which discrete variables should be sent to the EHR. Decisions on variables sent from vendors to EHRs are usually left up to the lead physician, with high variation between interfaces | |
| • Electronic PFT forms represent a streamlined version of a full report, potentially losing crucial data if certain variables are selected. However, more data fields (particularly if manual entry or curation is required) can lead to more errors | |
| • Poor interoperability at the health system level | |
| • Need for adequate connectivity to support data transfer from devices to EHRs | |
| • Lack of incentives by customer to link PFT data to the EHR (i.e., lack of incorporation of interoperability into laboratory certification) | |
| • Lack of variable-naming conventions, including multiple LOINC depending on the context of data acquisition | |
| • Lack of subject-matter expertise among clients. PFT directors often do not possess the IT knowledge needed for pre–interoperability-assessment needs, and IT departments do not possess the intimate knowledge needed to understand the needs of the PFT laboratory and/or healthcare stakeholders | |
| • Lack of common data model and interoperability standard by societies | |
| The EHR companies | • Data-output formats and fields vary across devices inputting into the EHR |
| • Compatibility with the EHR varies across devices | |
| • Variation in PFT orders linked to testing | |
| • Multiple PFT devices/vendors within single customer base | |
| • Lack of willingness of PFT vendors to interface discrete data with EHRs | |
| • Lack of variable-naming conventions and incomplete or duplicate LOINC | |
| • PFT data not prioritized for standardization by organizational bodies (FHIR) | |
| • Lack of common data model and interoperability standard by societies |
Definition of abbreviations: EHR = electronic health record; FHIR = Fast Healthcare Interoperability Resources; IT = information technology; LOINC = Logical Observation Identifiers Names and Codes; PFT = pulmonary function test.
Table 3.
Solutions by theme
| Lack of common data elements and definitions | 1. Creation of a standard library of terms |
| 2. Standard format | |
| 3. Identification of common data elements endorsed by ATS and academic thought leaders/societies | |
| 4. Standard application-programming interfaces in the future | |
| Lack of institutional priority and cost considerations and lack of motivators to influence stakeholders | 1. Patient groups as advocates |
| 2. Meaningful use metrics | |
| 3. Endorsement of medical societies | |
| 4. Demonstration that investment increases efficiency and reduces costs over long term | |
| 5. PFT data are recognized as a priority, similar to blood pressure or HbA1C | |
| Lack of flexible implementation | 1. Standard API |
| 2. Lower costs | |
| 3. Solutions to communicating quality | |
| 4. Consideration of legacy systems and retrofitting | |
| 5. Education and dissemination |
Definition of abbreviations: API = application-programming interface; ATS = American Thoracic Society; HbA1c = hemoglobin A1c; PFT = pulmonary function test.
Priority Obstacle 1: Lack of Infrastructure Standardization
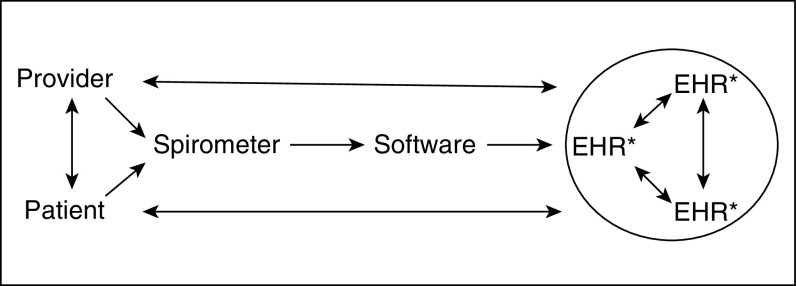
Lack of generally accepted standards for PFT data generated by testing equipment and maintained in EHRs is an important obstacle to achieving consistent availability and usability of PFT results. Lack of standardization also impedes the ability to share this information between providers and healthcare systems and to provide data to public health organizations and researchers. There is no generally accepted, standardized information model specifying how information should flow from ordering to generation of pulmonary function data by equipment during testing; storage, retrieval, and use in the medical record; and sharing between different health information-technology systems (Figure 1). In the absence of such a standard, equipment manufacturers and EHR developers must rely on proprietary solutions that limit prospects for interoperability.
Figure 1.
An overview of the flow of PFT–electronic health record (EHR) interoperability and integration. *The three EHRs represent large-healthcare-system, small-single-hospital, and outpatient-office relationships. PFT = pulmonary function test.
Availability of a standardized data vocabulary that labels and defines PFT elements would benefit both manufacturers of PFT equipment that generates pulmonary function data and developers of EHR software that receives the data and manages it. Moving from proprietary solutions to standardized information models and approaches to data exchange between PFT equipment and EHRs would have many benefits. It would promote interoperability, enabling data generated by pulmonary function equipment from a wide range of manufacturers to be recognized by a wide range of EHR systems and to be shared between different brands of EHR systems. Availability of standards would also support technological innovation by eliminating the costly overhead of developing proprietary solutions to issues already addressed in standards. Although some specific terms relevant to PFTs are named and defined in standardized systems, such as medical-device communication codes (36, 37), Logical Observation Identifiers Names and Codes (38), and SNOMED Clinical Terms (30), there is still a need to work through one or more of these systems as part of a standard-developing project (described below) to develop a well-organized, generally accepted vocabulary without gaps. Ideally, the vocabulary would define both essential/priority PFT data elements of interest to most users, such as those specified by the ATS for inclusion in standardized pulmonary function reports (16), and a more comprehensive set of data elements that might optionally be included in the EHR, such as those proposed by ATS for inclusion in standardized electronic spirometric data files (15) and those that are implemented as part of a harmonization protocol to pool studies from NHLBI cohort studies (39). Other standardized data elements to consider for naming and defining include data related to test ordering, type of testing site (e.g., hospital clinic, ambulatory center), interpretive findings, indicator variables for quality metrics, and other metadata.
Standards, such as those that are needed to enhance interoperability of pulmonary function data, are created by a complex system of standard-developing organizations (SDOs) (40). Internationally, the International Standards Organization (ISO) plays an important role, as most countries require the use of an ISO standard if one exists. Some SDOs have agreements with the ISO, providing pathways to bring their standards into the ISO. One example (41) relevant to PFT equipment is the Institute of Electrical and Electronics Engineers, which develops standards for a broad range of technologies, including medical devices. Another (40) is HL7, organized to create standards to support the development of health information systems. Three major standards (42) maintained by HL7 include the CDA standard, which specifies the structure and semantics of clinical documents; the version 2 standard, which provides a messaging standard allowing exchange of clinical data between systems; and the Fast Healthcare Interoperability Resources standard, which provides a content model and specification for information exchange via web-like REpresentational State Transfer (REST)-ful interfaces (43), messaging, and documents. Working through appropriate SDOs provides a realistic potential pathway to achieve standardization and improve interoperability of pulmonary function data in EHRs.
Priority Obstacle 2: Lack of Financial and Regulatory Incentives
As a second key obstacle, the committee identified lack of financial motivations by large and small healthcare systems to advance the goal of PFT–EHR interoperability. Despite the wide use of EHRs across large healthcare institutions, it was perceived that institutional prioritization of PFT–EHR interoperability was low, which was largely driven by the potential costs of required modifications to existing EHR systems. Beyond the potentially substantial financial impact of attempting to harmonize large and diverse EHRs, many of which are unique to a particular healthcare setting, the lack of clear governance of cost was identified as a meaningful obstacle. Specifically, it was believed that the costs for a harmonization process did not clearly reside within a single silo of the healthcare institutions. For example, these costs may be viewed by clinical departments as information-technology support costs, whereas hospital information-technology departments view these as clinical care costs. Moreover, the committee agreed that the lack of evidence of a clear return on investment associated with interoperability costs further impeded progress in this area. The workshop members were not aware of any studies that have evaluated the financial benefits of PFT–EHR interoperability. Such financial benefits are likely largely indirect, such as those obtained through increased clinical efficiency, improved clinical care, and overall risk mitigation. The availability of PFT data as discrete data elements within the EHR has the ability to positively impact value-based care systems (in which providers, including hospitals and physicians, are paid on the basis of patient health outcomes) through minimizing inappropriate under- and overdiagnosis and treatment of lung disease (44–46). Another driver of perceived value to any EHR system, large or small, is the presence of regulatory requirements. Currently, no meaningful use ascribed to PFT–EHR interoperability exists within the U.S. Centers for Medicare and Medicaid Services (CMS). Current professional-society guidelines do not mandate PFT–EHR interoperability as a component to guideline-adherent testing. Market demands, frequently driven by the consumer patient, are also not currently present. Without these external forces contributing to the perceived value of PFT–EHR interoperability, healthcare systems are likely to remain unmotivated to financially support this advancement.
Several solutions were identified to enhance the incentives for PFT–EHR interoperability. First, it was believed that a clear understanding of the challenges, benefits, and case studies in success should be captured in a single document to serve as a resource for those advocating change. This workshop report is intended to serve that purpose. Second, evidence-based studies should be conducted evaluating the potential impact of PFT–EHR interoperability on objective measures of efficiency, under- and overtreatment, and other discrete metrics to create a financial case for investment in interoperability. The committee believed there could be both “carrots” and “sticks” to motivate change in small and large healthcare systems. Carrots consisted of inclusion of interoperability as a component of healthcare scoring systems and accreditation, a core requirement of specialty pulmonary disease centers, and inclusion as a meaningful use metric by the CMS. Examples of sticks included requirement of EHR interoperability for spirometry as a component of professional-society core standards and PFT laboratory certification, as well as billing compliance. Societies should actively publish editorials highlighting the importance of this issue. The committee recognized that patient- and caregiver-advocacy groups are often drivers of change. Efforts to educate patients about the value of interoperability (e.g., for disease-tracking across different institutions and enhanced communication between providers) has the potential to create a grassroots movement to drive change within the healthcare system. An overall effort to increase patient awareness of the need for PFT interoperability, through requiring inclusion of serial PFT measures on patient reports and designation of centers meeting the benchmark of interoperability, can serve to increase awareness as a potential agent of change. Patient education materials on the topic of PFT–EHR interoperability, including personal stories of patients to bring a face to this issue, can be generated by societies and patient-advocacy groups.
Priority Obstacle 3: Lack of Flexible Implementation
The PFT–EHR workflow must serve various stakeholders, from individual healthcare professionals to large healthcare and research organizations. There is no clear roadmap to permit flexible implementation across this range of users. For PFTs, examples of heterogeneity of common practices range from variability in the workflow related to how orders for tests are created and implemented within the EHR to how results are routed to providers. There is currently no approach to retrofit existing systems with data archived in different formats, and this is necessary to preserve data trends over time and to accommodate the existing systems that are of variable age and costly to replace. To move forward with interoperability, systems will need to be able to contain some degree of flexibility while implementing standards to address retrospective and prospective PFT data as well as existing and new PFT testing systems.
Implementation strategies to achieve interoperability need to consider the heterogeneity of the healthcare landscape, ranging from small community practices to large healthcare systems. Although this workshop primarily focused on pulmonary function laboratories, content is highly relevant for future application to office spirometry. The heterogeneity and age of existing pulmonary function equipment also warrants consideration, with approaches to retrofitting systems and including data from legacy systems. Flexibility in implementation may include deliberate strategies to make adjustments over time to accommodate real-world obstacles. The timeline for adopting new standards should include consideration of existing systems and data while working in parallel to define interoperability standards for the present and future. To efficiently implement PFT software and EHR modifications, the healthcare system should support implementation coaches and training to enhance education on processes. Initiatives to quantity the impact of achieving interoperability must span multiple domains to substantiate investment in interoperability standards and the investment in adapting existing systems to meet these standards. Such initiatives should include patient, provider, and healthcare-system perspectives.
Conclusions
This workshop has demonstrated that PFT–EHR interoperability has tremendous potential value to all stakeholders in the healthcare system: patients, physicians and clinicians, government and healthcare organizations, and pulmonary function laboratories. EHR and PFT equipment and software companies are summarized in Table 4. Importantly, successful interoperability has already been demonstrated to be feasible across national and international settings. By identifying the priority obstacles to and solutions for successful PFT–EHR interoperability, this workshop report outlines priority obstacles and solutions on the roadmap to PFT–EHR interoperability. Many of the challenges identified by this workshop also apply to general EHR interoperability of laboratory and clinical data. As such, some of the identified solutions can be applied to the other data domains. To continue to advance the goal of PFT–EHR interoperability, several next steps are required: 1) invested professional organizations and government groups sponsoring additional stakeholder engagement opportunities, 2) a working group or committee developing an implementation framework to organize and operationalize the proposed solutions, 3) SDOs working with stakeholders to develop PFT data standards that can become required across software platforms, 4) healthcare organizations and government bodies funding studies evaluating the impact of PFT–EHR interoperability on healthcare efficiencies and treatment, and 5) accreditation bodies and payers incorporating interoperability into accreditation standards. Through multistakeholder efforts to improve standardization, motivation, and flexibility within the current PFT–EHR landscape, we can achieve early detection and monitoring of lung disease in patients and assessment of lung health in populations to improve healthcare delivery and discovery through research.
Table 4.
Examples of value of successful interoperability by stakeholder group
| The patient | • The patient is able to track PFT data from early childhood to adulthood with integration of data across healthcare systems throughout the lifetime. |
| • Results are accessible to providers in general and specialty care and in different geographic regions. | |
| The clinician | • Physicians have access to historical lung function information for a given patient who has been evaluated in different health systems to diagnose the onset of disease. |
| • Enhanced ability to observe trends in lung function over time to monitor patients with chronic disease. | |
| • Quality metrics provide a greater ability to factor in data quality. | |
| The clinician–researcher | • Data would be easily extractable within a healthcare system to foster discovery. |
| • Quality metrics would be more uniform to increase data quality and reduce bias. | |
| Device manufacturers | • Standard output would make output uniform between clients so that interfacing with EHR companies would be uniform. |
| • Enhanced efficiency. | |
| EHR | • Inputs would be standard so that interfacing data from PFT vendors would be uniform. Output of data to end-users’ healthcare providers and patients would be standardized, with minor customizations based on patient complexity. |
| • Enhanced efficiency. | |
| Population health/all stakeholders | • Population health research can be amplified using data analytics and big data. This will facilitate study of risk factors, protective factors to promote optimal lung development and lung health, and interventions. |
Definition of abbreviations: EHR = electronic health record; PFT = pulmonary function test.
Acknowledgment
This official workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Clinical Problems, Assembly on Behavioral Science and Health Services Research, and Assembly on Environmental, Occupational and Population Health.
Members of the subcommittee are as follows:
Meredith C. McCormack, M.D.1 (Co-Chair)
M. Bradley Drummond, M.D., M.H.S.2 (Co-Chair)
Rebecca Bascom, M.D., M.P.H.3
Michael Brandt4
Felip Burgos, Ph.D.5
Sam Butler, M.D.6
Christopher Caggiano, M.D.7
Anne E. F. Dimmock, M.S.3
Josh Fessel, M.D., Ph.D.8*
Adrian Fineberg9
Michelle Freemer, M.D., M.P.H.8*
Jeffrey Goldstein10
Francisco C. Guzman, B.S.11
Cara N. Halldin, Ph.D., M.P.H.12
J. D. Johnson13
Gwendolyn S. Kerby, M.D.14
Jerry A. Krishnan, M.D., Ph.D.15
Laura Kurth, Ph.D.12
Marrah Lachowicz-Scroggins, Ph.D.8*
Gareth Morgan, Ph.D.16
Richard A. Mularski, M.D., M.S.H.S., M.C.R.17
Cara B. Pasquale, M.P.H.18
Antonello Punturieri, M.D., Ph.D.8*
Julie Ryu, M.D.19
Tom Sinclair20
Nadia F. Stachowicz, B.S., R.R.T.11
Ann Taite, B.Sc.21
Jacob Tilles20
Jennifer R. Truta13
David N. Weissman, M.D.12
Tianshi David Wu, M.D., M.H.S.1
Barbara P. Yawn, M.D., M.Sc.22
1Johns Hopkins University, Baltimore, Maryland; 2University of North Carolina at Chapel Hill, Chapel Hill, North Carolina; 3Penn State Health, Hershey, Pennsylvania; 4MedGraphics Corporation, St. Paul, Minnesota; 5University of Barcelona, Barcelona, Spain; 6Epic, Verona, Wisconsin; 7Allscripts Healthcare Solutions, Inc., Chicago, Illinois; 8National Institutes of Health–National Heart, Lung, and Blood Institute, Washington, D.C.; 9Vitalograph, Lenexa, Kansas; 10Lung Transplant Foundation, Cary, North Carolina; 11Vyaire Medical, Chicago, Illinois; 12National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Washington, D.C.; 13Cerner Corporation, Kansas City, Missouri; 14Children’s Hospital Colorado, Aurora, Colorado; 15University of Illinois at Chicago, Chicago, Illinois; 16Morgan Scientific, Inc., Haverhill, Massachusetts; 17Kaiser Permanente Northwest Center for Health Research, Portland, Oregon; 18COPD Foundation, Miami, Florida; 19University of California at San Diego, San Diego, California; 20nDD Medical Technologies Inc., Andover, Massachusetts; 21Queen’s University, Ontario, Canada; and 22Olmsted Medical Center, Rochester, Minnesota
*Member of the subcommittee who was not part of the writing committee.
Footnotes
Supported by the American Thoracic Society and the National Heart, Lung, and Blood Institute (grant NHLBI R13 HL147437).
This official workshop report of the American Thoracic Society was approved September 2020
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/AnnalsATS.202010-1318ST.
This document has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: M.C.M. or spouse served on an advisory committee for Celgene, GlaxoSmithKline, United Therapeutics (spouse); as a consultant for Actelion (spouse), Arena (spouse); on a data safety and monitoring board for United Therapeutics (spouse); as a speaker for Talem Health; received royalties from UpToDate. R.B. received research support from Boehringer Ingelheim, MediciNova, Nitto Denko; travel support from Galapagos, Nitto Denko. M.B. is an employee of MGC Diagnostics Corporation. S.B. is an employee of Epic. C.C. was an employee of Allscripts. A.F. is an employee of Vitalograph. J.G. or spouse is an employee of Novartis (spouse). F.C.G. is an employee of Vyaire Medical. R.A.M. served on an advisory committee and received research support from GlaxoSmithKline. J.R. received research support from ResMed. N.F.S. is an employee of Vyaire Medical. B.P.Y. served on an advisory committee for AstraZeneca, GlaxoSmithKline; as a consultant for Boehringer Ingelheim, COPD Foundation, GlaxoSmithKline, NDD Medical Technologies, Novartis; received research support from GlaxoSmithKline, National Heart, Lung, and Blood Institute, PCORI. M.B.D. served on an advisory committee for American Society Hospital Pharmacists, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mylan; as a consultant for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Midmark, Mylan, NovaVax, Parion, Teva; on a roundtable for Respironics; received research support from AstraZeneca, Boehringer Ingelheim, Midmark, National Heart, Lung, and Blood Institute, Sanofi, Vertex; received royalties from Karger Publishing. F.B., A.E.F.D., J.G., C.N.H., J.D.J., G.S.K., J.A.K., L.K., G.M., C.B.P., T.S., A.T., J.T., J.R.T., D.N.W., and T.D.W. reported no commercial or relevant non-commercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Clinical Problems, Assembly on Behavioral Science and Health Services Research, and Assembly on Environmental, Occupational, and Population Health
References
- 1.Healthcare Information and Management Systems Society. Chicago, IL: Interoperability in Healthcare; 2020. The current state of interoperability. [accessed 2020 Nov 24]. Available from: https://www.himss.org/resources/interoperability-healthcare. [Google Scholar]
- 2.Healthcare Information and Management Systems Society Interoperability in the health ecosystem Chicago, IL: Healthcare Information and Management Systems Society; 2020. [accessed 2020 Mar 6]. Available from: https://www.himss.org/library/interoperability-standards/what-is-interoperability [Google Scholar]
- 3.Office of the National Coordinator for Health Information Technology Non-federal acute care hospital electronic health record adoptionHealth IT Quick-Stat #47. Washington, DC: Office of the National Coordinator for Health Information Technology; 2017[accessed 2020 Nov 24]. Available from: https://dashboard.healthit.gov/quickstats/pages/FIG-Hospital-EHR-Adoption.php [Google Scholar]
- 4.Spok Integration vs. interoperability: what’s the difference? Springfield, VA: Spok; 2019[accessed 2020 Mar 6]. Available from: https://www.spok.com/blog/integration-vs-interoperability-whats-difference [Google Scholar]
- 5.Roberts B.Integration vs interoperability: what’s the difference? Alpharetta, GA: Surgical Information Systems; 2017[accessed 2020 Mar 6]. Available from: https://blog.sisfirst.com/integration-v-interoperability-what-is-the-difference [Google Scholar]
- 6.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 7.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 8.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 9.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 11.Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49:1600016. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 12.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update: an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, et al. ATS Committee on Proficiency Standards for Pulmonary Function Laboratories. Recommendations for a standardized pulmonary function report: an official American Thoracic Society technical statement. Am J Respir Crit Care Med. 2017;196:1463–1472. doi: 10.1164/rccm.201710-1981ST. [DOI] [PubMed] [Google Scholar]
- 17.Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5:935–945. doi: 10.1016/S2213-2600(17)30434-4. [DOI] [PubMed] [Google Scholar]
- 18.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 19.Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194:607–612. doi: 10.1164/rccm.201604-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grol MH, Gerritsen J, Vonk JM, Schouten JP, Koëter GH, Rijcken B, et al. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years: a 30-year follow-up study. Am J Respir Crit Care Med. 1999;160:1830–1837. doi: 10.1164/ajrccm.160.6.9812100. [DOI] [PubMed] [Google Scholar]
- 22.Gauderman WJ, Gilliland GF, Vora H, Avol E, Stram D, McConnell R, et al. Association between air pollution and lung function growth in Southern California children: results from a second cohort. Am J Respir Crit Care Med. 2002;166:76–84. doi: 10.1164/rccm.2111021. [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention Coal Workers’ Health Surveillance Program Atlanta, GA: Centers for Disease Control and Prevention; 2020[accessed 2020 Mar 6]. Available from: https://www.cdc.gov/niosh/topics/cwhsp/default.html [Google Scholar]
- 24.Specifications for medical examinations of coal miners Fed Regist 20168173270[accessed 2020 Mar 6]. Available from: https://www.gpo.gov/fdsys/pkg/FR-2016-10-24/pdf/2016-24405.pdf [Google Scholar]
- 25.National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention Coal Workers’ Health Surveillance Program: spirometry manufacturer & model information for clinics Atlanta, GA: Centers for Disease Control and Prevention; 2018[accessed 2020 Mar 6]. Available from: https://www.cdc.gov/niosh/topics/cwhsp/ManufModelInfoClinics.html [Google Scholar]
- 26.National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention Spirometry: Spirometry Longitudinal Data Analysis (SPIROLA) software Atlanta, GA: Centers for Disease Control and Prevention; 2014[accessed 2020 Mar 6]. Available from: https://www.cdc.gov/niosh/topics/spirometry/spirola.html [Google Scholar]
- 27.Hnizdo E, Berry A, Hakobyan A, Beeckman-Wagner L-A, Catlett L. Worksite wellness program for respiratory disease prevention in heavy-construction workers. J Occup Environ Med. 2011;53:274–281. doi: 10.1097/JOM.0b013e31820b0ab1. [DOI] [PubMed] [Google Scholar]
- 28.Regenstrief Institute LOINC basics Indianapolis, IN: Regenstrief Institute; 2020[accessed 2020 Mar 6]. Available from: https://loinc.org/faq/basics/ [Google Scholar]
- 29.Dolin RH, Alschuler L, Boyer S, Beebe C, Behlen FM, Biron PV, et al. HL7 Clinical Document Architecture, release 2. J Am Med Inform Assoc. 2006;13:30–39. doi: 10.1197/jamia.M1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SNOMED International SNOMED International London, UK: SNOMED International; 2020[accessed 2020 Mar 6]. Available from: https://www.snomed.org [Google Scholar]
- 31.Melia U, Burgos F, Vallverdú M, Velickovski F, Lluch-Ariet M, Roca J, et al. Algorithm for automatic forced spirometry quality assessment: technological developments. PLoS One. 2014;9:e116238. doi: 10.1371/journal.pone.0116238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salas T, Rubies C, Gallego C, Muñoz P, Burgos F, Escarrabill J. Technical requirements of spirometers in the strategy for guaranteeing the access to quality spirometry. Arch Bronconeumol. 2011;47:466–469. doi: 10.1016/j.arbres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Marina Malanda N, López de Santa María E, Gutiérrez A, Bayón JC, Garcia L, Gáldiz JB. Telemedicine spirometry training and quality assurance program in primary care centers of a public health system. Telemed J E Health. 2014;20:388–392. doi: 10.1089/tmj.2013.0111. [DOI] [PubMed] [Google Scholar]
- 34.Marina N, Bayón JC, López de Santa María E, Gutiérrez A, Inchausti M, Bustamante V, et al. Economic assessment and budgetary impact of a telemedicine procedure and spirometry quality control in the primary care setting. Arch Bronconeumol. 2016;52:24–28. doi: 10.1016/j.arbres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Burgos F, Disdier C, de Santamaria EL, Galdiz B, Roger N, Rivera ML, et al. e-Spir@p Group. Telemedicine enhances quality of forced spirometry in primary care. Eur Respir J. 2012;39:1313–1318. doi: 10.1183/09031936.00168010. [DOI] [PubMed] [Google Scholar]
- 36.Institute of Electrical and Electronics Engineers Standards Association IEEE 11073-10101-2019: IEEE standard for health informatics—point-of-care medical device communication. Part 10101: nomenclature Piscataway, NJ: Institute of Electrical and Electronics Engineers Standards Association; 2019[accessed 2020 Mar 6]. Available from: https://standards.ieee.org/standard/11073-10101-2019.html. [Google Scholar]
- 37.RTM Management Service; National Institute of Standards and Technology About Rosetta New York, NY: Institute of Electrical and Electronics Engineers; 2016[accessed 2020 Mar 6]. Available from: https://rtmms.nist.gov/rtmms/index.htm [Google Scholar]
- 38.Regenstrief Institute LOINC: the international standard for identifying health measurements, observations, and documents Indianapolis, IN: Regenstrief Institute; 2020[accessed 2020 Mar 6]. Available from: https://loinc.org/ [Google Scholar]
- 39.Oelsner EC, Balte PP, Cassano PA, Couper D, Enright PL, Folsom AR, et al. Harmonization of respiratory data from 9 US population-based cohorts: the NHLBI pooled cohorts study. Am J Epidemiol. 2018;187:2265–2278. doi: 10.1093/aje/kwy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffe C, Hammond WE, Quinn J, Dolin RH. Healthcare IT standards and the standards development process: lessons learned from Health Level 7. Intel Technol J. 2009;13:58–79. [Google Scholar]
- 41.Institute of Electrical and Electronics Engineers IEEE standards New York, NY: Institute of Electrical and Electronics Engineers; 2020[accessed 2020 Mar 6]. Available from: https://www.ieee.org/standards/index.html [Google Scholar]
- 42.Health Level 7 International HL7 International Ann Arbor, MI: Health Level 7 International; 2020[accessed 2020 Mar 6]. Available from: http://www.hl7.org/ [Google Scholar]
- 43.Representational state transfer San Francisco, CA: Wikimedia Foundation; 2020[accessed 2020 Jul 29]. Available from: https://en.wikipedia.org/wiki/representational_state_transfer [Google Scholar]
- 44.Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. 2018;198:1012–1020. doi: 10.1164/rccm.201804-0682CI. [DOI] [PubMed] [Google Scholar]
- 45.Diab N, Gershon AS, Sin DD, Tan WC, Bourbeau J, Boulet LP, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198:1130–1139. doi: 10.1164/rccm.201804-0621CI. [DOI] [PubMed] [Google Scholar]
- 46.NEJM Catalyst. What is value-based healthcare? NEJM Catal. 2017;3(1) [Google Scholar]
- 47.O’Dowd E.Why application programming interfaces are key for healthcare Danvers, MA: HIT Infrastructure, Xtelligent Healthcare Media; 2016[accessed 2020 Mar 6]. Available from: https://hitinfrastructure.com/features/why-application-programming-interfaces-are-key-for-healthcare [Google Scholar]
- 48.Connectivity Springfield, MA: Merriam-Webster; 2020[accessed 2020 Mar 6]. Available from: https://www.merriam-webster.com/dictionary/connectivity [Google Scholar]
- 49.Office of the National Coordinator for Health Information Technology, Department of Health and Human Services; The National Alliance for Health Information Technology The National Alliance for Health Information Technology report to the Office of the National Coordinator for Health Information Technology on defining key health information technology terms Chicago, IL: Healthcare Information and Management Systems Society; 2008[accessed 2020 Nov 24]. Available from: https://s3.amazonaws.com/rdcms-himss/files/production/public/HIMSSorg/Content/files/Code%205%20Defining%20Key%20Health%20Information%20Technology%20Terms.pdf [Google Scholar]
- 50.Health Level 7 International Welcome to FHIR Ann Arbor, MI: Health Level 7 International; 2019[accessed 2020 Mar 6]. Available from: http://hl7.org/fhir/ [Google Scholar]
- 51.Health Level 7 International Overview FHIR Ann Arbor, MI: Health Level 7 International; 2019[accessed 2020 Mar 6]. Available from: http://hl7.org/fhir/overview.html [Google Scholar]
- 52.WebMD HEDIS Atlanta, GA: WebMD; 2019[accessed 2020 Mar 6]. Available from: https://www.webmd.com/health-insurance/terms/hedis [Google Scholar]
- 53.Health Level 7 International Introduction to HL7 standards Ann Arbor, MI: Health Level 7 International; 2020[accessed 2020 Mar 6]. Available from: https://www.hl7.org/implement/standards/ [Google Scholar]
- 54.Ehrens T, Rouse M.Integration Newton, MA: TechTarget; 2015[accessed 2020 Mar 6]. Available from: https://searchcustomerexperience.techtarget.com/definition/integration [Google Scholar]
- 55.Practice Fusion EHR (electronic health record) vs. EMR (electronic medical record) San Francisco, CA: Practice Fusion; 2019[accessed 2020 Mar 6]. Available from: https://www.practicefusion.com/blog/ehr-vs-emr/ [Google Scholar]
- 56.National Committee for Quality Assurance HEDIS and performance measurement Washington, DC: National Committee for Quality Assurance; 2020[accessed 2020 Mar 6]. Available from: https://www.ncqa.org/hedis/ [Google Scholar]
- 57.National Committee for Quality Assurance NCQA comments on CMS proposed rule for improving interoperability and patient access Washington, DC: National Committee for Quality Assurance; 2019[accessed 2020 Mar 6]. Available from: https://www.ncqa.org/comment-letter/ncqa-comments-on-cms-proposed-rule-for-improving-interoperability-and-patient-access/ [Google Scholar]
- 58.Landi H.NCQA updates HEDIS quality measures, new measure addresses opioid use Nashville, TN: Healthcare Innovation Group; 2018[accessed 2020 Mar 6]. Available from: https://www.hcinnovationgroup.com/population-health-management/telehealth/news/13030494/ncqa-updates-hedis-quality-measures-new-measure-addresses-opioid-use [Google Scholar]
- 59.National Library of Medicine SNOMED CT Bethesda, MD: National Library of Medicine; 2019[accessed 2020 Mar 29]. Available from: https://www.nlm.nih.gov/healthit/snomedct/index.html [Google Scholar]
- 60.Tutorials Point SQL: overview Hyderabad, Telangana, India: Tutorials Point; 2020[accessed 2020 Mar 29]. Available from: https://www.tutorialspoint.com/sql/sql-overview.htm [Google Scholar]