To the Editor:
TBX4 (T-box transcription factor 4) encodes an early embryonic transcription factor that plays an essential role in pulmonary vascular and airway branching (1, 2). TBX4 mutations are associated with pulmonary arterial hypertension (PAH); however, the cellular and molecular mechanisms behind this remain unknown (3–6). Decreased BMP (bone morphogenetic protein)–Smad1/5/8 phosphorylation and increased TGFβ (transforming growth factor β)–SMAD2/3 phosphorylation is linked to PAH in humans and animal models, highlighting the importance of this pathway (7). Interestingly, BMP–Smad signaling is closely related to TBX function, and several TBX family members are direct Smad targets (8–10). On the basis of the close relationship between the TBX family members and the BMP pathway and the critical role of the BMP pathway in PAH pathogenesis, we hypothesized that TBX4 mutations might affect intracellular BMP signaling.
TBX4 is expressed in pulmonary fibroblast, endothelial, and arterial smooth muscle cells (11). We first determined whether the TBX4 expression would affect phospho -Smad1/5 (p-Smad1/5) concentrations in cells. We found that overexpression of TBX4 in fetal lung fibroblast (FLF) and pulmonary artery smooth muscle cells (PASMCs) resulted in increased p-Smad 1/5 concentrations (Figures 1A, 1B and E1A in the data supplement) as determined by Western blot analysis, whereas TBX4 knockout (CRISPR-Cas9–mediated) decreased p-Smad1/5 (Figure 1C). Furthermore, TBX4 knockdown resulted in partial attenuation of the ID1, ID2, and ID3 mRNA, which are direct targets of p-Smad1/5, compared with controls (data not shown).
Figure 1.
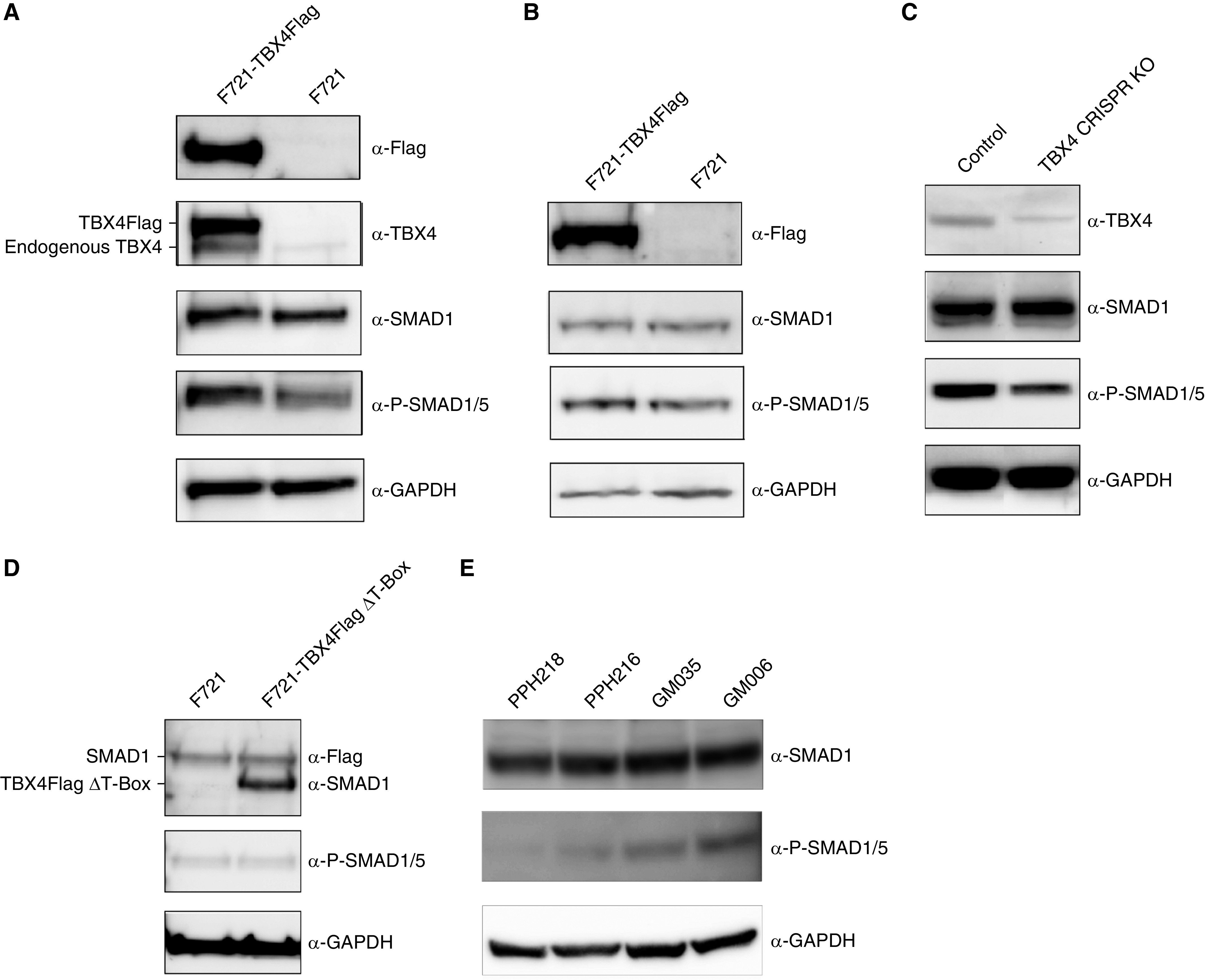
TBX4 (T-Box 4) enhances intracellular phospho-Smad 1/5 concentrations. (A–D) Western blot of cell lysate from TBX4 overexpression in fetal lung fibroblast (FLF) (A), pulmonary artery smooth muscle cells (B), CRISPR-Cas9–mediated TBX4-knockout FLF (C), and FLF cells transiently transfected with F721 or F721-TBX4Flag ΔT-Box (D). (E) Lymphoblasts derived from patients with primary pulmonary hypertension (PPH218 and PPH216) and healthy control subjects (GM035 and GM006). PPH216 is a c.538_547 (CCCTTTGGCC) deletion mutation that leads to frameshift; PPH218 is a TBX4 deletion mutation (chr17:57,972,342–60,472,864). The corresponding antibodies used, anti-Smad1, anti–phospho-Smad1/5, anti-Tbx4, and anti-GAPDH, are shown in the figure. KO = knockout; P = phospho.
To determine the functional TBX4 domain for this effect, we focused on the highly conserved DNA binding T-box domain. Seventy-five percent of missense mutations seen in TBX4 occur within the T-box domain (Figure E1B) (1, 12). We found that two-thirds of these mutations were in the conserved amino acids as identified by Ruvinsky and colleagues (Figure E1C) (13), and one-third were either adjacent to or just one amino acid away from the conserved amino acids (Figure E1C). We deleted the T-Bbox domain (71aa to 251aa of TBX4) in the expression vector F721-TBX4Flag ΔT-Box. The expression of this vector in FLF showed that TBX4 without T-box failed to increase p-Smad1/5 compared with control (compare Figure 1A to Figure 1D). These results showed that TBX4 affected intracellular p-Smad1/5 concentrations through its T-box domain.
We analyzed available RNA-sequencing expression data (GSE27661) (14) and found that TBX4 expression is upregulated when pulmonary artery smooth muscle cells PASMCs and human umbilical vein endothelial cells are exposed to BMPs (Figure E2A). To further confirm these data, we treated pulmonary artery smooth muscle cells with BMP4 and found that congruent with the RNA-sequencing data, TBX4 mRNA concentrations were higher in the cells treated with BMP4 (Figure E2B). These data suggested that TBX4 may be a target of Smad pathway. We scanned TBX4 proximal promoter region (−558 to −1) and found a TBX binding motif, a Smad binding element, as well as a cluster of BMP response elements/5GC Smad binding element sites (Figure E3A) (14, 15). To determine whether TBX4 and p-Smad1/5 bind to this region, we performed chromatin IP (ChIP)-qPCR assay. We found that p-Smad1/5 and TBX4 specifically bound the TBX4 upstream region compared with controls (Figure E3B). These data showed that the TBX4 upstream region is a direct target of p-Smad1/5 and TBX4, suggesting that TBX4 is autoregulated by TBX4 and regulated by p-Smad1/5.
We then used a luciferase reporter assay to confirm the ChIP data. We designed an expression vector in which the region −717 bp to −8 bp of TBX4 proximal promoter (contains all the TBX and Smad1/5 binding motifs) was inserted in front of a luciferase reporter herein referred to as pGL4.23-TBX4 reporter (Figure E3A). We then coexpressed this vector together with an increasing dose of vector F721-TBX4 (expressing TBX4) or F721-TBX4ΔT-Box in FLF and PASMCs. We found that in both PASMCs and FLF, pGL4.23-TBX4 reporter luciferase activity increased in a dose-dependent manner with an increasing dose of F721-TBX4 but not with the control reporter pGL4.23. Coexpression of F721-TBX4ΔT-Box did not increase pGL4.23-TBX4 luciferase expression (Figures E4A and E4B). These data show that TBX4 can transactivate its transcription, which is consistent with the ChIP data showing that TBX4 bound to TBX4 promoter (Figure E3B).
We then compared two PAH patient-derived cultured lymphocyte cell lines with two control lines. One cell line, PPH216, carries a frameshift deletion, whereas PPH218 has a complete deletion of TBX4. We found that the p-Smad1/5 concentrations were significantly lower in the TBX4-mutant cell lines compared with the control cell lines (Figures 1E and E5A).
The data presented in this letter show that overexpression of TBX4 increased intracellular concentrations of p-Smad1/5, and its downregulation decreased p-Smad1/5. Furthermore, BMP stimulation upregulated TBX4 transcription and increased p-Smad1/5 concentrations. And, TBX4 bound to the TBX4 upstream proximal promoter region, suggesting a positive feedback loop between TBX4 and p-Smad1/5. Importantly p-Smad1/5 concentrations were significantly lower in the TBX4-mutant patient with PAH–derived cell lines (see model in Figure E5B).
Given these data, we hypothesize that TBX4 mutations may lead to a lower baseline intracellular p-SMAD1/5 concentration, resulting in decreased activity of downstream BMP signaling cascades. Diminished BMP signaling may subsequently increase the risk for abnormal lung development, response to injury, or both, which could increase the risk of developing PAH. This hypothesis could provide an initial framework to explore how TBX4 mutations may cause PAH in future studies.
Supplementary Material
Footnotes
Supported by the U.S. National Institutes of Health awards P01 HL 108800 (E.D.A.), R01 HL134802 (E.D.A.) and R01 HL 102020 (R.H.) as well as the Cardiovascular Medical Research and Education Fund (E.D.A.).
Author Contributions: Y.C. conducted experiments, analyzed data, and wrote the manuscript. L.Y. conducted experiments and analyzed data. J.D.C. analyzed data. M.J.K., L.K.H., and B.N. conducted experiments. Y.C., L.Y., J.W., E.D.A., and R.H. conceived the study; designed the experiments; acquired, analyzed, and interpreted the data; and wrote and edited the manuscript.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–2693. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- 2.Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 2012;8:e1002866. doi: 10.1371/journal.pgen.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy M, Eyries M, Szezepanski I, Ladouceur M, Nadaud S, Bonnet D, et al. Genetic analyses in a cohort of children with pulmonary hypertension. Eur Respir J. 2016;48:1118–1126. doi: 10.1183/13993003.00211-2016. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Rivas G, Jerjes-Sánchez C, Rodriguez D, Garcia-Pelaez J, Trevino V. A systematic review of genetic mutations in pulmonary arterial hypertension. BMC Med Genet. 2017;18:82. doi: 10.1186/s12881-017-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N, Gonzaga-Jauregui C, Welch CL, Ma L, Qi H, King AK, et al. Exome sequencing in children with pulmonary arterial hypertension demonstrates differences compared with adults. Circ Genom Precis Med. 2018;11:e001887. doi: 10.1161/CIRCGEN.117.001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew JD, Loyd JE, Austin ED. Genetics of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2017;38:585–595. doi: 10.1055/s-0037-1606201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tielemans B, Delcroix M, Belge C, Quarck R. TGFβ and BMPRII signalling pathways in the pathogenesis of pulmonary arterial hypertension. Drug Discov Today. 2019;24:703–716. doi: 10.1016/j.drudis.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Cho KW, Kim JY, Song SJ, Farrell E, Eblaghie MC, Kim HJ, et al. Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development. Proc Natl Acad Sci USA. 2006;103:16788–16793. doi: 10.1073/pnas.0604645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirai M, Imanaka-Yoshida K, Schneider MD, Schwartz RJ, Morisaki T. T-box 2, a mediator of Bmp-Smad signaling, induced hyaluronan synthase 2 and Tgfbeta2 expression and endocardial cushion formation. Proc Natl Acad Sci USA. 2009;106:18604–18609. doi: 10.1073/pnas.0900635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behesti H, Holt JK, Sowden JC. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol. 2006;6:62. doi: 10.1186/1471-213X-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie T, Liang J, Liu N, Huan C, Zhang Y, Liu W, et al. Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J Clin Invest. 2016;126:3063–3079. doi: 10.1172/JCI85328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galambos C, Mullen MP, Shieh JT, Schwerk N, Kielt MJ, Ullmann N, et al. Phenotype characterisation of TBX4 mutation and deletion carriers with neonatal and paediatric pulmonary hypertension. Eur Respir J. 2019;54:1801965. doi: 10.1183/13993003.01965-2018. [DOI] [PubMed] [Google Scholar]
- 13.Ruvinsky I, Oates AC, Silver LM, Ho RK. The evolution of paired appendages in vertebrates: T-box genes in the zebrafish. Dev Genes Evol. 2000;210:82–91. doi: 10.1007/s004270050014. [DOI] [PubMed] [Google Scholar]
- 14.Morikawa M, Koinuma D, Tsutsumi S, Vasilaki E, Kanki Y, Heldin CH, et al. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res. 2011;39:8712–8727. doi: 10.1093/nar/gkr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldron L, Steimle JD, Greco TM, Gomez NC, Dorr KM, Kweon J, et al. The cardiac TBX5 interactome reveals a chromatin remodeling network essential for cardiac septation. Dev Cell. 2016;36:262–275. doi: 10.1016/j.devcel.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


