Abstract
Rationale: Patients undergoing cardiac surgery often require vasopressor or inotropic (“vasoactive”) medications, but patterns of postoperative use are not well described.
Objectives: This study aimed to describe vasoactive medication administration throughout hospitalization for cardiac surgery, to identify patient- and hospital-level factors associated with postoperative use, and to quantify variation in treatment patterns among hospitals.
Methods: Retrospective study using the Premier Healthcare Database. The cohort included adult patients who underwent coronary artery bypass grafting or open valve repair or replacement (or in combination) from January 1, 2016, to June 30, 2018. Primary outcome was receipt of vasoactive medication(s) on the first postoperative day (POD1). We identified patient- and hospital-level factors associated with receipt of vasoactive medications using multilevel mixed-effects logistic regression modeling. We calculated adjusted median odds ratios to determine the extent to which receipt of vasoactive medications on POD1 was determined by each hospital, then calculated quotients of Akaike Information Criteria to compare the relative contributions of patient and hospital characteristics and individual hospitals with observed variation.
Results: Among 104,963 adults in 294 hospitals, 95,992 (92.2%) received vasoactive medication(s) during hospitalization; 30,851 (29.7%) received treatment on POD1, most commonly norepinephrine (n = 11,427, 37.0%). A median of 29.0% (range, 0.0–94.4%) of patients in each hospital received vasoactive drug(s) on POD1. After adjustment, hospital of admission was associated with twofold increased odds of receipt of any vasoactive medication on POD1 (adjusted median odds ratio, 2.07; 95% confidence interval, 1.93–2.21). Admitting hospital contributed more to observed variation in POD1 vasoactive medication use than patient or hospital characteristics (quotients of Akaike Information Criteria 0.58, 0.44, and <0.001, respectively).
Conclusions: Nearly all cardiac surgical patients receive vasoactive medications during hospitalization; however, only one-third receive treatment on POD1, with significant variability by institution. Further research is needed to understand the causes of variability across hospitals and whether these differences are associated with outcomes.
Keywords: cardiotonic agents, cardiovascular surgical procedures, physicians’ practice patterns, vasoconstrictor agents
More than 400,000 adults undergo coronary artery bypass grafting (CABG) or open valve surgery in the United States every year (1). These patients typically require vasopressor and inotropic (“vasoactive”) medications to treat perioperative vasodilatory hypotension (2) or myocardial dysfunction (3). Although the use of vasoactive medications during cardiac surgery is common (4–7), little is known about postoperative patterns of use across the United States. Previous epidemiologic studies focused primarily on the administration of inotropes during separation from cardiopulmonary bypass (CPB) or in the first hours after surgery (4, 5, 8–10), and variation in practice among clinicians (5, 11, 12) and hospitals (4, 5, 10). Although it is well established that some patients receive vasoactive medications for one or more days after cardiac surgery (7, 13–15), little is known about the patterns of postoperative administration of these medications. There is limited trial (16, 17) and observational evidence (18–21) and few available clinical guidelines specific to cardiac surgery (22, 23) to inform administration of vasoactive medications in this population. In light of recent work in other populations suggesting that use of vasoactive medication may be unnecessary or associated with harm (24), understanding current use of these medications in cardiac surgery is a necessary first step in designing future comparative effectiveness studies.
This study aims to describe the administration of vasoactive medications throughout hospitalization for cardiac surgery, to identify patient- and hospital-level factors associated with use postoperatively (focusing on postoperative Day 1 [POD1]), and to quantify variation in treatment patterns between hospitals. We hypothesized that observed variation in vasoactive medication use would be significantly associated with patients’ hospital of admission.
A portion of this work in abstract form was presented at the 2020 American Thoracic Society International Conference (25).
Methods
The Baystate Health Institutional Review Board granted permission to conduct this study, including a waiver of informed consent (#777703-1).
Study Design and Cohort Selection
We performed a retrospective analysis of the Premier Healthcare Database, which includes diagnosis, procedure, and charge data from approximately 20% of acute care hospital admissions in the United States annually (26). We identified adult patients admitted to a participating hospital between January 1, 2016, and June 30, 2018, who underwent principal procedures of CABG or open cardiac valve repair or replacement in any combination. We excluded patients <18 years old on the day of hospital admission and those admitted to pediatric specialty hospitals (defined as hospitals with median patient age <18 yr). We also excluded patients undergoing complex cardiac, aortic, transplant, and permanent mechanical circulatory support surgeries with expected differences in management and perioperative course. We excluded patients admitted to low-volume hospitals (contributing fewer than 25 adult cardiac surgery patients to the data set (the 10th percentile of admissions among eligible hospitals)), patients admitted to hospitals in which no patients had any charge codes for a vasoactive medication, and individual patients missing key variables.
Variable Identification and Definitions
Medications were identified from pharmacy charge codes. We identified CABG and valve replacement or repair surgery using International Classification of Disease, Ninth Revision (ICD-9) procedure codes as previously described (27, 28), then mapped these codes to International Classification of Disease, Tenth Revision (ICD-10) procedure classification system codes in the data set (29). We used the same approach to identify patients undergoing CABG with and without CPB (30) and to identify and exclude patients undergoing heart transplantation and implantation or repair of permanent left ventricular assist devices (31), open aortic surgery (32), and transcatheter valve replacement (33) as well as patients with congenital heart disease (34). We identified comorbid disease and acute diagnoses present on admission relevant to CABG outcomes (35) and infectious endocarditis using validated ICD-10 codes (36). All ICD-10 codes were adjudicated by an investigator (E.A.V.) for clinical relevance.
Clinical Care and Patient Outcome Variables
Admissions were classified as elective, urgent/emergent, or other according to the Premier Healthcare Database schema. For primary analyses, we classified cardiac surgery into four types: 1) CABG with CPB (at least one coronary artery bypass graft without repair or replacement of any valve[s]), 2) CABG without CPB, 3) valve repair(s) or replacement(s) without CABG and, 4) valve repair(s) or replacement(s) with CABG. Mechanical ventilation, renal replacement therapy, and intensive care unit length of stay (in whole calendar days) were identified from charge codes (37, 38). We identified receipt of temporary mechanical circulatory support (intraaortic balloon pump, temporary ventricular assist device, or venoarterial extracorporeal membrane oxygenation) from ICD-10 codes (39–41). Relevant patient outcome variables included new onset of atrial fibrillation or renal failure during hospital admission (42, 43), in-hospital mortality, and discharge disposition among survivors as defined by Premier (26). As the focus of the study was on clinical practice, we did not examine other patient outcomes captured in the data set but less plausibly associated with receipt of vasoactive medication. The online supplement contains a full set of ICD-10 codes used in the study (Table E1 in the online supplement).
Study Outcomes
As the data set captures medication charges by calendar day of hospital admission, we were unable to distinguish between pre-, intra-, and postoperative use of study drugs on the day of cardiac surgery. To focus our analyses on postoperative drug administration, the primary outcome was the receipt of one or more vasopressor (defined as epinephrine, dopamine, norepinephrine, phenylephrine, or vasopressin) or inotrope (dobutamine or milrinone) medications on POD1. Other vasoactive drugs, such as terlipressin and angiotensin II, were not available in the United States during the full study period. Other outcomes were receipt of one or more vasoactive medication(s) at any time during hospitalization (including intraoperatively), receipt of vasopressors and inotropic medications separately, receipt of individual vasoactive medications, receipt of vasoactive medications by day of hospitalization, and use of adjunctive therapies for shock or chronic vasodilatory hypotension (including hydrocortisone, methylene blue, thiamine, ascorbic acid, vitamin B12 and midodrine, as well as temporary mechanical circulatory support [intraaortic balloon pumps, percutaneous ventricular assist devices, and venoarterial extracorporeal membrane oxygenation]).
Statistical Analysis
Components of the study data set have been used previously by the investigators in other studies examining practice and outcomes after cardiac surgery (27, 28). Initial data analysis plan was written before data were specifically accessed for use in this study. After study cohort creation in May 2019, investigators met and reviewed available data. After determining that pre-, intra-, and postoperative medications could not be distinguished in the data set, the investigators agreed to change primary outcome to receipt of vasoactive medication(s) on POD1. Planned analyses are reflected in the final data analysis plan, which was written, date-stamped (permanent dated electronic signature June 25, 2019), and recorded in the investigators’ files. During manuscript preparation, investigators decided post hoc to perform one additional sensitivity analysis to adjust for unmeasured differences in case mix between cohort hospitals by expanding cardiac surgery classification into nine types.
We first summarized cohort patient and clinical characteristics and outcomes for the whole cohort of cardiac surgery patients and then stratified the cohort according to receipt of at least one vasoactive medication during hospital admission. Next, we described the overall rate of vasoactive medication use for the whole cohort and then assessed the rate and duration of vasoactive medication use by drug class and individual drug. We plotted the proportion of patients who received each class of vasoactive medication on each hospital day (referenced to the date of cardiac surgery). We also examined receipt of adjunctive treatments by day of hospital admission.
We next assessed patients who received one or more vasoactive medications on POD1, without consideration for receipt of vasoactive medications on other hospital days. We repeated descriptive analyses for the POD1 group, then compared demographic and clinical characteristics and outcomes between patients who did and did not receive vasoactive medication(s) on POD1 (but remained alive). Next, we described rates and patterns of vasoactive medication use (by individual drug and drug combinations) among POD1 group patients on the first postoperative day and throughout the hospital stay overall and in each cohort hospital.
To identify patient and hospital covariates associated with receipt of vasoactive drugs on POD1, we created three multilevel multivariable mixed logistic regression models, with outcomes: 1) receipt of any vasoactive medication, 2) receipt of vasopressor(s) (with or without inotropes), and 3) receipt of inotrope(s) (with or without vasopressors), with hospital of admission as a random intercept. Patient-level model covariates included demographic variables, comorbid disease, acute diagnoses present on admission, and cardiac surgery type. Hospital-level model covariates included geographic region, number of hospital beds, teaching status, and urban location. We calculated the adjusted median odds ratio (AMOR) for each model to describe the role of individual hospital of admission in a patient’s chance of receiving vasoactive medication(s) on POD1 (44, 45). In multilevel mixed-effects logistic regression modeling, AMOR describes the relative contribution of individual hospital of admission to observed variation in the study outcome. More specifically, it describes the median change in the odds of an outcome that would be observed if a patient were moved between two randomly selected study hospitals. Although it is conceptually similar to the intraclass correlation coefficient (used for continuous outcomes), AMOR is independent of treatment prevalence and its value may be directly compared with the adjusted effects of other model covariates. We determined the relative contributions of patient and hospital characteristics and individual hospital of admission to observed variation using quotients of Akaike Information Criteria (AIC) (see online supplement for expanded description) (46, 47).
We performed two sensitivity analyses using the full study cohort: 1) repeating the main model examining receipt of at least one vasoactive drug on POD1 after subdividing index procedures into nine specific surgeries (to account for potential residual differences in case mix between cohort hospitals) (see Table E7 for list) and 2) repeating the model examining receipt of one or more inotrope(s) on POD1 after reclassifying dopamine and epinephrine as inotropes.
We described continuous variables as mean ± standard deviation and median (interquartile range or full range). Missing data were minimal and were not imputed. We excluded one patient with unrecorded sex from the cohort and classified those with missing race or insurance status into the category of “Other.” We compared groups with chi-square tests and unpaired t tests or Wilcoxon rank-sum tests according to normality. All hypothesis tests were two-sided; P values <0.05 were considered statistically significant. We did not adjust for multiple comparisons; therefore, all secondary analyses should be regarded as exploratory. Analyses were performed using SAS 9.4 software (SAS Institute); graphs were produced with Excel for Mac (Version 16.27) (Microsoft Corp).
Results
Cohort Characteristics
The cohort included 104,063 patients in 294 hospitals (Figure E1). Mean age was 66.1 years ± standard deviation 10.4; 28.7% were female (Table 1). A majority of patients (60.9%) underwent CABG with CPB without valve repair or replacement. In-hospital mortality was 1.9%, and 77.6% of hospital survivors were discharged home.
Table 1.
Cohort patient characteristics, clinical care, and outcomes stratified by receipt of vasoactive medications
| Whole Cohort | Received Vasoactive Medication(s) during Hospital Admission | Did Not Receive Any Vasoactive Medication on POD1* | Received Vasoactive Medication(s) on POD1† | |
|---|---|---|---|---|
| n (% of cohort) | 104,063 | 95,992 (92.2) | 72,902 (70.3) | 30,851 (29.7) |
| Patient characteristics | ||||
| Age, mean ± SD, yr | 66.1 ± 10.4 | 66.2 ± 10.4 | 65.9 ± 10.4 | 66.7 ± 10.4 |
| Sex, F, n (% of group) | 29,889 (28.7) | 27,561 (28.7) | 20,231 (27.8) | 9,512 (30.8) |
| Race or ethnicity, n (% of group) | ||||
| White | 80,413 (77.3) | 74,679 (77.8) | 56,464 (77.5) | 23,710 (76.9) |
| Black | 6,706 (6.4) | 6,055 (6.3) | 4,685 (6.4) | 1,999 (6.5) |
| Hispanic | 5,957 (5.7) | 5,758 (6.0) | 3,998 (5.5) | 1,944 (6.3) |
| Other or unknown | 10,987 (10.6) | 9,500 (9.9) | 7,755 (10.6) | 3,198 (10.4) |
| Primary insurance provider, n (% of group) | ||||
| Private | 28,790 (27.7) | 26,438 (27.5) | 21,041 (28.9) | 7,684 (24.9) |
| Medicare | 60,738 (58.4) | 56,292 (58.6) | 41,764 (57.3) | 18,770 (60.8) |
| Medicaid | 8,289 (8.0) | 7,362 (7.7) | 5,803 (8.0) | 2,464 (8.0) |
| Other/unknown/uninsured | 6,246 (6.0) | 5,900 (6.1) | 4,294 (5.9) | 1,933 (6.3) |
| Comorbid disease‡ | ||||
| Malignancy | 2,182 (2.1) | 2,035 (2.1) | 1,481 (2.0) | 699 (2.3) |
| Diabetes with complications | 27,818 (26.7) | 25,638 (26.7) | 18,702 (25.7) | 9,039 (29.3) |
| Cerebrovascular disease | 8,606 (8.3) | 8,014 (8.3) | 5,780 (7.9) | 2,792 (9.0) |
| Acute/chronic renal failure | 23,619 (22.7) | 21,962 (22.9) | 14,657 (20.1) | 8,873 (28.8) |
| COPD | 19,342 (18.6) | 18,237 (19.0) | 12,636 (17.3) | 6,644 (21.5) |
| Acute diagnoses present on admission‡ | ||||
| Congestive heart failure | 36,611 (35.2) | 34,047 (35.5) | 21,891 (30.0) | 14,578 (47.3) |
| Cardiogenic shock | 1,794 (1.7) | 1,707 (1.8) | 642 (0.9) | 1,134 (3.7) |
| Arrhythmia | 24,491 (23.5) | 22,830 (23.8) | 15,531 (21.3) | 8,871 (28.8) |
| Pulmonary edema | 843 (0.8) | 793 (0.8) | 483 (0.7) | 355 (1.2) |
| Type of admission§ | ||||
| Elective | 57,585 (55.3) | 53,509 (55.7) | 41,927 (57.5) | 15,514 (50.3) |
| Urgent/emergent | 45,619 (43.8) | 41,639 (43.4) | 30,390 (41.7) | 15,065 (48.8) |
| Other | 859 (0.8) | 844 (0.9) | 585 (0.8) | 272 (0.9) |
| Clinical care | ||||
| Cardiac surgery type, n (% of group) | ||||
| CABG with CPB | 63,363 (60.9) | 58,817 (61.3) | 45,033 (61.8) | 18,182 (58.9) |
| CABG without CPB | 13,422 (12.9) | 11,978 (12.5) | 10,028 (13.8) | 3,360 (10.9) |
| CABG with valve repair(s) or replacement(s) | 10,203 (9.8) | 9,609 (10.0) | 5,940 (8.2) | 4,202 (13.6) |
| Valve repair(s) or replacement(s), without CABG | 17,075 (16.4) | 15,588 (16.2) | 11,901 (16.3) | 5,107 (16.6) |
| Mechanical ventilation‖ | 96,654 (92.9) | 89,324 (93.1) | 67,094 (92.0) | 29,344 (95.1) |
| Renal replacement therapy during admission | 4,915 (4.7) | 4,695 (4.9) | 2,543 (3.5) | 2,357 (7.6) |
| Receipt of temporary mechanical circulatory support | ||||
| IABP | 5,949 (5.7) | 5,639 (5.9) | 2,618 (3.6) | 3,259 (10.6) |
| Temporary VAD | 314 (0.3) | 311 (0.3) | 63 (0.1) | 242 (0.8) |
| VA ECMO | 203 (0.2) | 202 (0.2) | 34 (0.0) | 154 (0.5) |
| ICU length of stay, median (IQR), d | ||||
| ICU survivors | 3 (1–5) | 3 (1–5) | 2 (1–4) | 4 (2–6) |
| ICU nonsurvivors | 6 (3–14) | 6 (3–14) | 6 (3–14) | 7 (3–15) |
| Patient outcomes | ||||
| New-onset atrial fibrillation, n (% of group) | 22,963 (22.1) | 21,386 (22.3) | 15,324 (21.0) | 7,617 (24.7) |
| New-onset acute renal failure, n (% of group) | 14,842 (14.3) | 16,226 (16.9) | 9,546 (13.1) | 7,588 (24.6) |
| Hospital mortality | 1,934 (1.9) | 1,911 (2.0) | 450 (0.6) | 1,301 (4.2) |
| Location after hospital discharge among survivors, n (% of group) | ||||
| Home | 79,215 (77.6) | 72,388 (76.9) | 58,881 (81.3) | 20,252 (68.5) |
| Nursing facility | 21,623 (21.2) | 20,452 (21.7) | 12,853 (17.7) | 8,749 (29.6) |
| Other acute care hospital | 565 (0.6) | 548 (0.6) | 270 (0.4) | 273 (0.9) |
| Hospice | 130 (0.1) | 128 (0.1) | 57 (0.1) | 73 (0.3) |
Definition of abbreviations: CABG = coronary artery bypass graft; COPD = chronic obstructive pulmonary disease; CPB = cardiopulmonary bypass; IABP = intraaortic balloon pump; ICU = intensive care unit; IQR = interquartile range; POD1 = postoperative Day 1; SD = standard deviation; VAD = ventricular assist device; VA ECMO = venoarterial extracorporeal membrane oxygenation.
Among 103,753 patients who remained alive and admitted to the hospital on POD1, whether or not they received vasoactive medication(s) on other day(s) of hospitalization.
Patients who received at least one vasoactive medication, alone or in combination with others, on the day after index cardiac surgery, whether or not they received vasoactive medications on other days of hospitalization.
As per Abildstrøm (35).
All patients admitted from home who underwent surgery on the first day of hospital admission classified as elective. All others classified as urgent/emergent.
Receipt of preoperative and/or postoperative mechanical ventilation, identified from charge codes.
Receipt of Vasoactive Medications during the Hospitalization
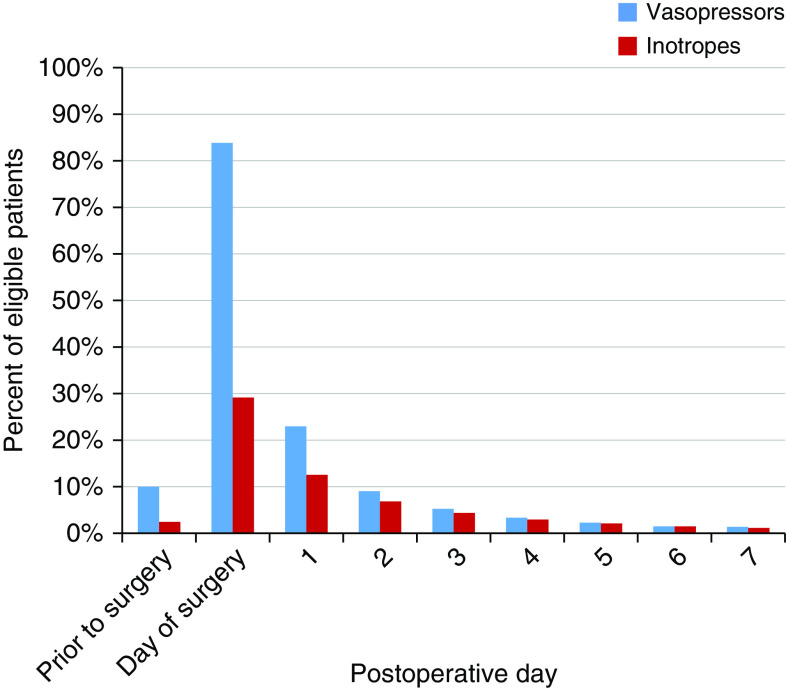
Nearly all cohort patients (92.2%) received at least one vasoactive medication, alone or in combination with others, during hospital admission (Table E2). Rates of vasoactive medication use were highest on the day of surgery (85.7% of patients); 84.0% received at least one vasopressor, and 29.2% received one or more inotropes (Figure 1). The receipt of vasoactive medications decreased to 29.7% on POD1 and continued to decline thereafter. Cohort patients received vasoactive drugs for a median of 1 day (interquartile range, 1–2). Phenylephrine was the most commonly administered vasoactive drug during hospitalization (76.6% of patients). On POD1, administration of norepinephrine was most common (37.0%), followed by phenylephrine (32.3%).
Figure 1.
Receipt of vasoactive medications by day of hospitalization among cohort patients. The figure shows the percentage of cohort patients alive and admitted to hospital on each hospital day (referenced to date of principal procedure) who received any vasopressor or inotrope, alone or in combination with other vasoactive medications, on that day.
In addition to vasopressors and inotropes, 13,378 cardiac surgery patients (12.9% of cohort) received at least one adjunctive treatment for shock. Of these, hydrocortisone was most common (5,959 patients, 5.7%) (Table E3). Hydrocortisone and methylene blue were most frequently administered on the day of surgery (3.2% and 2.3% of cohort, respectively).
Patients Receiving Vasoactive Medications on POD1
Compared with patients who did not receive a vasoactive medication on POD1, those who did were more likely to have an admission diagnosis of congestive heart failure (47.3% vs. 30.0%, P < 0.001) or an arrhythmia (28.8% vs. 21.3%, P < 0.001) (Table 1). They were also more likely to have undergone an urgent or emergent surgical procedure (48.8% vs. 41.7%, P < 0.001), to receive mechanical circulatory support during the hospitalization (11.4% vs. 3.7%, P < 0.001), and to die during hospital admission (4.2% vs. 0.6%, P < 0.001). Comparisons of vasoactive drug use patterns between all patients that had surgery and the POD1 group are included (Table E2).
Hospital-Level Variability in Use of Vasoactive Medications on POD1
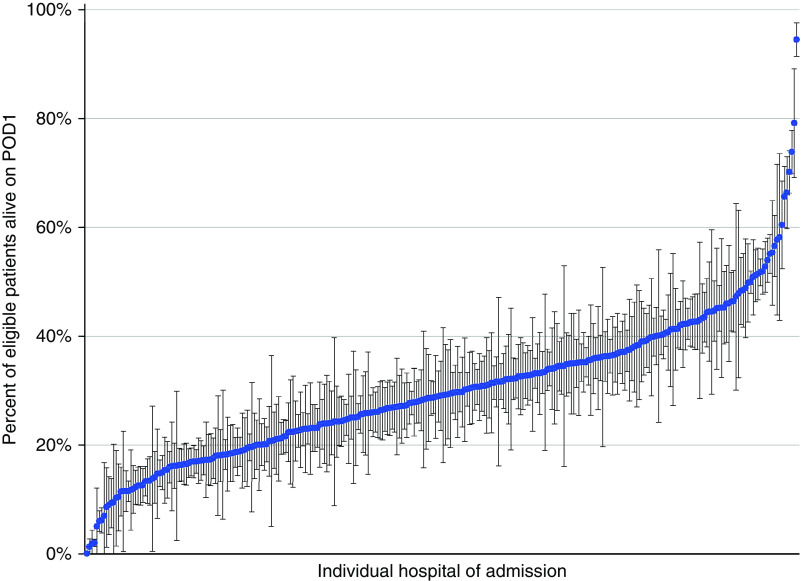
Cohort hospitals admitted a median of 252 cardiac surgery patients (full range, 29–2,248) during the study period. The majority of hospitals were large (50.3% had >400 beds), urban (90.5%), and located in the U.S. South (41.5%) (Table E4). There was substantial interhospital variation in rates and combinations of vasoactive drug delivery on POD1. Cohort hospitals administered vasoactive drugs to a median of 29.0% of patients (full range, 0.0–94.4%) alive and hospitalized on POD1 (Figure 2). Figure E2 depicts patterns of POD1 vasoactive medication administration (by drug class) across study hospitals.
Figure 2.
Percentage of patients at each study hospital who received at least one vasoactive medication on POD1. Blue dots represent point estimates. Bars represent 95% confidence intervals. POD1 = postoperative Day 1. Reprinted from Reference 25.
Patient and Hospital-Level Factors Associated with Receipt of Vasoactive Medications on POD1
After multivariable adjustment, receipt of at least one vasoactive medication on POD1 was associated with congestive heart failure on admission (adjusted odds ratio [aOR], 1.85; 95% confidence interval [95% CI], 1.79–1.91; P < 0.001), cardiogenic shock on admission (aOR, 2.38; 95% CI, 2.13–2.66; P < 0.001), and receipt of temporary mechanical circulatory support on or before the day of surgery (aOR, 3.00; 95% CI, 2.82–3.19; P < 0.001) (Table 2). Receipt of vasoactive medication on POD1 was also associated with individual hospital of admission (AMOR, 2.07; 95% CI, 1.93–2.21). Individual hospital of admission contributed more to an individual patient’s likelihood of receipt of vasoactive medication(s) than patient- or hospital-level characteristics (quotients of AIC, 0.58 for hospital of admission vs. 0.44 for patient characteristics vs. <0.001 for hospital characteristics) (Table 3). Reclassification of cardiac surgery into nine subtypes (Table E5) did not change the relative contribution of individual hospital of admission (AMOR, 2.08; 95% CI, 1.94–2.22; quotient of AIC, 0.57), patient characteristics (quotient of AIC, 0.45), or hospital characteristics (quotient of AIC, <0.001) to observed variation in vasoactive drug use. We observed similar covariate effect sizes and AMORs in models examining factors associated with receipt of vasopressors (with or without inotropes) and inotropes (with or without vasopressors) (Tables E6 and E7). In the inotrope model and a sensitivity analysis in which epinephrine and dopamine were reclassified as inotropes (Table E8), patient characteristics were more strongly associated with observed variation than individual hospital of admission (AIC 0.53 vs. 0.47 and AIC 0.57 vs. 0.45, respectively) (Table 3).
Table 2.
Adjusted odds ratios from multilevel multivariable mixed-effects model describing patient- and hospital-level covariates associated with receipt of one or more vasoactive medication(s) on POD1
| aOR | 95% CI | P Value | |
|---|---|---|---|
| Patient-level characteristics | |||
| Age | 1.00 | 1.00–1.01 | <0.001 |
| Sex, M (ref. F) | 1.15 | 1.11–1.18 | <0.001 |
| Race | |||
| White (ref.) | 1.00 | — | — |
| Black | 0.97 | 0.91–1.03 | 0.31 |
| Hispanic | 0.99 | 0.92–1.07 | 0.87 |
| Other or unknown | 1.05 | 0.99–1.11 | 0.12 |
| Insurance type | |||
| Private (ref.) | 1.00 | — | — |
| Medicare | 1.04 | 0.99–1.08 | 0.09 |
| Medicaid | 1.07 | 1.01–1.14 | 0.02 |
| Other/unknown/uninsured | 1.07 | 1.00–1.15 | 0.04 |
| Comorbid disease present on admission* | |||
| Malignancy | 1.00 | 0.91–1.10 | >0.99 |
| Diabetes with complications | 1.01 | 0.97–1.04 | 0.78 |
| Cerebrovascular disease | 1.02 | 0.97–1.08 | 0.40 |
| Acute or chronic renal failure | 1.37 | 1.32–1.42 | <0.001 |
| COPD | 1.11 | 1.07–1.15 | <0.001 |
| Diagnoses present on admission* | |||
| Congestive heart failure | 1.85 | 1.79–1.91 | <0.001 |
| Cardiogenic shock | 2.38 | 2.13–2.66 | <0.001 |
| Arrhythmia | 1.27 | 1.23–1.32 | <0.001 |
| Pulmonary edema | 1.22 | 1.05–1.43 | 0.01 |
| Principal procedure | |||
| CABG alone (ref.) | 1.00 | — | — |
| CABG without CPB | 0.77 | 0.73–0.81 | <0.001 |
| Valve repair(s) or replacement(s) without CABG | 1.01 | 0.97–1.06 | 0.59 |
| CABG + valve(s) | 1.66 | 1.59–1.75 | <0.001 |
| Receipt of temporary mechanical circulatory support on or before the day of index surgery | 3.00 | 2.82–3.19 | <0.001 |
| Hospital-level characteristics | |||
| U.S. region† | |||
| West (ref.) | 1.00 | — | — |
| Midwest | 1.42 | 1.08–1.86 | 0.01 |
| South | 1.35 | 1.05–1.73 | 0.02 |
| Northeast | 1.05 | 0.74–1.49 | 0.79 |
| Number of beds | |||
| >400 (ref.) | 1.00 | — | — |
| 200–400 | 0.91 | 0.74–1.12 | 0.38 |
| <200 | 0.96 | 0.69–1.33 | 0.80 |
| Teaching hospital† | 0.83 | 0.67–1.03 | 0.09 |
| Urban location† | 0.94 | 0.69–1.29 | 0.71 |
| AMOR | 2.07 | 1.93–2.21 | — |
Definition of abbreviations: aOR = adjusted odds ratio; AMOR = adjusted median odds ratio; CABG = coronary artery bypass graft; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CPB = cardiopulmonary bypass; POD1 = postoperative Day 1; ref. = referent.
As per Abildstrøm (35).
As defined by Premier (26).
Table 3.
Sources of observed variation in outcomes in selected study models
| Outcome | Quotient of Akaike Information Criteria* |
AMOR (95% CI) | ||
|---|---|---|---|---|
| Patient Characteristics | Hospital Characteristics | Individual Hospital of Admission | ||
| Receipt of one or more vasoactive medication(s)† on POD1 | 0.44 | <0.001 | 0.58 | 2.07 (1.93–2.21) |
| Receipt of one or more vasopressor(s)‡ on POD1 | 0.34 | <0.001 | 0.69 | 2.26 (2.09–2.43) |
| Receipt of one or more inotrope(s)§ on POD1 | 0.53 | <0.001 | 0.47 | 2.81 (2.54–3.09) |
| Sensitivity analysis. Receipt of one or more vasoactive medications† on POD1 with an expanded set of cardiac surgery type covariates | 0.45 | <0.001 | 0.57 | 2.08 (1.94–2.22) |
| Sensitivity analysis. Receipt of one or more inotrope(s)§ on POD1 with epinephrine and dopamine reclassified as inotropes | 0.57 | <0.001 | 0.45 | 2.59 (2.36–2.92) |
Definition of abbreviations: AMOR = adjusted median odds ratio; CI = confidence interval; POD1 = postoperative Day 1.
Describes the relative contribution of each group of variables to observed variation in the multivariable mixed-effects regression model (46, 47). See online supplement for additional details.
Alone or in combination with one or more vasopressor(s) and/or inotrope(s).
Alone or in combination with one or more inotrope(s).
Alone or in combination with one or more vasopressor(s).
Discussion
In this retrospective study of a large cohort of adult patients undergoing cardiac surgery in the United States, we observed significant variation in the types, combinations, and timing of vasopressors and inotropic medications administered postoperatively. Although several patient characteristics and care variables, including diagnoses present on admission and type of cardiac surgery, were associated with receipt of vasoactive drugs on the first postoperative day, individual hospital of admission contributed more to observed variation in use of vasopressors and inotropes than most patient or hospital characteristics captured in the data set.
Our study found variability both in the likelihood of receipt of any vasoactive support on POD1 and in the choice of vasoactive medication. The presence of significant interhospital variation in vasoactive medication use may suggest the presence of unmeasured differences (including case mix, pharmacy practices, clinician preference, or local drug shortage) between hospitals but may also reflect the lack of evidence and guidelines supporting administration of specific vasoactive medications in this population and equipoise among clinicians. It is possible that observed variation in administration of vasoactive medication in cardiac surgical patients has implications for both patient outcomes (including direct harm from adverse effects (48), use of less effective agents, or exposure to unnecessary medications that are associated with worse outcomes in cardiac surgical patients (18) and other populations (24)) and medication and hospitalization costs. The variability in overall use represents a potential area for further study and improvement; given that that receipt of vasoactive medications is a frequent cause of continued care in an intensive care unit setting, a better understanding of factors associated with this use may identify potentially modifiable variables that could reduce the need for vasoactive support, both reducing exposure to potential harm and expediting transitions to the ward. It is also notable that on POD1, both norepinephrine and phenylephrine were frequently used to provide vasoactive support.
Compared with prior observational studies, several aspects of this study are unique. Not only does it describe a substantially larger and more current patient and hospital cohort, it focuses on postoperative vasoactive medication use, includes vasopressor drugs, and quantifies observed practice variation. Direct comparison of our study findings with most previous work is prevented by differences in populations, types of cardiac surgery, individual drugs studied, classification of specific vasoactive drugs as inotropes or vasopressors, and perioperative timing of administration (5, 6, 8, 9, 13, 15, 18, 49). Some aspects of this study are consistent with the work of Hernandez and colleagues, which examined use of vasodilators, inotropes, and vasopressors within 12 postoperative hours in a U.S. cohort of “high-risk” patients undergoing CABG (4). Compared with their study, we observed similar rates of vasoactive medication use but different patterns of individual drugs administered.
Limitations
Limitations of this study include those inherent to secondary analyses of administrative data sets. Specifically, this study is at risk of misclassification bias; not all of the ICD-10 codes used to identify study variables have been previously used or validated. Although we attempted to mitigate this with a sensitivity analysis using an expanded cardiac surgery classification, the potential for residual confounding remains. Moreover, we were unable to identify drug doses, or hemodynamic or echocardiographic parameters, or another clinical rationale for selection of a specific drug. Additionally, the data set captures both surgery and drug administration in calendar days. Expected variation in duration and timing of surgery between cohort patients and hospitals increases the likelihood that equal durations of vasoactive infusions may have been inconsistently classified as use on POD1, potentially confounding the degree of interhospital practice variation we observed. Furthermore, this lack of granularity in timing limits our ability to distinguish between intraoperative and perioperative use on the day of index surgery or between simultaneous or sequential use on a given hospital day. We addressed this problem by focusing our analyses on the group of patients who received vasoactive drugs on the day after index surgery. Still, this approach leaves unanswered questions about intraoperative and immediate postoperative patterns of medication administration patterns and clinically meaningful duration of use. Given the relatively short (2.5 yr) study period, we did not conduct analyses to examine trends in medication use over time. Furthermore, we did not evaluate whether observed variations in clinical practice were associated with differences in patient outcomes. Although U.S. cardiac surgery outcomes are closely tracked and publicly reported for individual hospitals (50), the study data set does not identify individual hospitals and does not contain a specific severity of illness variable necessary for adequate adjustment.
Despite these limitations, our study results have face validity; patient characteristics most strongly associated with vasoactive drug use included conditions and therapies (such as mechanical circulatory support) associated with higher severity of illness. Although the analyses were exploratory, we found that perioperative use of vasoactive drugs was associated with markers of increased severity of illness, more complex surgery, and worse patient outcomes, similar to other observational studies (7, 8, 13–15, 18, 20, 49). Future work is needed to validate these associations and to adjust for plausible confounders not captured in this study data set. This study was not designed to compare outcomes associated with use of individual medications or medication classes but may establish the groundwork for future comparative effectiveness analyses.
Conclusions
In a large cohort of adult patients undergoing cardiac surgery in U.S. hospitals, almost all patients were exposed to vasoactive drugs on the day of cardiac surgery and one-third of patients remained on a vasoactive medication on the first day after surgery. Use of vasoactive drugs after coronary artery bypass grafting and open cardiac valve surgery—if they are used and, if so, which ones are used—varies widely between hospitals. Further research is necessary to determine whether the observed variation in practice is associated with clinical outcomes.
Acknowledgments
Acknowledgment
The authors thank Dr. Ruxandra Pinto (University of Toronto) for her assistance.
Footnotes
Supported by grant K24 HL132008 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (P.K.L.).
Author Contributions: Study conception and design: E.A.V., H.B.G., A.J.W., P.K.L., and H.W. Data acquisition, analysis, or interpretation: E.A.V., M.-S.S., P.S.P., H.B.G., A.J.W., P.K.L., and H.W. Manuscript drafting: E.A.V., H.B.G., A.J.W., P.K.L., and H.W. Manuscript revision: E.A.V., M.-S.S., P.S.P., H.B.G., A.J.W., P.K.L., and H.W. All authors have approved this version of the manuscript and agree to be accountable for all aspects of the work. All listed authors meet International Committee of Medical Journal Editors criteria for authorship.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Gomes WJ, Carvalho AC, Palma JH, Teles CA, Branco JN, Silas MG, et al. Vasoplegic syndrome after open heart surgery. J Cardiovasc Surg (Torino) 1998;39:619–623. [PubMed] [Google Scholar]
- 3.Lomivorotov VV, Efremov SM, Kirov MY, Fominskiy EV, Karaskov AM. Low-cardiac-output syndrome after cardiac surgery. J Cardiothorac Vasc Anesth. 2017;31:291–308. doi: 10.1053/j.jvca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez AF, Li S, Dokholyan RS, O’Brien SM, Ferguson TB, Peterson ED. Variation in perioperative vasoactive therapy in cardiovascular surgical care: data from the Society of Thoracic Surgeons. Am Heart J. 2009;158:47–52. doi: 10.1016/j.ahj.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen DV, Johnsen SP, Madsen M, Jakobsen CJ. Variation in use of peroperative inotropic support therapy in cardiac surgery: time for reflection? Acta Anaesthesiol Scand. 2011;55:352–358. doi: 10.1111/j.1399-6576.2010.02382.x. [DOI] [PubMed] [Google Scholar]
- 6.Bastien O, Vallet B French Study Group AGIR. French multicentre survey on the use of inotropes after cardiac surgery. Crit Care. 2005;9:241–242. doi: 10.1186/cc3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahin J, DeVarennes B, Tse CW, Amarica DA, Dial S. The relationship between inotrope exposure, six-hour postoperative physiological variables, hospital mortality and renal dysfunction in patients undergoing cardiac surgery. Crit Care. 2011;15:R162. doi: 10.1186/cc10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinlay KH, Schinderle DB, Swaminathan M, Podgoreanu MV, Milano CA, Messier RH, et al. Predictors of inotrope use during separation from cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2004;18:404–408. doi: 10.1053/j.jvca.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Müller M, Junger A, Bräu M, Kwapisz MM, Schindler E, Akintürk H, et al. Incidence and risk calculation of inotropic support in patients undergoing cardiac surgery with cardiopulmonary bypass using an automated anaesthesia record-keeping system. Br J Anaesth. 2002;89:398–404. doi: 10.1093/bja/89.3.398. [DOI] [PubMed] [Google Scholar]
- 10.Williams JB, Hernandez AF, Li S, Dokholyan RS, O’Brien SM, Smith PK, et al. Postoperative inotrope and vasopressor use following CABG: outcome data from the CAPS-care study. J Card Surg. 2011;26:572–578. doi: 10.1111/j.1540-8191.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterworth JF, IV, Legault C, Royster RL, Hammon JW., Jr Factors that predict the use of positive inotropic drug support after cardiac valve surgery. Anesth Analg. 1998;86:461–467. doi: 10.1097/00000539-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kastrup M, Markewitz A, Spies C, Carl M, Erb J, Grosse J, et al. Current practice of hemodynamic monitoring and vasopressor and inotropic therapy in post-operative cardiac surgery patients in Germany: results from a postal survey. Acta Anaesthesiol Scand. 2007;51:347–358. doi: 10.1111/j.1399-6576.2006.01190.x. [DOI] [PubMed] [Google Scholar]
- 13.Weis F, Kilger E, Beiras-Fernandez A, Nassau K, Reuter D, Goetz A, et al. Association between vasopressor dependence and early outcome in patients after cardiac surgery. Anaesthesia. 2006;61:938–942. doi: 10.1111/j.1365-2044.2006.04779.x. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen DV, Hansen MK, Johnsen SP, Hansen M, Hindsholm K, Jakobsen CJ. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 2014;120:1098–1108. doi: 10.1097/ALN.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 15.Koponen T, Karttunen J, Musialowicz T, Pietiläinen L, Uusaro A, Lahtinen P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122:428–436. doi: 10.1016/j.bja.2018.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumann J, Henrich EC, Strobl H, Prondzinsky R, Weiche S, Thiele H, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;1:CD009669. doi: 10.1002/14651858.CD009669.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dünser MW, Bouvet O, Knotzer H, Arulkumaran N, Hajjar LA, Ulmer H, et al. Vasopressin in cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2018;32:2225–2232. doi: 10.1053/j.jvca.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Fellahi JL, Parienti JJ, Hanouz JL, Plaud B, Riou B, Ouattara A. Perioperative use of dobutamine in cardiac surgery and adverse cardiac outcome: propensity-adjusted analyses. Anesthesiology. 2008;108:979–987. doi: 10.1097/ALN.0b013e318173026f. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y, Pan T, Ge M, Chen T, Ye J, Lu L, et al. Evaluation of vasopressin for vasoplegic shock in patients with preoperative left ventricular dysfunction after cardiac surgery: a propensity-score analysis. Shock. 2018;50:519–524. doi: 10.1097/SHK.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen DV, Torp-Pedersen C, Skals RK, Gerds TA, Karaliunaite Z, Jakobsen CJ. Intraoperative milrinone versus dobutamine in cardiac surgery patients: a retrospective cohort study on mortality. Crit Care. 2018;22:51. doi: 10.1186/s13054-018-1969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming GA, Murray KT, Yu C, Byrne JG, Greelish JP, Petracek MR, et al. Milrinone use is associated with postoperative atrial fibrillation after cardiac surgery. Circulation. 2008;118:1619–1625. doi: 10.1161/CIRCULATIONAHA.108.790162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carl M, Alms A, Braun J, Dongas A, Erb J, Goetz A, et al. S3 guidelines for intensive care in cardiac surgery patients: hemodynamic monitoring and cardiocirculary system. Ger Med Sci. 2010;8:Doc12. doi: 10.3205/000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mebazaa A, Pitsis AA, Rudiger A, Toller W, Longrois D, Ricksten SE, et al. Clinical review: practical recommendations on the management of perioperative heart failure in cardiac surgery. Crit Care. 2010;14:201. doi: 10.1186/cc8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamontagne F, Richards-Belle A, Thomas K, Harrison DA, Sadique MZ, Grieve RD, et al. 65 trial investigators. Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA. 2020;323:938–949. doi: 10.1001/jama.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vail EA, Shieh MS, Gershengorn HB, Walkey AJ, Lindenauer PK, Wunsch H. Use of vasopressor and inotrope drugs after cardiac surgery in the United States [abstract] Am J Respir Crit Care Med. 2020;201:A3605. [Google Scholar]
- 26.Premier Inc. Charlotte, NC: Premier Inc.; 2020. Premier healthcare database white paper: data that informs and performs. [updated 2020 Mar 2; accessed 2020 May 5]. Available from: https://learn.premierinc.com/white-papers/premier-healthcaredatabase-whitepaper. [Google Scholar]
- 27.Atreya AR, Priya A, Stefan MS, Pack QR, Lagu T, Lofti AS, et al. Association between perioperative amiodarone and postoperative atrial fibrillation following cardiac surgery [abstract] Circ Cardiovasc Qual Outcomes. 2017;10:A132. [Google Scholar]
- 28.Pack QR, Lahr BD, Squires RW, Lopez-Jimenez F, Greason KL, Michelena HI, et al. Survey reported participation in cardiac rehabilitation and survival after mitral or aortic valve surgery. Am J Cardiol. 2016;117:1985–1991. doi: 10.1016/j.amjcard.2016.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roth J. Cambridge, MA: National Bureau of Economic Research; 2016. Center for Medicare and Medicaid Services ICD-9-CM to and from ICD-10-CM and ICD-10-PCS crosswalk or general equivalence mappings. [accessed 2020 May 5]. Available from: https://www.cms.gov/medicare/coding/icd10/downloads/icd-10_gem_fact_sheet.pdf. [Google Scholar]
- 30.Benedetto U, Angelini GD, Caputo M, Feldman DN, Kim LK, Lau C, et al. Off- vs. on-pump coronary artery bypass graft surgery on hospital outcomes in 134,117 octogenarians. J Thorac Dis. 2017;9:5085–5092. doi: 10.21037/jtd.2017.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkler AE, Chen ML, Parikh NS, Murthy SB, Yaghi S, Goyal P, et al. Association between heart transplantation and subsequent risk of stroke among patients with heart failure. Stroke. 2019;50:583–587. doi: 10.1161/STROKEAHA.118.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachs T, Pomposelli F, Hagberg R, Hamdan A, Wyers M, Giles K, et al. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample J Vasc Surg 201052860–866.[Discussion, p. 866.] [DOI] [PubMed] [Google Scholar]
- 33.Kundi H, Strom JB, Valsdottir LR, Elmariah S, Popma JJ, Shen C, et al. Trends in isolated surgical aortic valve replacement according to hospital-based transcatheter aortic valve replacement volumes. JACC Cardiovasc Interv. 2018;11:2148–2156. doi: 10.1016/j.jcin.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins KJ, Newburger JW, Lock JE, Davis RB, Coffman GA, Iezzoni LI. In-hospital mortality for surgical repair of congenital heart defects: preliminary observations of variation by hospital caseload. Pediatrics. 1995;95:323–330. [PubMed] [Google Scholar]
- 35.Abildstrøm SZ, Hvelplund A, Rasmussen S, Nielsen PH, Mortensen PE, Kruse M Danish Heart Register. Prognostic information in administrative co-morbidity data following coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;38:573–576. doi: 10.1016/j.ejcts.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Tan C, Hansen M, Cohen G, Boyle K, Daneman N, Adhikari NK. Accuracy of administrative data for identification of patients with infective endocarditis. Int J Cardiol. 2016;224:162–164. doi: 10.1016/j.ijcard.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Vail EA, Gershengorn HB, Hua M, Walkey AJ, Wunsch H. Epidemiology of vasopressin use for adults with septic shock. Ann Am Thorac Soc. 2016;13:1760–1767. doi: 10.1513/AnnalsATS.201604-259OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindenauer PK, Stefan MS, Johnson KG, Priya A, Pekow PS, Rothberg MB. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest. 2014;145:1032–1038. doi: 10.1378/chest.13-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal S, Sud K, Martin JM, Menon V. Trends in the use of mechanical circulatory support devices in patients presenting with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:1772–1774. doi: 10.1016/j.jcin.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 40.McIlvennan CK, Lindenfeld J, Kao DP. Sex differences and in-hospital outcomes in patients undergoing mechanical circulatory support implantation. J Heart Lung Transplant. 2017;36:82–90. doi: 10.1016/j.healun.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huesch MD. Volume-outcome relationships in extracorporeal membrane oxygenation: retrospective analysis of administrative data from Pennsylvania, 2007-2015. ASAIO J. 2018;64:450–457. doi: 10.1097/MAT.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 42.Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.So L, Evans D, Quan H. ICD-10 coding algorithms for defining comorbidities of acute myocardial infarction. BMC Health Serv Res. 2006;6:161. doi: 10.1186/1472-6963-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med. 2017;36:3257–3277. doi: 10.1002/sim.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrell FE., Jr . Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. 1st ed. New York: Springer Science+Business Media; 2001. Overview of maximum likelihood estimation; pp. 203–206. [Google Scholar]
- 47.Garland A, Connors AF. Physicians’ influence over decisions to forego life support. J Palliat Med. 2007;10:1298–1305. doi: 10.1089/jpm.2007.0061. [DOI] [PubMed] [Google Scholar]
- 48.Russell JA. Vasopressor therapy in critically ill patients with shock. Intensive Care Med. 2019;45:1503–1517. doi: 10.1007/s00134-019-05801-z. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed I, House CM, Nelson WB. Predictors of inotrope use in patients undergoing concomitant coronary artery bypass graft (CABG) and aortic valve replacement (AVR) surgeries at separation from cardiopulmonary bypass (CPB) J Cardiothorac Surg. 2009;4:24. doi: 10.1186/1749-8090-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.New York State Department of Health. Cardiovascular disease data and statistics: cardiac surgery and angioplasty outcomes reports in New York state. 2019 [accessed 2020 May 5]. Available from: https://www.health.ny.gov/statistics/diseases/cardiovascular/