Abstract
Rationale: The combination of lumacaftor (LUM) and ivacaftor (IVA) is an approved CFTR (cystic fibrosis [CF] transmembrane conductance regulator) modulator treatment for homozygous F508del patients with CF.
Objectives: To evaluate the effectiveness of LUM/IVA in children (6 yr or more) and adults (more than 18 yr) in a postapproval setting.
Methods: This longitudinal cohort study, performed at 38 centers in the U.S. CF Therapeutics Development Network, enrolled homozygous F508del patients with CF ages 6 years old and older with no prior exposure to LUM/IVA. Study assessments were performed at baseline and at 1, 3, 6, and 12 months after LUM/IVA initiation.
Results: A total of 193 patients initiated LUM/IVA, and 85% completed the study through 1 year. Baseline mean percent-predicted forced expiratory volume in 1 second (ppFEV1) was 85 (standard deviation, 22.4) in this cohort. No statistically significant change in ppFEV1 was observed from baseline to any of the follow-up time points, with a mean absolute change at 12 months of −0.3 (95% confidence interval [CI], −1.8 to 1.2). Body mass index improved from baseline to 12 months (mean change, 0.8 kg/m2; P < 0.001). Sweat chloride decreased from baseline to 1 month (mean change, −18.5 mmol/L; 95% CI, −20.7 to −16.3; P < 0.001), and these reductions were sustained through the study period. There were no significant changes in hospitalization rate for pulmonary exacerbations and Pseudomonas aeruginosa infection status with treatment.
Conclusions: In this real-world multicenter cohort of children and adults, LUM/IVA treatment was associated with significant improvements in growth and reductions in sweat chloride without statistically significant or clinically meaningful changes in lung function, hospitalization rates, or P. aeruginosa infection.
Clinical trial registered with www.clinicaltrials.gov (NCT 02477319).
Keywords: cystic fibrosis, lumacaftor, ivacaftor, postapproval study, clinical effectiveness
Cystic fibrosis (CF) is an autosomal recessive genetic disease caused by mutations in the gene encoding the CFTR (CF transmembrane conductance regulator) protein, an anion channel at the apical surface of epithelial cells that transports chloride and bicarbonate and regulates ion and fluid transport (1–3). As a result of CFTR dysfunction, people with CF develop multisystem disease characterized by muco-obstructive airway disease accompanied by progressive respiratory decline, pancreatic insufficiency, and gastrointestinal dysfunction among other manifestations (4). The most common mutation in CFTR is F508del, and nearly 50% of individuals followed in the U.S. CF Foundation Patient Registry (CFFPR) are homozygous for the mutation (5).
Treatment of the basic protein defect with small molecule therapies to restore function to the CFTR protein has emerged as a key therapeutic approach for people with CF (6). The corrector lumacaftor (LUM) increases trafficking of the F508del CFTR to the cell surface, whereas the potentiator ivacaftor (IVA) augments channel gating of CFTR on the cell surface (7, 8). Large multinational clinical trials demonstrated that the combination of LUM and IVA modestly improved lung function, growth, and quality of life and significantly reduced pulmonary exacerbations in individuals homozygous for the F508del CFTR (9, 10), leading to its approval by the U.S. Food and Drug Administration (FDA) in 2015. However, questions remain regarding the clinical effectiveness of LUM/IVA combination treatment in the larger CF population and the mechanistic basis for these improved outcomes with treatment. Recent studies from France have reported on the real-world experience of LUM/IVA treatment, highlighting the fact that a significant number of patients discontinued treatment because of adverse events, but those who continued treatment experienced clinical benefits comparable with what was observed in the pivotal clinical trials (11, 12). Further investigation of LUM/IVA combination treatment in a less selective population than was studied during drug development is warranted.
Previous real-world studies evaluating the effects of IVA in patients with the G551D and other related gating mutations provided important clinical and mechanistic insights, including confirmation of the benefits in a broader CF population compared with those enrolled in registrational trials, demonstration of improved mucociliary clearance and intestinal pH, and provocative observations regarding improvements in respiratory microbiology that have been confirmed in subsequent studies. These results helped set the stage for future research and informed clinical practice related to airway microbiology, airway and systemic inflammation, mucus biology, digestive health, endocrine function, and liver disease in the post–CFTR modulator era (13–17).
To examine the clinical effectiveness of LUM/IVA in a more real-world setting, we conducted a similar effort after drug approval in the United States. Clinical outcomes were captured before and up to 1 year after the initiation of LUM/IVA in children (6 yr or older) and adults (18 yr or older) homozygous for the F508del-CFTR mutation. Some of the results have been previously reported in the form of an abstract (18).
Methods
Study Design and Population
This study is a before and after evaluation of a prospective longitudinal cohort from 38 U.S. centers within the CF Therapeutics Development Network. This study, embedded within a two-part multicenter prospective longitudinal study of CFTR-dependent disease profiling (PROSPECT) (http://clinicaltrials.gov/ct2/show/NCT02477319), enrolled participants from July 2015 until April 2017. Institutional review boards at each participating center approved the study, and each subject and/or his/her parent provided written informed consent (assent when applicable). Persons with CF aged 6 years or older with two F508del CFTR mutations who qualified to start on LUM/IVA treatment on the basis of FDA labeling were eligible to participate. Inclusion criteria included being clinically stable for at least 2 weeks before the baseline study visit and enrollment in the CFFPR (19). Key exclusion criteria included acute lower respiratory symptoms treated with antibiotics within 2 weeks of the baseline study visit, initiation of any new chronic therapy or use of an investigational agent within 4 weeks of baseline, use of chronic oral corticosteroids, history of lung or liver transplantation, and prior exposure to CFTR-modulator therapy.
Five study visit assessments were performed, timed at baseline (pre-LUM/IVA) and at 1, 3, 6, and 12 months after the initiation of LUM/IVA. Spirometry, sweat chloride testing, and height and weight measurements were performed at each study visit. Sputum induction was done at baseline and 1, 6, and 12 months post-LUM/IVA initiation. Blood, urine, and induced sputum were collected for banking purposes. American Thoracic Society guidelines were implemented for spirometry, and Global Lung Initiative equations (20) were applied for standardization and the calculation of percent-predicted forced expiratory volume in 1 second (ppFEV1). Spirometry was performed in concert with sputum induction and occurred between 15 ± 5 minutes post–bronchodilator administration and before the sputum induction procedure. Body mass index (BMI) percentiles were based on U.S. Centers for Disease Control and Prevention equations and calculated for only pediatric participants (less than 20 yr old) (21). Hospitalization and respiratory culture data were extracted from the CFFPR for the 1-year period before and after the initiation of LUM/IVA. At a subset of sites, nasal epithelial cells were collected and banked on a one-time basis from consenting participants. The PROSPECT study also included the following optional substudies, which were performed in a subset of study participants: multiple breath washout and fractional exhaled nitric oxide testing, measurement of mucociliary clearance, and evaluation of gastrointestinal health with intestinal pH determinations. Results from these substudies will be reported separately.
Statistical Analyses
A sample size of up to 200 was chosen on the basis of feasibility to support the embedded substudies and provide enough power for correlations with potential changes in clinical outcome measures, including ppFEV1, weight, and BMI. Summary statistics are reported as follows: all continuous data are based on the mean, standard deviation (SD), and 95% confidence interval (CI), whereas categorical data are shown as proportions. Mean absolute changes comparing baseline data with postbaseline visits were analyzed by paired t tests. To assess baseline covariates that might be associated with change in ppFEV1, we arbitrarily defined “responders” as participants who experienced an increase in ppFEV1 greater than 5 at 6 months (49 of 176 [27.8%]) and “nonresponders” as those who experienced a decrease less than 5 (44 of 176 [25%]) (i.e., based on highest and lowest quartiles of the data). A multivariate relative risk model was fit to evaluate the risk of being a responder against the following baseline covariates: age, sweat chloride, sex, and BMI. We excluded baseline ppFEV1 from the model because of high collinearity between age and ppFEV1. One-year before and after comparisons (based on 6-mo intervals) of the percentage of participants who were hospitalized for pulmonary exacerbations or had positive Pseudomonas aeruginosa cultures were made by McNemar’s exact test. For each 6 month interval with at least one culture, participants were classified in terms of P. aeruginosa infection category defined as the following: “P. aeruginosa free” (no positive cultures), “intermittent” (>0% to ≤50% P. aeruginosa+ cultures), and “chronic” (>50% P. aeruginosa+ cultures). Comparison of participants change in classification from pre to post LUM/IVA was made by McNemar-Bowker’s test. All analyses were performed using SAS version 9.4 (SAS Institute) and R version 3.5.2 (R Foundation for Statistical Computing). Two-sided P values ≤0.05 were considered statistically significant, and no adjustments for multiple comparisons were made.
Results
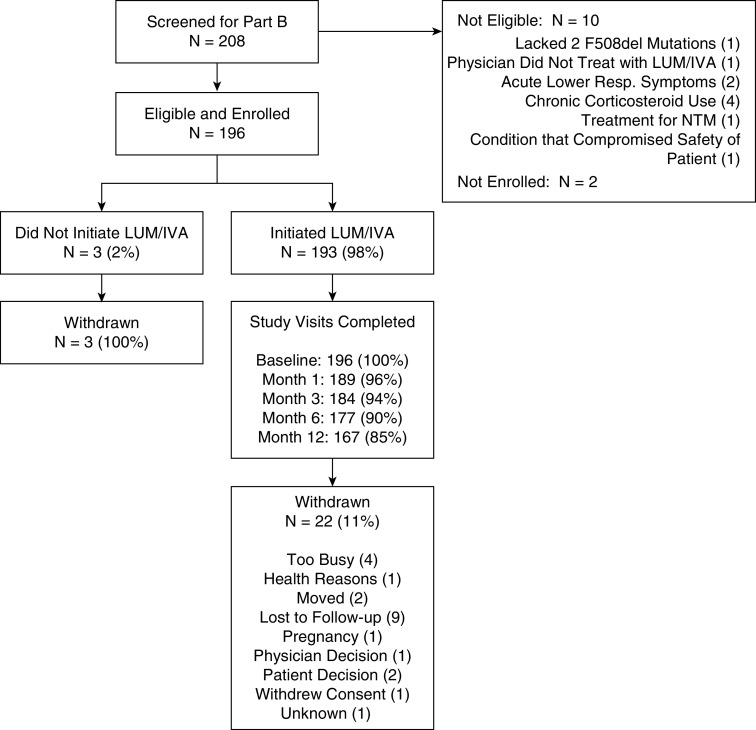
Of the 208 F508del-CFTR homozygous participants screened, 196 enrolled and met the protocol eligibility criteria before LUM/IVA was initiated. Of the 196 enrolled participants, 193 initiated LUM/IVA. Among those initiating LUM/IVA, 90% of study participants completed study visits through 6 months, with 85% completing their 1-year follow-up visit (Figure 1). Participant demographics and baseline clinical characteristics for those who initiated LUM/IVA therapy and those who withdrew from the study are shown in Table 1. The cohort who received LUM/IVA (N = 193) was 55% (n = 106) female and 98% (n = 189) white, and the mean age was 19.0 years (minimum, 6.1 yr; maximum, 57.6 yr). Baseline lung function in the cohort was high (mean ppFEV1 = 85; SD, 22.4). Fifty-two participants (27%) had a ppFEV1 of100 or greater, and only 10% had a ppFEV1 below 50. The mean follow-up time in the treated group was 336 days (SD, 93). In addition, blood, sputum, stool, and urine biospecimens were obtained from these study participants during the study and banked for future investigation (Table 1) (accessible at https://www.cff.org/Research/Researcher-Resources/Tools-and-Resources/CF-Foundation-Biorepository/).
Figure 1.
Consolidated Standards of Reporting Trials diagram of the study participants. IVA = ivacaftor; LUM = lumacaftor; NTM = nontuberculous mycobacteria; Resp. = respiratory.
Table 1.
Participant demographics and characteristics at baseline for those who initiated LUM/IVA therapy and those who withdrew from the study
| Characteristics | Initiated LUM/IVA (n = 193) | Withdrawal Patients (n = 22) |
|---|---|---|
| Sex, n (%) | ||
| F | 106 (54.9) | 13 (59.1) |
| M | 87 (45.1) | 9 (40.9) |
| Age distribution, n (%) | ||
| ≥6 to <12 yr | 45 (23.3) | 5 (22.7) |
| ≥12 to <18 yr | 72 (37.3) | 6 (27.3) |
| ≥18 to <30 yr | 42 (21.8) | 6 (27.3) |
| ≥30 yr | 34 (17.6) | 5 (22.7) |
| Age, yr | ||
| Mean (SD) | 19.0 (10.6) | 20.7 (10.7) |
| Median | 15.4 | 18.7 |
| Minimum–maximum | 6.1–57.6 | 6.6–44.9 |
| Race, n (%) | ||
| White | 189 (97.9) | 22 (100) |
| ppFEV1, n (%)* | ||
| <25% | 1 (0.5) | 0 (0.0) |
| ≥25% to <50% | 18 (9.3) | 2 (9.1) |
| ≥50% to <75% | 35 (18.1) | 3 (13.6) |
| ≥75% to <100% | 87 (45.1) | 13 (59.1) |
| ≥100% | 52 (26.9) | 4 (18.2) |
| Diabetes diagnosis, n (%) | 19 (9.8) | 3 (13.6) |
| Enrolled in substudy | ||
| MBW/FENO | 59 (30.6) | 4 (18.2) |
| MCC | 24 (12.4) | 4 (18.2) |
| GIFT | 43 (22.3) | 7 (31.8) |
| pH pill | 10 (5.2) | 3 (13.6) |
| Follow-up time (d)† | ||
| Mean (SD) | 336 (93) | 124 (87) |
| BMI percentile (age <20 yr) | ||
| Mean (SD) | 58.3 (23.5) | 55.9 (27.5) |
| BMI kg/m2 (age ≥20 yr) | ||
| Mean (SD) | 22.5 (3.2) | 21.7 (1.8) |
| Specimen collection, mean # of samples per participant‡ | ||
| Blood | 4.61 | 2.73 |
| Urine | 4.61 | 2.68 |
| Sputum | 2.86 | 1.59 |
| Participants, n (%) | ||
| Human nasal epithelial cell | 115 (60) | 5 (22.7%) |
Definition of abbreviations: BMI = body mass index; GIFT = gastrointestinal and glucose/insulin functional testing; IVA = ivacaftor; LUM = lumacaftor; MBW/FENO = multiple breath washout and fractional exhaled nitric oxide testing; MCC = measurement of mucociliary clearance; pH pill = intestinal pH measurements; ppFEV1 = percent-predicted forced expiratory volume in 1 second; SD = standard deviation.
ppFEV1 was based on Global Lung Initiative equations.
Follow-up time was calculated as number of days from LUM/IVA start date until last study visit.
The mean number of samples per subject is reported. Blood and urine were expected to be collected at each visit (five total), whereas sputum was expected to be collected at up to four visits.
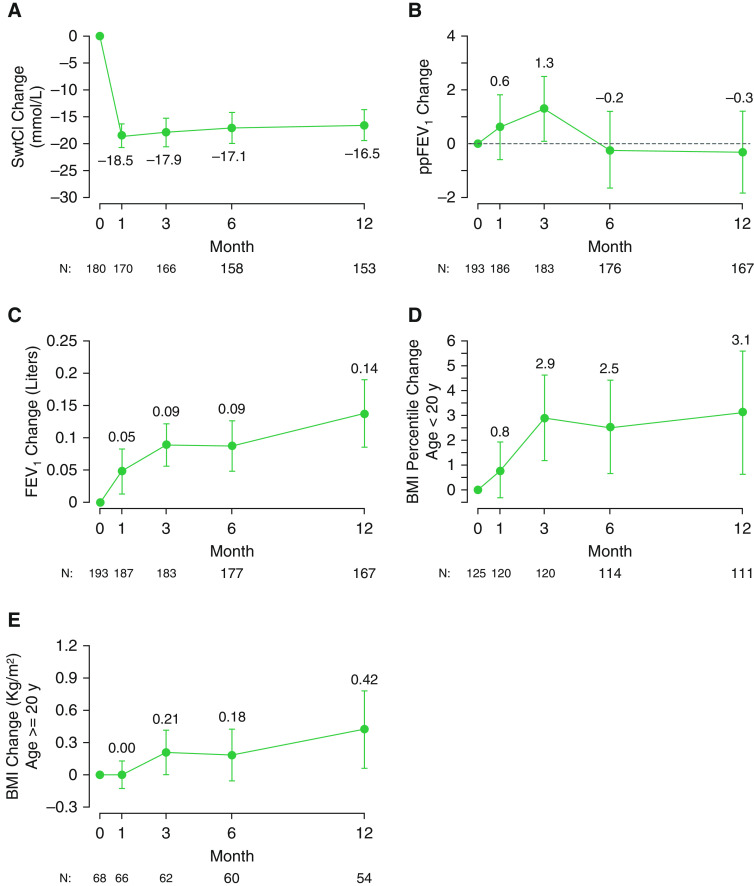
Results of the core clinical outcome measures are shown in Figure 2 and Table E1 (see online supplement). By 1 month, sweat chloride dropped an average of −18.5 mmol/L (95% CI, −20.7 to −16.3) from a baseline mean of 100.4 (SD, 11.5) to a 1 month mean of 81.6 mmol/L (SD, 14.4) (Figure 2A). No statistically significant change in ppFEV1 from baseline to 1 or 12 months was observed, with a mean change at 1 month of 0.6 (95% CI, −0.6 to 1.8) and at 12 months of −0.3 (95% CI, −1.8 to 1.2) (Figure 2B). The lack of improvement in ppFEV1 did not appear to be due either to subjects who experienced a large decrease in ppFEV1 (see Figure E1) or to subjects who withdrew from the study (Table E2). Absolute FEV1 (in liters) did increase throughout the study, likely reflecting the influence of lung growth in the pediatric population (Figure 2C). In contrast to the lack of significant changes in lung function, nutritional status improved, as indicated by BMI percentile in children and absolute BMI in adults. BMI percentile in those less than 20 years of age (n = 125) steadily increased throughout the study from a baseline mean of 58.3% (SD, 23.5%), increasing by 0.8% (95% CI, −0.3 to 1.9%) at 1 month, by 2.9% (95% CI, 1.2–4.6%) at 3 months, and by 3.1% (95% CI, 0.6 –5.6%) at 12 months (see Figure 2D and Table E1). Among those 20 years of age or older (n = 68), BMI at 3 months increased by 0.21 kg/m2 (95% CI, 0.01–0.41 kg/m2), and BMI at 12 months increased by 0.42 kg/m2 (95% CI, 0.07–0.78 kg/m2) (Figure 2E).
Figure 2.
Mean absolute change from baseline in clinical measures at 1, 3, 6, and 12 months after initiation of lumacaftor/ivacaftor. The bars represent the 95% confidence intervals. (A) Sweat chloride (in mmol/L). (B) ppFEV1. (C) FEV1 (in liters). (D) Body mass index (BMI) percentile (n = 125 subjects < 20 yr of age). (E) BMI (in kg/m2) (n = 68 subjects ≥ 20 yr of age). FEV1 = forced expiratory volume in 1 second; ppFEV1 = percent-predicted FEV1; SwtCl = sweat chloride.
Change in clinical outcomes were also evaluated by age category (6–less than 12 yr, 12–less than 18 yr, 18–less than 30 yr, and 30 yr or older) and by baseline ppFEV1 category (less than 50, 50–less than 90, and 90 or greater) (Tables E3 and E4). Stratification by age and baseline lung function revealed that significant improvements in ppFEV1 were only observed in adolescents (between 12 and less than 18 yr) and young adults (between 18 and less than 30 yr) at the 3 month post-LUM/IVA time point and in those with a baseline ppFEV1 between 50 and less than 90 at the 3 and 6 month time points. In contrast, nutritional benefits were observed across age and disease severity but were most evident in younger patients. We did not observe significant differences in sweat chloride responses in the different age categories. None of the baseline covariates (age, sex, sweat chloride, and BMI) were statistically associated with being a responder in terms of change in ppFEV1 (defined as an increase in ppFEV1 of more than 5 at 6 mo) compared with a nonresponder (defined as a decrease in ppFEV1 of less than 5 at 6 mo). The variable most associated with being a responder was male sex, with a relative risk of 1.46 of being a responder (95% CI, 0.98–2.18). Sixty-six percent of male subjects (27 of 41) were responders whereas 42% of (22 of 52) female subjects were responders.
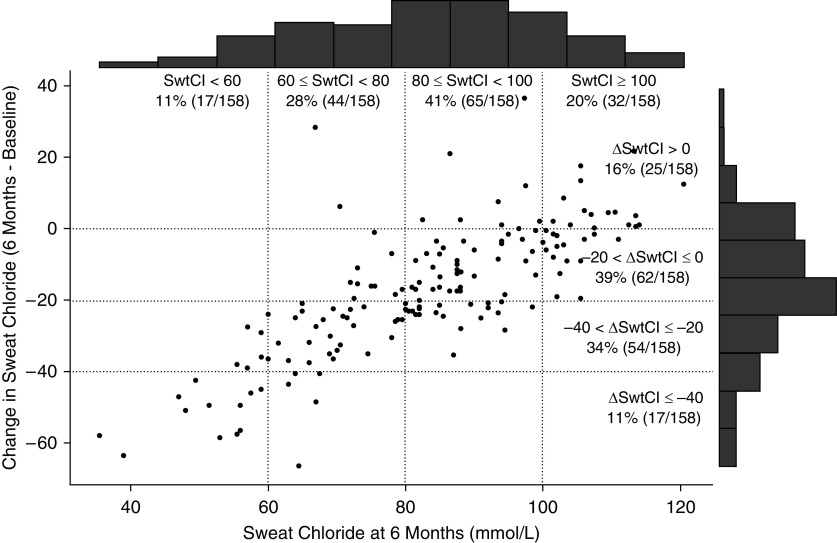
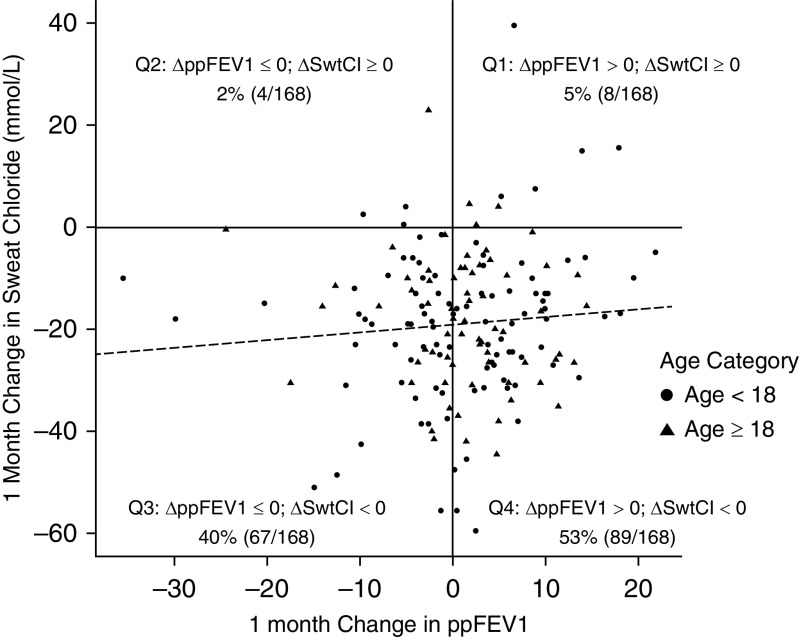
The distribution of sweat chloride values at 6 months is shown in Figure 3, with 11% of participants having sweat chloride levels at 6 months of less than 60 mmol/L, 28% having levels between 60 and less than 80 mmol/L, 41% having levels between 80 and less than 100 mmol/L, and 20% having levels of 100 mmol/L or greater. These data are presented as compared with change from baseline on an individual level and show a strong relationship between posttreatment sweat chloride and the change experienced with the initiation of LUM/IVA. The comparison of 1 month change in ppFEV1 against 1 month change in sweat chloride did not reveal any consistent trends (Figure 4). Although 93% (156 of 168) of participants had some reduction in sweat chloride (∆ sweat chloride of less than 0) at 1 month, only 58% (97 of 168) had some improvement in ppFEV1 change (∆ppFEV1 > 0) over 1 month. There were no statistically significant associations between change in sweat chloride and nutritional outcomes measured by absolute BMI or BMI percentile.
Figure 3.
Distribution of SwtCl at 6 months plotted against absolute change from baseline after initiation of lumacaftor/ivacaftor. SwtCl = sweat chloride.
Figure 4.
One-month change in percent-predicted forced expiratory volume in 1 second (ppFEV1) versus 1-month change in sweat chloride (SwtCl; mmol/L). The gray dashed line represents the following model fit: 1-month change in sweat chloride, ∼ 1-month change ppFEV1 + age category. The 1-month change in SwtCl (i.e., slope estimate) corresponding to a 1% increase in ppFEV1 was +0.15 (95% confidence interval, −0.13 to 0.42) mmol/L.
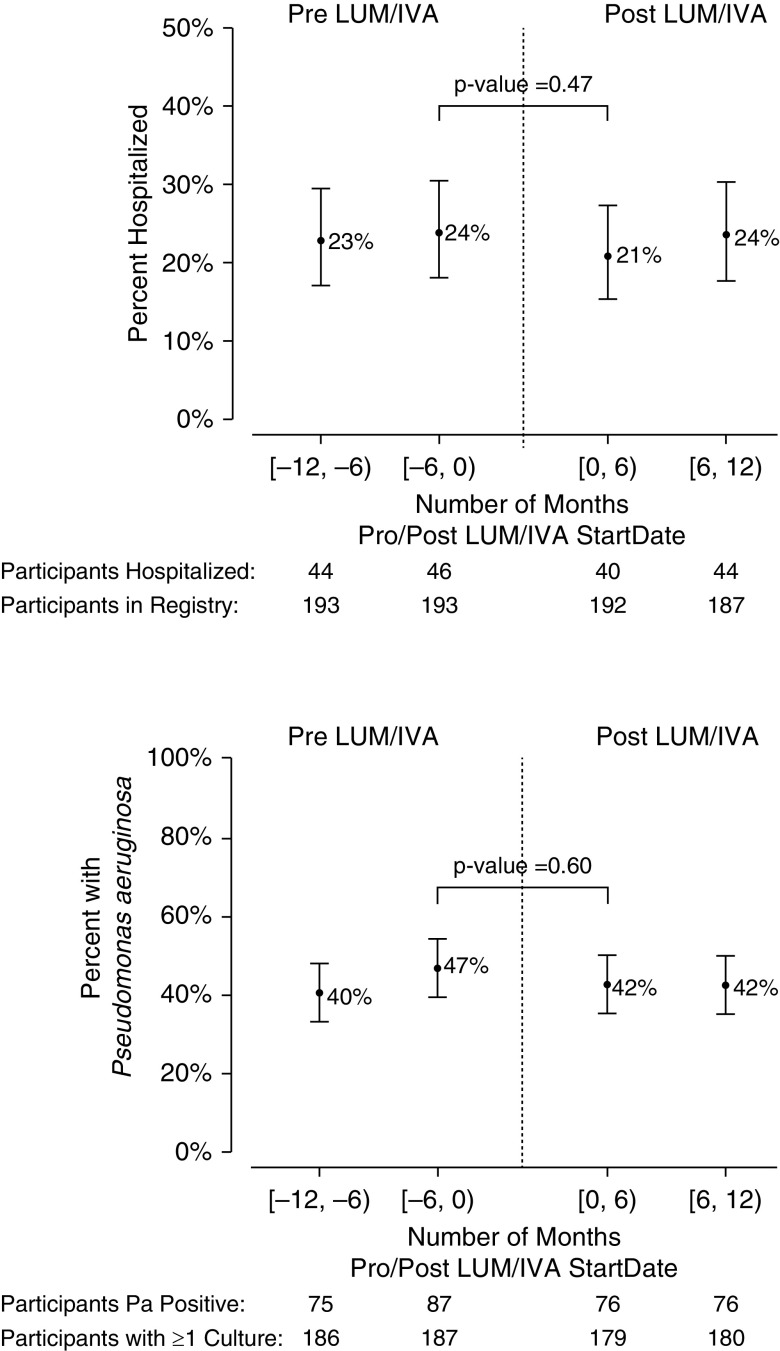
Figure 5 shows the percentage of participants who were hospitalized for pulmonary exacerbation (PEx) in the 1-year period before and after LUM/IVA initiation (based on 6-mo intervals) and a similar analysis for the percentage of patients whose respiratory cultures were P. aeruginosa+. Of the 193 participants who initiated LUM/IVA, 187 (97%) had CFFPR data for at least 1 year before and after LMU/IVA initiation. The percentage of patients hospitalized for PEx was not different at 6 months before and after initiation of LUM/IVA (before, 24%; after, 21%; P = 0.47; Figure 5A). We also examined the mean number of PEx events treated with intravenous antibiotics either in the hospital or at home and found that the mean number of these PEx events per participant in the year before LUM/IVA was 0.67 versus 0.62 in the subsequent year, representing a 7.5% reduction in number of events (difference, 0.05; 95% CI, −0.09 to 0.19; P value: 0.49).
Figure 5.
Hospitalizations for pulmonary exacerbation and P. aeruginosa culture positivity in the 1 year before and after initiation of lumacaftor/ivacaftor (in 6-mo intervals). P values are based on paired data (i.e., data available in each 6-mo interval before and after lumacaftor/ivacaftor). IVA = ivacaftor; LUM = lumacaftor; Pa = Pseudomonas aeruginosa.
Similarly, the percentage of participants who were P. aeruginosa+ 6 months before LUM/IVA was 47%, compared with 42% who were P. aeruginosa+ 6 months after LUM/IVA (P value = 0.60; Figure 5B). 188 participants had one or more cultures in the year before LUM/IVA initiation available for analysis as follows: 47% (n = 89) were classified as P. aeruginosa free, 22% (n = 41) had intermittent P. aeruginosa, and 34% (n = 63) had chronic P. aeruginosa. In the year after treatment, 11% (n = 21 of 188) worsened in culture status (more P. aeruginosa detection), 72% (n = 136 of 188) had no change in status, and 16% (n = 31 of 188) improved categories (less P. aeruginosa detection; P = 0.11; Table 2). Restricting this analysis to participants with three or more cultures in both years (before and after LUM/IVA) resulted in a similar percentage worsening of 12% (n = 19 of 153), whereas 15% (n = 23 of 153) improved in status from the year prior (P = 0.66; Table E5).
Table 2.
Shift in P. aeruginosa infection category 1 year before and after LUM/IVA initiation
| n |
P. aeruginosa Infection 1 yr after LUM/IVA Initiation |
||||
|---|---|---|---|---|---|
| Free [n (%)] | Intermittent [n (%)] | Chronic [n (%)] | No Cultures [n (%)] | ||
| P. aeruginosa infection 1 yr before LUM/IVA initiation | |||||
| Free* | 89 | 75 (84.3) | 12 (13.5) | 1 (1.1) | 1 (1.1) |
| Intermittent† | 41 | 18 (43.9) | 14 (34.1) | 8 (19.5) | 1 (2.4) |
| Chronic‡ | 63 | 7 (11.1) | 6 (9.5) | 47 (74.6) | 3 (4.8) |
Definition of abbreviations: IVA = ivacaftor; LUM = lumacaftor; P. = Pseudomonas.
McNemar-Bowker test P value = 0.112.
Free is defined as having no P. aeruginosa+ cultures with at least one or more culture available.
Intermittent is defined as >0% to ≤50% of cultures P. aeruginosa+.
Chronic is defined as >50% of cultures P. aeruginosa+.
Discussion
Here, we report the results of a large postapproval observational study to examine the clinical impact of combination CFTR-modulator treatment with LUM/IVA on individuals with CF caused by the most common disease-causing mutation; furthermore, we established a framework for a concomitant biomarker evaluation program coupled with a large biorepository to examine the mechanistic effects of CFTR-modulator therapy. A similar effort in patients with CF and the G551D and other gating mutations who initiated IVA therapy revealed a number of important findings (13–15, 22), including documenting multiple measures of improved outcomes that closely mirrored smaller phase 3 trials (23, 24).
Retention of study subjects was generally successful and extended over a longer period of time than the aforementioned G551D Observational (GOAL) study (14). The magnitude of clinical benefit, especially that related to lung function, observed in our study cohort was less than that reported in the pivotal clinical trials, which is likely due in part to the broad range of baseline lung function in our study population, some of whom have less dynamic spirometry (i.e., ppFEV1 greater than 90%), and our considerably smaller sample size relative to the clinical trials. Lacking a comparator group limits the conclusions that can be made regarding the clinical impact of LUM/IVA in our real-world population. Bioactivity of LUM/IVA was clearly established by the changes in sweat chloride, an important marker of CFTR function, that were evident at 1 month and sustained through the course of the study. Because phase 3 studies of LUM/IVA did not include sweat chloride evaluation (10) and the PROSPECT study was substantially larger than the phase 2 trial of LUM/IVA that included sweat chloride (9), the current study provides the most robust estimate of sweat chloride changes with LUM/IVA in a F508del homozygous population to date, an important estimate of the level of CFTR activity achieved in patients. Because sweat chloride changes were closely related to posttreatment sweat chloride concentrations, consistent with expectations based on in vitro efficacy but not previously demonstrated (7), posttreatment sweat chloride could be used as a long-term indicator of drug effect in future analyses. Posttreatment sweat chloride was also similar to that in pediatric studies that were conducted concurrently, serving as a validation for these estimates (25).
In the current study, we did not observe significant changes in lung function with LUM/IVA in our overall study cohort. These results contrast with findings from the large phase 3 trials (10) and postapproval studies completed in Europe (12, 26). In addition, sensitivity analyses excluding patients with highly unstable spirometry did not alter our conclusions. This discrepancy in results may be due in part to the higher baseline lung function of our cohort (mean ppFEV1 of 85) that limited the ability to detect further improvements compared with the other reported study populations. The lack of improvement in lung function in our real-world patients, who did not necessarily meet the rigorous entry criteria often required in clinical trials, also highlights the heterogeneity in clinical response to LUM/IVA among F508del homozygous patients with CF. Given that the phase 3 clinical trials enrolled over 1,000 subjects to demonstrate statistically significant improvements compared with placebo (10), our study was underpowered to reveal lung function improvements in the magnitude of a 3.5% change. Although it is possible that LUM/IVA attenuated a decline in lung function that would have otherwise occurred in these patients without therapy (12, 27), the lack of a control group prevents us from answering this definitively. Responder analyses did not reveal specific demographic or clinical features associated with a beneficial response to therapy. As expected in a study enrolling a pediatric population that is growing, absolute FEV1 did improve over the course of the study, but this cannot be attributed to LUM/IVA use. As with prior single-therapy studies, there was no correlation between individual change in sweat chloride and FEV1 (14, 28, 29). Of note, studies of larger populations have begun to demonstrate correlations between changes in sweat chloride and improvements in clinical outcome measures across a broader range of treatment responses (30).
In contrast to measures of spirometry, improved nutritional status, as observed by increases in BMI percentile, absolute BMI, and weight gain, were consistent and conclusive, demonstrating a steady improvement over the 12-month observation period. The beneficial effects of LUM/IVA on nutritional status were most evident in younger patients. We speculate that this is due to the growth potential of the younger population, based on previous data showing that ivacaftor improves linear growth (31). These data support the notion that CFTR modulators may be particularly beneficial if they are initiated early in the life of individuals with CF (31).
Exacerbation rates, as estimated by hospitalization occurrences, did not change with LUM/IVA use in our study. Again, these results contrast with findings from the phase 3 trials (10) and the French real-world study, in which patients who tolerated and continued LUM/IVA treatment experienced a 35% reduction in intravenous antibiotic courses (12). Because hospitalizations generally reflect the most severe exacerbations, it is possible that LUM/IVA was associated with a reduction in the overall pulmonary exacerbation rate, including in those treated with outpatient therapy, but these events were not captured in the current study. Furthermore, this study was not powered to determine an effect on exacerbations. An interesting observation from the previously published GOAL study was the reduction of P. aeruginosa detection rates in a proportion of individuals (14), particularly those with early evidence of infection (based on Leed’s criteria) or nonmucoid P. aeruginosa (13, 22). It is uncertain whether this represented clearance of infection or reduced expectoration/production of lower airway specimens, but recent observational reports in the United Kingdom have validated these findings (32). Changes in P. aeruginosa infection status were not observed in patients enrolled in this study. We speculate that higher levels of CFTR activity may be needed to result in reductions in P. aeruginosa detection.
It is important to acknowledge the limitations of this study. First, the study was underpowered for changes in spirometry and did not include a control group, limiting conclusions that can be drawn from this longitudinal cohort. Second, the population enrolled was younger and healthier than those of previous phase 3 studies, each of which reduce the likelihood of observing a change in FEV1. This same population may have been particularly well suited to experience improvements in nutritional status, as observed herein. We did not systematically track drug tolerance or side effects. Also, we did not monitor medication adherence, although the reductions in sweat chloride observed across the overall study suggest general adherence to therapy; however, we cannot estimate the degree of adherence. To this point, the approximate 18% decrease in sweat chloride observed in our study participants exceeds the reference change value of sweat chloride (13.7%) in this population (33), indicating a true change has occurred because of LUM/IVA therapy beyond the inherent analytical and biological variation of sweat chloride.
It is evident that LUM/IVA treatment was not associated with the magnitude of the beneficial response observed with IVA in a similar postapproval study design in the G551D population (14), reflecting less efficacy of this treatment combination for the homozygous F508del patient population. That said, improved nutritional outcomes observed here could be particularly relevant to pediatric patients with CF, for whom LUM/IVA remains the only available CFTR-modulator regimen in certain age groups and geographic areas. For these reasons, we do not believe this substantially alters the risk/benefit ratio for LUM/IVA CFTR-modulator therapy in patients homozygous for F508del. Nevertheless, these results justify further development of more efficacious CFTR-modulator combinations, as recently accomplished with elexacaftor–tezacaftor–IVA therapy (34, 35). Still, the ongoing analysis of serum and sputum biomarkers, future studies with the biospecimen repository, and final analysis of several associated substudies could reveal additional insights into the partial correction of the F508del protein and are resources available to the research community made possible by the conduct of this study.
Acknowledgments
Acknowledgment
The authors thank the study-site principal investigators and coordinators who participated in conducting this study (see online supplement).
Footnotes
Supported by Cystic Fibrosis Foundation Therapeutics (SAGEL14K1, HAMBLE14K1, and ROWE19R0) and the U.S. National Institutes of Health (UL1 TR002535, DK089507, P30DK072482, and R35HL135816).
A complete list of PROSPECT Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network may be found in the online supplement.
Author Contributions: S.D.S., U.K., S.L.H., J.P.C., D.B., D.G., S.H.D., A.M., F.R., J.M.V., and S.M.R. contributed to the study design, execution, and data interpretation. U.K. and S.L.H. performed statistical analyses. S.D.S., U.K., S.L.H., and S.M.R. drafted the manuscript. All other authors contributed to the review and revision of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the PROSPECT Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network
References
- 1.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [Published erratum appears in Science 245:1437.] [DOI] [PubMed] [Google Scholar]
- 3.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 4.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 5.Cystic Fibrosis Foundation. Bethesda, MD: Cystic Fibrosis Foundation; 2018. Patient registry 2018 annual data report. [Google Scholar]
- 6.Clancy JP. Rapid therapeutic advances in CFTR modulator science. Pediatr Pulmonol. 2018;53:S4–S11. doi: 10.1002/ppul.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, et al. VX09-809-102 Study Group. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med. 2014;2:527–538. doi: 10.1016/S2213-2600(14)70132-8. [DOI] [PubMed] [Google Scholar]
- 10.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. TRAFFIC Study Group; TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubert D, Chiron R, Camara B, Grenet D, Prévotat A, Bassinet L, et al. Real-life initiation of lumacaftor/ivacaftor combination in adults with cystic fibrosis homozygous for the Phe508del CFTR mutation and severe lung disease. J Cyst Fibros. 2017;16:388–391. doi: 10.1016/j.jcf.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Burgel PR, Munck A, Durieu I, Chiron R, Mely L, Prevotat A, et al. French Cystic Fibrosis Reference Network Study Group. Real-life safety and effectiveness of lumacaftor-ivacaftor in patients with cystic fibrosis. Am J Respir Crit Care Med. 2020;201:188–197. doi: 10.1164/rccm.201906-1227OC. [DOI] [PubMed] [Google Scholar]
- 13.Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, et al. GOAL (the G551D Observation-AL) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis. 2015;60:703–712. doi: 10.1093/cid/ciu944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guimbellot J, Solomon GM, Baines A, Heltshe SL, VanDalfsen J, Joseloff E, et al. GOALe(2) Investigators. Effectiveness of ivacaftor in cystic fibrosis patients with non-G551D gating mutations. J Cyst Fibros. 2019;18:102–109. doi: 10.1016/j.jcf.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratjen F, Klingel M, Black P, Powers MR, Grasemann H, Solomon M, et al. Changes in lung clearance index in preschool-aged patients with cystic fibrosis treated with ivacaftor (GOAL): a clinical trial. Am J Respir Crit Care Med. 2018;198:526–528. doi: 10.1164/rccm.201802-0243LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris JK, Wagner BD, Zemanick ET, Robertson CE, Stevens MJ, Heltshe SL, et al. Changes in airway microbiome and inflammation with ivacaftor treatment in patients with cystic fibrosis and the G551D mutation. Ann Am Thorac Soc. 2020;17:212–220. doi: 10.1513/AnnalsATS.201907-493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe SM, Khan U, Heltshe S, Donaldson SH, Borowitz D, Gelfond D, et al. Results of a multicenter prospective longitudinal study evaluating the effectiveness of lumacaftor/ivacaftor in F508del homozygous CF patients following FDA approval (PROSPECT Part B Core Study): North American cystic fibrosis conference, Indianapolis, IN. Pediatr Pulmonol. 2017;52:437. [Google Scholar]
- 19.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The cystic fibrosis foundation patient Registry: design and methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 20.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 22.Heltshe SL, Rowe SM, Skalland M, Baines A, Jain M GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Ivacaftor-treated patients with cystic fibrosis derive long-term benefit despite no short-term clinical improvement. Am J Respir Crit Care Med. 2018;197:1483–1486. doi: 10.1164/rccm.201710-2046LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, et al. VX08-770-103 (ENVISION) Study Group. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–1225. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milla CE, Ratjen F, Marigowda G, Liu F, Waltz D, Rosenfeld M VX13-809-011 Part B Investigator Group *. Lumacaftor/ivacaftor in patients aged 6-11 years with cystic fibrosis and homozygous for F508del-CFTR. Am J Respir Crit Care Med. 2017;195:912–920. doi: 10.1164/rccm.201608-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loukou I, Moustaki M, Plyta M, Douros K. Longitudinal changes in lung function following initiation of lumacaftor/ivacaftor combination. J Cyst Fibros. 2020;19:534–539. doi: 10.1016/j.jcf.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5:107–118. doi: 10.1016/S2213-2600(16)30427-1. [DOI] [PubMed] [Google Scholar]
- 28.Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, et al. Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13:139–147. doi: 10.1016/j.jcf.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest. 2013;143:14–18. doi: 10.1378/chest.12-1430. [DOI] [PubMed] [Google Scholar]
- 30.Fidler MC, Beusmans J, Panorchan P, Van Goor F. Correlation of sweat chloride and percent predicted FEV1 in cystic fibrosis patients treated with ivacaftor. J Cyst Fibros. 2017;16:41–44. doi: 10.1016/j.jcf.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Stalvey MS, Pace J, Niknian M, Higgins MN, Tarn V, Davis J, et al. Growth in prepubertal children with cystic fibrosis treated with ivacaftor. Pediatrics. 2017;139:e20162522. doi: 10.1542/peds.2016-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frost FJ, Nazareth DS, Charman SC, Winstanley C, Walshaw MJ. Ivacaftor is associated with reduced lung infection by key cystic fibrosis pathogens: a cohort study using National Registry Data. Ann Am Thorac Soc. 2019;16:1375–1382. doi: 10.1513/AnnalsATS.201902-122OC. [DOI] [PubMed] [Google Scholar]
- 33.LeGrys VA, Moon TC, Laux J, Rock MJ, Accurso F. Analytical and biological variation in repeated sweat chloride concentrations in clinical trials for CFTR modulator therapy. J Cyst Fibros. 2018;17:43–49. doi: 10.1016/j.jcf.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]