To the Editor:
Lymphangioleiomyomatosis (LAM) is a female-predominant, low-grade metastatic neoplasm that results in progressive cystic lung remodeling. LAM occurs in patients harboring germline TSC (tuberous sclerosis complex) mutations (TSC-LAM) and also in a sporadic form (S-LAM) in patients with somatic TSC2 mutations (1). The vascular endothelial growth factors VEGF-C and VEGF-D play key roles in the tissue remodeling, metastasis, and invasion that occurs in LAM (2–4). Serum VEGF-D has proven to be a clinically useful diagnostic, prognostic, and predictive biomarker in LAM (5–9). However, serum VEGF-D is elevated in only about 60–70% of patients with LAM, and there is an unmet need for additional biomarkers that may improve the sensitivity and specificity for diagnosis, inform disease management, and facilitate trial conduct in LAM. Based on the role of VEGF-C in the pathogenesis of LAM (2–4), we postulate that VEGF-C may be a useful adjunctive biomarker in LAM. The primary objective of our study was to determine whether VEGF-C concentrations correlate with measures of disease severity, the rate of disease progression, and the response to therapy and whether VEGF-C has the potential to aid in LAM diagnosis. In addition, because VEGF-C is known to be a component of platelets, as a secondary objective, we aimed to assess the relative performance of serum and plasma VEGF-C values as biomarkers in LAM and determine the optimal blood compartment for future measurements.
Methods
Our study population comprised participants from the following two cohorts: 1) patients enrolled in the MILES (Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus) trial (NCT00414648) and 2) women with S-LAM, TSC-LAM, and TSC without LAM and healthy control subjects enrolled from the University of Cincinnati (UC) LAM and the Cincinnati Children’s Medical Center (CCHMC) TSC clinics (UC/CCHMC cohort, UC institutional review board number 05–12–19–01 and CCHMC institutional review board number 2008–0932). The MILES trial was a double-blind, placebo-controlled trial in which women with moderate to severe LAM were randomized to receive sirolimus or placebo for 12 months, followed by 12 months of observation off therapy. Pulmonary function tests (PFTs) were obtained at baseline and at 3-month intervals in the first year and 6-month intervals in the second year. Serum and plasma samples were obtained at 0, 6, 12, and 24 months (10).
Plasma and serum VEGF-C were measured in triplicate by enzyme-linked immunosorbent assay using the human VEGF-C DuoSet kits (R&D systems). Data from the MILES cohort was used to assess the relationship between VEGF-C concentrations and baseline disease features at enrollment, rate of disease progression, and response to sirolimus treatment. A linear regression model was used to assess the relationship between baseline VEGF-C concentrations and baseline PFTs in the MILES cohort. The Wilcoxon rank-sum test was used to test for differences in baseline serum VEGF-C concentrations between subgroups, which were defined by the presence or absence of categorical variables such as angiomyolipoma, pneumothorax, and supplemental oxygen use. General linear models were used to assess absolute changes in PFTs from baseline to 12 months, and linear mixed-effects models were used to assess changes with time. The Wilcoxon matched pairs signed rank test was used to compute differences in baseline, 6-month, and 12-month values of VEGF-C for the MILES cohort. To assess the value of VEGF-C as a diagnostic biomarker in LAM, serum VEGF-C concentrations in the UC/CCHMC cohort were combined with the baseline values in the MILES cohort. A Kruskal-Wallis test was conducted to detect overall group differences, and a Mann-Whitney test was used to detect differences between individual categories. Statistical analyses were conducted with SAS version 9.3 and GraphPad Prism 8, and a P value of 0.05 or less was considered significant.
Results
The baseline characteristics of the MILES trial participants have been published previously (10). The trial consisted of 89 subjects, including 81 with S-LAM and eight with TSC-LAM. Forty-three subjects were randomized to the placebo arm, and 46 subjects were randomized to the sirolimus arm (10).
The median baseline serum VEGF-C concentration in the MILES cohort was 6,957 (interquartile range [IQR], 6,072–8,528) pg/ml. After segregating the cohort into S-LAM and TSC-LAM subgroups, the median (IQR) serum VEGF-C values were 6,957 (6,072–8,339) pg/ml for the S-LAM subgroup and 6,854 (5,811–10,794) pg/ml for the TSC-LAM subgroup. Baseline median (IQR) plasma VEGF-C concentrations were approximately 30-fold lower than the serum values, with 237 (154–370) pg/ml for the entire MILES cohort; 233 (145–367) pg/ml for the S-LAM subgroup, and 316 (293–2,003) pg/ml for the TSC-LAM subgroup.
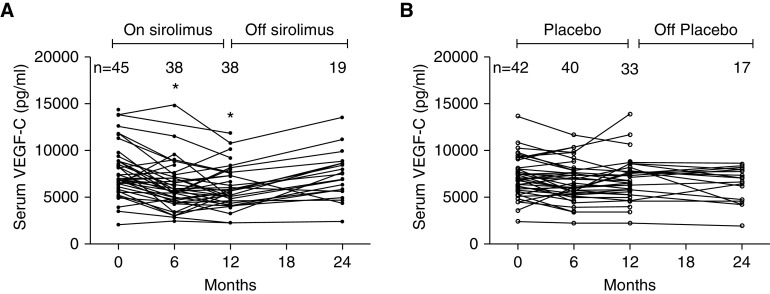
There was no correlation between baseline serum VEGF-C and VEGF-D concentrations in the MILES cohort (Spearman r = −0.19; P = 0.08). The median serum VEGF-C concentrations were similar at baseline in the sirolimus and placebo groups (Table 1). Baseline serum VEGF-C correlated with the need for supplemental oxygen (P = 0.007) but not with other disease features at enrollment, such as PFTs, angiomyolipomas, and history of pneumothorax. Longitudinal analysis during the treatment year revealed that a one-unit increase in baseline log (serum VEGF-C) was associated with an increase in the rate of decline in forced expiratory volume in 1 second by 27 ml/mo in the placebo group (P = 0.06). VEGF-C concentrations remained stable over the 2-year study period in the placebo group. Serum VEGF-C concentrations decreased by approximately 20% from the baseline value in the sirolimus group at 6 (P < 0.0001) and 12 months (P < 0.0001) and returned to baseline values when the drug was held during the observation year (Figure 1).
Table 1.
Baseline and 12-month values of serum VEGF-C and VEGF-D and plasma VEGF-C concentrations in the MILES trial
| Biomarker | Sirolimus Group |
Placebo Group |
||||
|---|---|---|---|---|---|---|
| Baseline [Median (95% CI)] | 12 mo [Median (95% CI)] | P Value | Baseline [Median (95% CI)] | 12 mo [Median (95% CI)] | P Value | |
| Serum VEGF-D, pg/ml | 1,345 (924–2,006) | 709 (508–1,010) | <0.0001 | 1,329 (722–1,735) | 1,231 (734–2,546) | 0.30 |
| Serum VEGF-C, pg/ml | 6,860 (6,600–8,198) | 5,453 (4,804–6,248) | <0.0001 | 7,028 (6,337–7,490) | 6,752 (5,814–7,622) | 0.08 |
| Plasma VEGF-C, pg/ml | 268 (231–367) | 204 (147–266) | 0.03 | 217 (177–313) | 238 (157–303) | 0.14 |
Definition of abbreviations: CI = confidence interval; MILES = Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus; VEGF-C = vascular endothelial growth factor C; VEGF-D = vascular endothelial growth factor D.
Figure 1.
Trends in serum VEGF-C (vascular endothelial growth factor C) values in the MILES (Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus) trial. (A) In the treatment group, serum VEGF-C concentrations decreased in the first 12 mo when patients were receiving sirolimus and returned toward the baseline values in the observation period off sirolimus. (B) In the placebo group, serum VEGF-C concentrations remained stable throughout the 2-yr study duration. *P < 0.05.
Baseline plasma VEGF-C concentrations did not correlate with PFTs and other disease features at enrollment, longitudinal disease progression (in the placebo arm), or treatment response (in the sirolimus arm). Plasma VEGF-C concentrations remained stable in the placebo arm and decreased after treatment with sirolimus, although the magnitude of the response was less pronounced than that for serum VEGF-C concentrations (Table 1).
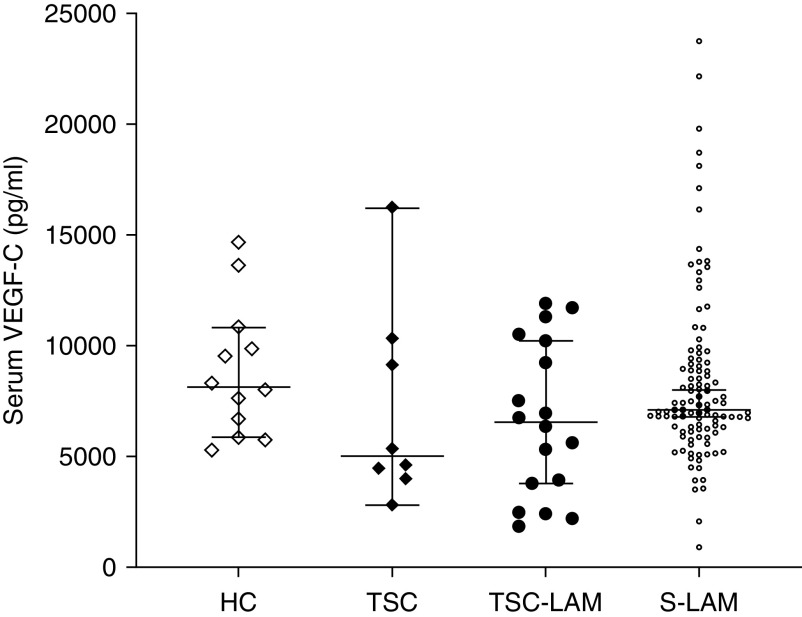
The UC/CCHMC cohort consisted of eight women with TSC without LAM, 10 women with TSC-LAM, 28 women with S-LAM, and 12 healthy female control subjects. Serum VEGF-C values obtained from patients with S-LAM (n = 79) and TSC-LAM (n = 8) at their baseline MILES visit were combined with those from the UC/CCHMC cohort for a final LAM patient composition of 18 patients with TSC-LAM and 107 patients with S-LAM. The median serum VEGF-C values of this combined cohort were 7,110 pg/ml (IQR, 912–23,754) pg/ml for S-LAM, 6,553 (IQR, 1,861–11,910) pg/ml for TSC-LAM, 5,017 (IQR, 2,827–16,231) pg/ml for women with TSC without LAM, and 8,143 (IQR, 5,286–14,682) pg/ml for healthy adult women. There were no significant differences in serum VEGF-C values between these groups (P = 0.20) (Figure 2). Finally, we analyzed the serum VEGF-C values obtained only from the UC/CCHMC cohort; there was no difference in the serum VEGF-C values in patients with S-LAM and healthy control subjects (P = 0.31) or in women with TSC-LAM and women with TSC without LAM (P = 0.46).
Figure 2.
Serum VEGF-C (vascular endothelial growth factor C) concentrations in women with sporadic lymphangioleiomyomatosis (LAM), TSC (tuberous sclerosis complex)-LAM, TSC without LAM, and healthy control subjects. Serum VEGF-C concentrations (median and 95% confidence intervals) were similar in all groups (P = 0.20). HC = healthy control subjects; S-LAM = sporadic LAM.
Discussion
Serum VEGF-D is a clinically useful diagnostic biomarker for LAM, and a value of 800 pg/ml or greater in women with a compatible chest computed tomographic scan is almost 100% specific in distinguishing LAM from other cystic lung diseases (6, 8, 9). However, additional biomarkers are needed to facilitate noninvasive diagnosis of LAM in the 30–40% of patients who have VEGF-D values of less than 800 pg/ml, and our hope was that VEGF-C would be useful for this purpose. Our analysis indicates that serum VEGF-C values are similar in patients with S-LAM compared with healthy control subjects and in women with TSC with and without LAM. These findings are consistent with those previously reported in a Japanese cohort (11) and suggest that serum VEGF-C holds little promise as a diagnostic biomarker in LAM.
In the MILES trial, serum VEGF-C remained stable over time in the placebo group, was suppressed by sirolimus treatment, and returned toward baseline when the drug was held, similar to the VEGF-D response (7). This pattern is consistent with a reversible inhibition of the mechanistic target of rapamycin pathway and with the known cytostatic action of sirolimus in LAM. Plasma VEGF-C exhibited a similar but less pronounced pattern of suppression on sirolimus during the period of drug exposure compared with serum VEGF-C. Because VEGF-C is contained within platelets and is released upon clotting (12), plasma VEGF-C may more accurately reflect the fraction of VEGF-C that is free in circulation. It is quite likely, however, that VEGF-C within the platelet compartment is also biologically relevant and bioavailable, and serum VEGF-C (which presumably includes platelet-derived VEGF-C) appears to be more responsive to mechanistic target of rapamycin pathway activation and suppression than plasma VEGF-C.
There is considerable variation in VEGF-C concentrations measured using two different commercially available assays, Quantikine and DuoSet (both R&D Systems). Because we were primarily interested in the longitudinal trends of VEGF-C in the MILES trial as opposed to absolute values, we chose the DuoSet kits for our analysis to reduce cost and to ensure uniformity in measurement methodology. If VEGF-C is to be used for clinical purposes in future, the basis for discrepancies in VEGF-C measurement between various assays will need to be resolved, and standard sample processing and quantification procedures will need to be developed.
Conclusions
Serum VEGF-C is a potentially useful biomarker of treatment response to sirolimus in patients with LAM, but there appears to be little diagnostic utility of VEGF-C in patients with suspected LAM.
Footnotes
Supported by U.S. National Institutes of Health/U.S. National Heart, Lung, and Blood Institute grant U01HL131022–01 (F.X.M.), U.S. National Institutes of Health grant K24HL143281 (L.R.Y.), and the Lymphangioleiomyomatosis Foundation (L.R.Y.).
Author Contributions: L.R.Y. and F.X.M. conceived the study idea and design. H.W. performed the biomarker quantification assays. N.G., M.H., A.P., and R.D.S. performed the data analysis. All authors made substantial contributions to writing and editing the manuscript. N.G. and F.X.M. have access to all of the study data and assume final responsibility for the data integrity as well as the decision to submit for publication.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease: part I. Am J Respir Crit Care Med. 2015;191:1354–1366. doi: 10.1164/rccm.201411-2094CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta R, Kitaichi M, Inoue Y, Kotloff R, McCormack FX. Lymphatic manifestations of lymphangioleiomyomatosis. Lymphology. 2014;47:106–117. [PubMed] [Google Scholar]
- 3.Kumasaka T, Seyama K, Mitani K, Sato T, Souma S, Kondo T, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28:1007–1016. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 4.Kumasaka T, Seyama K, Mitani K, Souma S, Kashiwagi S, Hebisawa A, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005;29:1356–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 5.Gupta N, Lee HS, Young LR, Strange C, Moss J, Singer LG, et al. NIH Rare Lung Disease Consortium. Analysis of the MILES cohort reveals determinants of disease progression and treatment response in lymphangioleiomyomatosis. Eur Respir J. 2019;53:1802066. doi: 10.1183/13993003.02066-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack FX, Gupta N, Finlay GR, Young LR, Taveira-DaSilva AM, Glasgow CG, et al. ATS/JRS Committee on Lymphangioleiomyomatosis. Official American Thoracic Society/Japanese Respiratory Society clinical practice guidelines: lymphangioleiomyomatosis diagnosis and management. Am J Respir Crit Care Med. 2016;194:748–761. doi: 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young L, Lee HS, Inoue Y, Moss J, Singer LG, Strange C, et al. MILES Trial Group. Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1:445–452. doi: 10.1016/S2213-2600(13)70090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young LR, Inoue Y, McCormack FX. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008;358:199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young LR, Vandyke R, Gulleman PM, Inoue Y, Brown KK, Schmidt LS, et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138:674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seyama K, Kumasaka T, Souma S, Sato T, Kurihara M, Mitani K, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;4:143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 12.Wartiovaara U, Salven P, Mikkola H, Lassila R, Kaukonen J, Joukov V, et al. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost. 1998;80:171–175. [PubMed] [Google Scholar]