Case Vignette
A 64-year-old woman with a history of severe emphysema and prior tobacco use presented to the hospital with acute mixed hypoxemic and hypercapneic respiratory failure after an incidental inhalational exposure. She was sanitizing her bathroom with a mixed solution containing bleach (active ingredient 5% sodium hypochlorite) and 2-butoxyethanol when her family observed acute dyspnea with subsequent unresponsiveness. She arrived at the emergency department with severe hypoxia via pulse oximetry. Venous blood gas was remarkable for a pH of 7.16 and carbon dioxide partial pressure (Pco2) of 83 mm Hg, for which she was emergently intubated. Postintubation arterial blood gas demonstrated a pH of 7.19, Pco2 of 81 mm Hg, and oxygen pressure (Po2) of 81 mm Hg on 40% fractional inspired oxygen (FiO2). The patient was noted to have severe bilateral chemical conjunctivitis requiring immediate bedside ocular irrigation. The remainder of her admission labs were unremarkable, including the urine drug screen. Plain chest radiography was clear (Figure 1), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was negative. Upon arrival to the intensive care unit, assisted volume control with tidal volumes of 360 ml and positive end expiratory pressure (PEEP) of 5 cm H2O generated peak inspiratory pressure of 47 cm H2O, plateau pressure of 34 cm H2O, static compliance of 12.9 ml/cm H2O, dynamic compliance of 11.3 ml/cm H2O, and resistance of 17.6 cm H2O/L/s. Despite adjustments to provide lung protective ventilation, she had significant dynamic hyperinflation (intrinsic PEEP of 13 cm H2O) with hypotension and ventilator dyssynchrony that required lowering the set ventilator rate, deep sedation, and paralysis.
Figure 1.
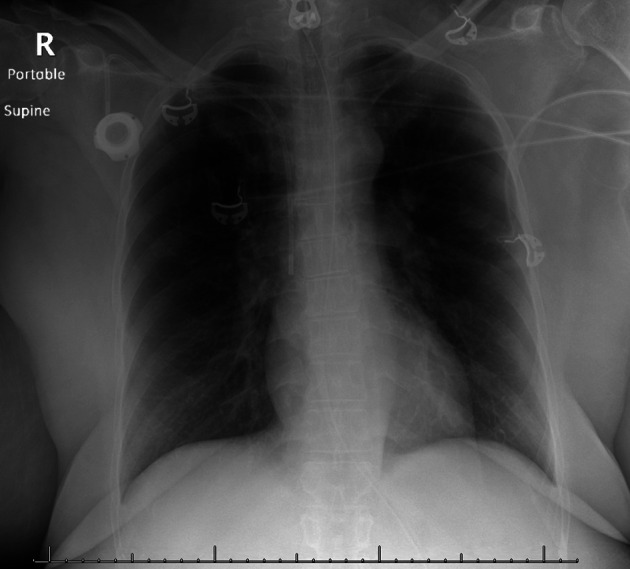
Chest radiography. Anteroposterior film obtained in the emergency department.
Questions
1. Which inhaled toxin is responsible for this patient’s mixed hypoxemic and hypercapnic respiratory failure?
2. What are the clinical characteristics of inhalational gas exposure?
3. What are the most appropriate management strategies for this patient?
Clinical Reasoning
Acute inhalational gas injuries may present with a variety of respiratory signs and symptoms depending on the chemical, dose or concentration, and duration of exposure. There was high clinical suspicion for chemical injury from inhalational toxin exposure. Common household agents causing direct lung injury include chlorine gas produced from the reaction of bleach with other cleaning products (such as the mixing of 5% sodium hypochlorite in bleach and 2-butoxyethanol), trichloramine gas produced from the reaction of bleach with ammonia, direct inhalation of certain products such as sodium dichloroisocyanurate, reduction of bleach compounds such as sodium hydrosulfite, and oxygen gas–induced lung injury from cleaning products containing hydrogen peroxide gas or sodium percarbonate. Additional considerations include acute exacerbation of chronic obstructive pulmonary disease with severe bronchospasm given the patient’s medical history, chemical pneumonitis, aspiration pneumonia, SARS-CoV-2 pneumonia, and acute respiratory distress syndrome (ARDS), which can present initially with a clear chest radiograph (1, 2).
In addition to early intubation, mechanical ventilation, and ocular irrigation, the patient was started on a combination of intravenous methylprednisolone, nebulized bronchodilators for underlying emphysema, and 70%:30% helium:oxygen mixture. Early bronchoscopy demonstrated collapsible proximal airways, scant thin white secretions with minor patches of mucosal erythema, and an abbreviated injury scale score of 1. There was no evidence of carbonaceous deposits, purulence, or endobronchial lesions. Her lung mechanics and gas exchange gradually improved after 1 week of paralysis. On hospital Day 13, she was extubated but quickly required reintubation for hypoxia with stridor. She was continued on systemic corticosteroid and bronchodilator therapy. On hospital Day 16, she underwent a tracheostomy for prolonged ventilator weaning. Repeat airway inspection 1 month into hospitalization demonstrated significant laryngeal edema (Figure 2).
Figure 2.
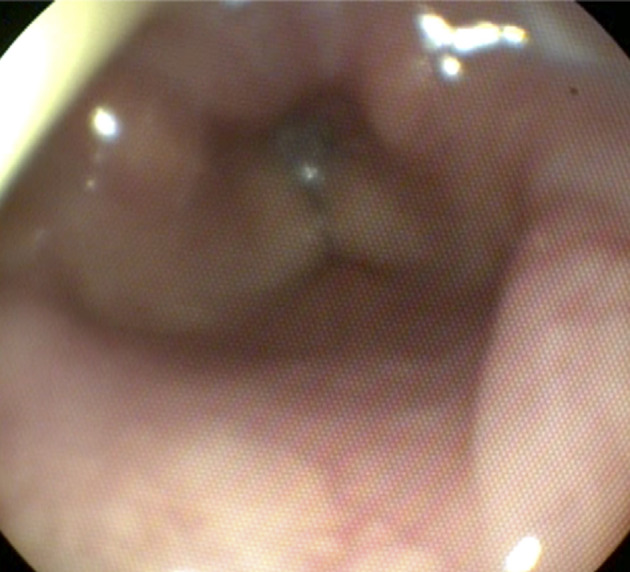
Video laryngoscopy. Laryngoscopic image obtained 1 month post–chlorine gas exposure course demonstrates continued significant supraglottic airway edema.
Discussion
The coronavirus disease (COVID-19) pandemic has led to concerted efforts to educate and mitigate disease spread through public health campaigns about proper hand hygiene and cleanliness (3). Unfortunately, cleaning product supply-chain shortages have led many to pursue makeshift attempts at mixing various store-purchased chemicals to obtain desired disinfection (3). These factors have coincided with a 20% year-over-year increase in cleaner and disinfectant exposures reported to the National Poison Data System and the American Association of Poison Control Centers between January and March (45,550 cases in 2020 vs. 37,822 cases in 2019) and a 43% increase in the incidence of inhalational exposures specifically, driven largely by bleach product use (4, 5).
In our patient, 5% sodium hypochlorite, the main active ingredient in bleach, reacted with 2-butoxyethanol, a widely used commercial solvent in paints and cleaning products, to form chlorine gas (6). Alone, 2-butoxyethanol is associated with respiratory irritation, rapid shallow breathing, and lung congestion (7). At the cell epithelial surface, chlorine gas further reacts with water to form hydrochloric acid, hypochlorous acid, and unstable oxidizing agents (6), damaging mucous membranes and upper airway epithelium, resulting in significant inflammation and bronchospasm (8). Neutrophils are recruited to damaged airway epithelium and release proteolytic enzymes, reactive oxygen species, and free radicals, further contributing to injury (8). Chlorine gas has a dose-dependent effect on the lungs, as follows: mucosal irritation at concentrations of 1–30 ppm, bronchospasm and pulmonary edema at 30–60 ppm, and acute lung injury and ARDS at >60 ppm (9). Exposures of >400 ppm can be lethal (9). Patients with underlying obstructive pulmonary disease are more susceptible to severe symptomatology (10).
Patients with chlorine gas exposure can present with bilateral chemical conjunctivitis, chest discomfort, and respiratory symptoms, including hypoxia, dyspnea, and nonproductive cough. Physical examination may reveal rhonchi and expiratory wheezing. On presentation, patients often have clear chest radiographs despite hypoxia, and the degree of lung injury may be underestimated even in those with abnormal findings (11). Pulmonary function testing typically reveals obstructive physiology (6). Bronchoalveolar lavage may show elevated eosinophil count and inflammatory markers (12). In animal models, histopathology has demonstrated small airways disease, organizing pneumonia, and bronchiolitis obliterans up to 7 days after the initial inhalational insult (9).
Although commercial household cleaning products routinely have labels cautioning users against mixing and about possible toxicities, the wide variety of products available and the critical global supply-chain shortages since the emergence of COVID-19 can change patient safety behavior. Furthermore, even lower range exposures of these products or reaction mixtures can induce severe clinical syndromes in patients with underlying chronic lung disease, with increased risk of respiratory-related inhalational gas injury complications compared with the general population (10). Patients with obstructive lung physiology may confuse the effects of an inhalational injury as an exacerbation of their underlying lung disease and delay medical care.
The management of chlorine gas–induced lung injury is largely supportive (13). However, early recognition of these lung injuries is critical because of the potential for severe airway injury and edema necessitating rapid intubation (14) as well as the potential for unidentified ocular injury in patients with altered mentation (15). Patients with underlying obstructive lung disease have an increased risk of reactive airways disease and bronchospasm (10). Supplemental humidified oxygen therapy should be given for hypoxemia. Inhaled or nebulized β-adrenergic agonists should be given for bronchospasm (8, 13). In severe cases, mechanical ventilation may be necessary. Permissive hypercapnia may be required—balancing low set respiratory rate with adequate alveolar ventilation to avoid dynamic hyperinflation. Airway clearance and secretion management using bronchodilators, nebulized hypertonic saline, airway clearance devices, chest physiotherapy, mechanical insufflation–exsufflation, and early ambulation are believed to be helpful. The combination of 70%:30% helium:oxygen mixture may be used to improve airway laminar flow, although randomized trials have not been performed.
There is limited evidence supporting the use of corticosteroids. Animal models have shown improved lung compliance, mixed venous oxygen saturation, and recovery time, but there are no randomized human trials supporting its use (13). Nebulized sodium bicarbonate has been postulated to neutralize hydrochloric acid at the airway mucosal surface (16), but larger studies show no demonstrable benefit (13). Inhaled mucolytic agents may induce further airway irritation and bronchoconstriction (13). There is no role for antibacterial therapy unless microbiologic data are present to guide use.
Current literature is conflicting on long-term outcomes after chlorine gas exposure; some report uncomplicated recovery (17), whereas others report chronic airflow obstruction and airway hyperresponsiveness years after exposure (8). Critically ill patients, especially those requiring paralysis, are at risk of deconditioning, critical illness myopathy, and psychological disorders from prolonged hospitalization.
The increasing incidence of inhalational gas injuries in the COVID-19 era is concerning despite ongoing cautions and labeling surrounding the dangers of mixing cleaning and disinfectant solutions (3, 4). The medical community needs to remain vigilant in combating the rise of incidental exposures to bleach and other cleaning products during this pandemic.
Answers
1. Which inhaled toxin is responsible for this patient’s mixed hypoxemic and hypercapnic respiratory failure?
The mixing of bleach (active ingredient 5% sodium hypochlorite) and 2-butoxyethanol produces chlorine gas, the toxin almost certainly responsible for her clinical presentation.
2. What are the clinical characteristics of inhalational gas exposure?
Mucocutaneous and respiratory symptoms include bilateral chemical conjunctivitis and upper or lower airway injury that causes nonproductive cough with expiratory wheezing. Airway edema can produce stridor or progressive airway obstruction. The initial chest radiograph is usually clear despite hypoxia.
3. What are the most appropriate management strategies for this patient?
Supportive care with humidified supplemental oxygen, bronchodilation, and airway clearance may avoid intubation with mechanical ventilation. Although there may be limited data supporting corticosteroid therapy, there is no role for nebulized sodium bicarbonate, inhaled mucolytic agents, or empiric antibiotic therapy.
Follow-Up
The patient required frequent respiratory care for heavy secretion burden and was hospitalized for approximately 60 days after her initial inhalational exposure. She was eventually discharged to a skilled nursing facility with a tracheostomy for continued respiratory care and physical therapy.
Insights
• There is an alarming rise in the incidence of bleach and cleaning product misuse in the COVID-19 era. Misuse is likely common despite ongoing public cautions and warnings.
• Early identification of chlorine gas–induced lung injury is important because of the risk of significant mucosal injury that may require airway protection and care for ocular injuries.
• Treatment of chlorine gas–induced lung injury is supportive. Early care focuses on correcting hypoxemia and bronchospasm. Severe cases may require lung protective ventilation.
Footnotes
Supported by U.S. National Institutes of Health grant K08 HL136857 (J.J.R.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J. 2011;87:612–622. doi: 10.1136/pgmj.2011.118398. [DOI] [PubMed] [Google Scholar]
- 2.Endo S, Shibata S, Sato N, Hashiba E, Tajimi K, Saito K, et al. Tohoku ALI Study Group. A prospective cohort study of ALI/ARDS in the Tohoku district of Japan (second report) J Anesth. 2010;24:351–358. doi: 10.1007/s00540-010-0881-x. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2020. Reopening guidance for cleaning and disinfecting public spaces, workplaces, businesses, schools, and homes. [2020 Jun 1]. Available from: cdc.gov/coronavirus/2019-ncov/community/reopen-guidance.html. [Google Scholar]
- 4.Chang A, Schnall AH, Law R, Bronstein AC, Marraffa JM, Spiller HA, et al. Cleaning and disinfectant chemical exposures and temporal associations with COVID-19 - National Poison Data System, United States, January 1, 2020-March 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:496–498. doi: 10.15585/mmwr.mm6916e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuehn BM. Spike in poison control calls related to disinfectant exposures. JAMA. 2020;323:2240. doi: 10.1001/jama.2020.8307. [DOI] [PubMed] [Google Scholar]
- 6.Chester EH, Kaimal J, Payne CB, Jr, Kohn PM. Pulmonary injury following exposure to chlorine gas: possible beneficial effects of steroid treatment. Chest. 1977;72:247–250. doi: 10.1378/chest.72.2.247. [DOI] [PubMed] [Google Scholar]
- 7.Harris O, Wilbur S, George J, Eisenmann C. Toxicological profile for 2-butoxyethanol and 2-butoxyethanol acetate. Washington, DC: U.S. Department of Health and Human Services; 1998. [Google Scholar]
- 8.White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 2010;7:257–263. doi: 10.1513/pats.201001-008SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musah S, Schlueter CF, Humphrey DM, Jr, Powell KS, Roberts AM, Hoyle GW. Acute lung injury and persistent small airway disease in a rabbit model of chlorine inhalation. Toxicol Appl Pharmacol. 2017;315:1–11. doi: 10.1016/j.taap.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mapp CE, Pozzato V, Pavoni V, Gritti G. Severe asthma and ARDS triggered by acute short-term exposure to commonly used cleaning detergents. Eur Respir J. 2000;16:570–572. doi: 10.1034/j.1399-3003.2000.016003570.x. [DOI] [PubMed] [Google Scholar]
- 11.Rehberg S, Maybauer MO, Enkhbaatar P, Maybauer DM, Yamamoto Y, Traber DL. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3:283–297. doi: 10.1586/ERS.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda N, Maghni K, Daigle S, L’Archevêque J, Castellanos L, Al-Ramli W, et al. Long-term pathologic consequences of acute irritant-induced asthma. J Allergy Clin Immunol. 2009;124:975–81.e1. doi: 10.1016/j.jaci.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Huynh Tuong A, Despréaux T, Loeb T, Salomon J, Mégarbane B, Descatha A. Emergency management of chlorine gas exposure - a systematic review. Clin Toxicol (Phila) 2019;57:77–98. doi: 10.1080/15563650.2018.1519193. [DOI] [PubMed] [Google Scholar]
- 14.Madnani DD, Steele NP, de Vries E. Factors that predict the need for intubation in patients with smoke inhalation injury. Ear Nose Throat J. 2006;85:278–280. [PubMed] [Google Scholar]
- 15.Jones R, Wills B, Kang C. Chlorine gas: an evolving hazardous material threat and unconventional weapon. West J Emerg Med. 2010;11:151–156. [PMC free article] [PubMed] [Google Scholar]
- 16.Aslan S, Kandiş H, Akgun M, Cakir Z, Inandi T, Görgüner M. The effect of nebulized NaHCO3 treatment on “RADS” due to chlorine gas inhalation. Inhal Toxicol. 2006;18:895–900. doi: 10.1080/08958370600822615. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Chlorine gas release associated with employee language barrier—Arkansas, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:981–985. [PubMed] [Google Scholar]


