Abstract
Recently, we characterized blue light–mediated relaxation (photorelaxation) of airway smooth muscle (ASM) and implicated the involvement of opsin 3 (OPN3), an atypical opsin. In the present study, we characterized the cellular signaling mechanisms of photorelaxation. We confirmed the functional role of OPN3 in blue light photorelaxation using trachea from OPN3 null mice (maximal relaxation 52 ± 13% compared with wild-type mice 90 ± 4.3%, P < 0.05). We then demonstrated colocalization of OPN3 and Gαs using co-IP and proximity ligation assays in primary human ASM cells, which was further supported by an increase in cAMP in mouse trachea treated with blue light compared with dark controls (23 ± 3.6 vs. 14 ± 2.6 pmol cAMP/ring, P < 0.05). Downstream PKA (protein kinase A) involvement was shown by inhibiting photorelaxation using Rp-cAMPS (P < 0.0001). Moreover, we observed converging mechanisms of desensitization by chronic β2-agonist exposure in mouse trachea and correlated this finding with colocalization of OPN3 and GRK2 (G protein receptor kinase) in primary human ASM cells. Finally, an overexpression model of OPN1LW (a red light photoreceptor in the same opsin family) in human ASM cells showed an increase in intracellular cAMP levels following red light exposure compared with nontransfected cells (48 ± 13 vs. 13 ± 2.1 pmol cAMP/mg protein, P < 0.01), suggesting a conserved photorelaxation mechanism for wavelengths of light that are more tissue penetrant. Together, these results demonstrate that blue light photorelaxation in ASM is mediated by the OPN3 receptor interacting with Gαs, which increases cAMP levels, activating PKA and modulated by GRK2.
Keywords: photorelaxation, blue light, OPN3, nonvisual opsin, OPN1LW
Over the past decade, there have been a number of unconventional sensory GPCRs (G protein–coupled receptors) found to be endogenously expressed in airway smooth muscle (ASM) (1, 2). Interestingly, these atypical sensory GPCRs have been determined to exert prorelaxant effects. For example, bitter taste receptors have been found to be expressed in ASM and their activation has been shown to mediate potent relaxation (1). Similarly, odorant receptors were also found to modulate ASM tone (2). In our recent study, we demonstrated “photorelaxation” of ASM by blue wavelength light (3). We also determined that this effect is mediated through the GPCR opsin 3 (OPN3). Opsins were first observed in smooth muscle more than a half century ago in rabbit aortic smooth muscle and have been described in an increasing number of tissues, such as brain, skin, and a variety of smooth muscle beds, including but not limited to those found in vasculature, the uterus, and the airway (3–9). In the current study, we investigate the mechanism of a visual sensory receptor, specifically OPN3, which is a light receptor that has been demonstrated to relax ASM in response to blue wavelengths of light (3).
Opsins are typically associated with the retina in mediating vision. A well-studied opsin is rhodopsin (OPN2), which facilitates scotopic vision. Photopic vision is mediated through opsin 1 (OPN1), which are stratified as red (OPN1LW), green (OPN1MW), and blue (OPN1SW). Opsins that mediate dark–light and color vision in the retina are considered visual opsins. In contrast, there has been recent interest in understanding what are called “atypical opsins,” which are located in extraocular tissues, many of which are located in deep tissue beds. The functional role of atypical opsins in physiology is poorly understood. A well-studied atypical opsin is melanopsin, also known as opsin 4 (OPN4). It was recently determined that blue light exhibits a relaxation effect in mouse tail artery through OPN4 (4). The same group implicated a similar blue light–mediated vasorelaxation in pulmonary vasculature that occurs through both OPN4 and OPN3 (5). These photorelaxation effects were also shown to be regulated by GRK2 (G protein receptor kinase) activity, which is well known to phosphorylate and desensitize a variety of activated GPCRs including the β2 adrenoceptor (β2AR) (10, 11). We recently demonstrated in ASM the expression of OPN3, blue light–mediated relaxation, and modulation by GRK2. However, the molecular mechanisms of photorelaxation in ASM remain unclear.
Unique to opsins is the coactivation of the receptor by light through an associated chromophore ligand. Chromophores associate with the opsin apoprotein in its binding pocket and mediate phototransduction typically by the photoisomerization of the chromophore from cis to trans upon the absorption of light energy. The subsequent downstream signaling occurs through a variety of G proteins, such as transducin (Gαt), which is well known to mediate visual pigment phototransduction in the eye. Although we had initially demonstrated expression of Gαt mRNA, we and others have been unable to substantiate protein-level expression (12). Thus, the classic pathway of phototransduction is likely not responsible for ASM photorelaxation by OPN3. OPN3 has been observed to couple with Gαi in melanocytes and Gαs in jellyfish (7, 13). We sought to determine the G protein alpha subunit that associates with OPN3 during ASM light-mediated relaxation. In ASM, these signaling pathways are often classified as procontractile or prorelaxant, depending on the associated G protein (10). A canonical prorelaxant GPCR classically couples to Gαs, which activates adenylyl cyclase (AC) to synthesize cAMP which in turn activates PKA (protein kinase A), exchange proteins activated by cAMP (EPACs), and perhaps additional targets to facilitate relaxation (14–17).
In the present study, we demonstrate that blue light photorelaxation is mediated through OPN3 interactions with Gαs to increase cAMP through AC leading to the activation of PKA, with OPN3 receptor regulation by GRK2. Additionally, we demonstrate conservation of cAMP synthesis mediated by OPN1LW, a related opsin with wavelength sensitivities in the red light spectrum, in human ASM cells.
Methods
Detailed methods are described in the data supplement.
Reagents and Chemicals
G protein receptor kinase 2 inhibitor (GRK2i) (Methyl-5-[(E)-2-(5-nitrofuran-2-yl)ethenyl]furan-2-carboxylate) was purchased from Santa Cruz. 9-cis retinal was purchased from Sigma Aldrich. GRK2i and 9-cis-retinal was used to minimize receptor desensitization and chromophore bleaching, respectively, to environmental light. 9-cis retinal is commonly used instead of 11-cis retinal, the endogenous chromophore in the retina, owing to increased chromophore stability of 9-cis retinal in presence of ambient light (18, 19). We have previously demonstrated significantly enhanced photorelaxation upon inclusion of GRK2i and 9-cis-retinal in our previous study (3).
Cell Culture
Primary ASM cells were a kind gift from Dr. Reynold A. Panettieri, and the immortalized ASM cells were a kind gift from Dr. William Gerthoffer. Both lines were previously characterized (20, 21). Cells were cultured in 5% CO2/95% O2 at 37°C with 10% FBS, antibiotics, and Ham’s F12 or M199 basal media, respectively.
OPN3 Null Mouse Model
OPN3 null mice (OpnlacZ/lacZ) were established and characterized by Dr. Richard Lang’s group (22). Excised tracheas were shipped overnight in media along with trachea from control wild-type mice for wire myography and Western blotting.
Tracheal Ring Preparation and Wire Myography
Animal tissue procedures were approved by Columbia University Institutional Animal Care and Use Committee. C57BL/6 male and female mice were killed with 50 mg/kg pentobarbital. Tracheas were excised and prepared for wire myography as previously described (3). See data supplement for experimental details.
Mouse Trachea ASM Lysate Preparation
See data supplement.
Western Blot
See data supplement.
Proximity Ligation Assay
Primary human ASM cells were grown to subconfluence. Cells were washed with PBS, fixed with 4% paraformaldehyde in PBS for 10 minutes, and washed again. Cells were permeabilized with 0.1% Triton-X in PBS for 10 minutes and washed with PBS. The Duolink proximity ligation assay (PLA) kit in red with rabbit/mouse secondary antibodies (Sigma Aldrich) was used according to the manufacturer’s instructions, with a custom 2-hour primary antibody incubation. Punctate red fluorescence indicates that the targeted antigens are within 40 nm. Details of PLA and imaging are further described in the data supplement.
Co-IP
See data supplement.
Cell Transfection
See data supplement.
Statistical Analysis
Student’s two-tailed t test, one-tailed ANOVA with Bonferroni post hoc test, and nonlinear curve fitting was performed using Prism 4 (GraphPad). Data are presented as mean ± SEM with α = 0.05.
Results
OPN3 Expression Is Critical for Blue Light Photorelaxation in Mouse Trachea
We previously implicated OPN3 in blue light–mediated photorelaxation in ASM (3). Here, we sought to confirm OPN3 involvement using mice with global genetic deletion of OPN3. Excised tracheal rings from wild-type and OPN3 null mice were mounted on a wire myograph, contracted with an EC50 concentration of acetylcholine, and pretreated with 9-cis retinal before blue light treatment to discern function by basal OPN3 expression. OPN3 null mice demonstrated a 52 ± 13% relaxation normalized to the maximum relaxation achieved with isoproterenol in comparison with tracheal rings from wild-type mice, which exhibited 90 ± 4.3% relaxation (*P < 0.05) (n = 4–5) (Figure 1A). The absence of the OPN3 protein in trachea from OPN3 null mice was confirmed by Western blot (Figure 1B), with β-actin used as a protein loading control, demonstrating equivalent protein loading. A representative tracing of photorelaxation attenuation in null mice is demonstrated in Figure 1C. A representative tracing of isoproterenol-mediated relaxation (maximal relaxation) is demonstrated in Figure 1D. These data reveal the importance of OPN3 in blue light–mediated relaxation.
Figure 1.
Mice globally genetically deficient in OPN3 demonstrate significantly reduced blue light–induced photorelaxation of an acetylcholine-induced contraction in tracheal rings ex vivo. (A) Muscle force in wire myography demonstrating decreased photorelaxation in tracheal rings from OPN3 null compared with wild-type mice (52 ± 13% vs. 90 ± 4.3% of maximal isoproterenol relaxation). *P < 0.05. n = 4–5. (B) Immunoblot of OPN3 from null mice confirming absence of OPN3 protein and from wild-type mice demonstrating an immunoreactive band at the predicted molecular mass. Immunoblot of β-actin as loading control demonstrated equivalent total protein loading. Lysate of this immunoblot represents tracheal muscle strips from 9 null or wild-type mice. (C) Representative muscle force tracing of decreased photorelaxation of an acetylcholine-induced contraction in tracheal rings from OPN3 null (gray) compared with wild-type mice (black). Arrowhead indicates the time point where light was administered. (D) Representative muscle force tracing of comparable maximal relaxation of an acetylcholine-induced contraction by the β-agonist isoproterenol in tracheal rings from OPN3 null (gray) and wild-type mice (black). Max = maximum; OPN3 = opsin 3.
Blue Light Photorelaxation Increases cAMP in Mouse Tracheal ASM
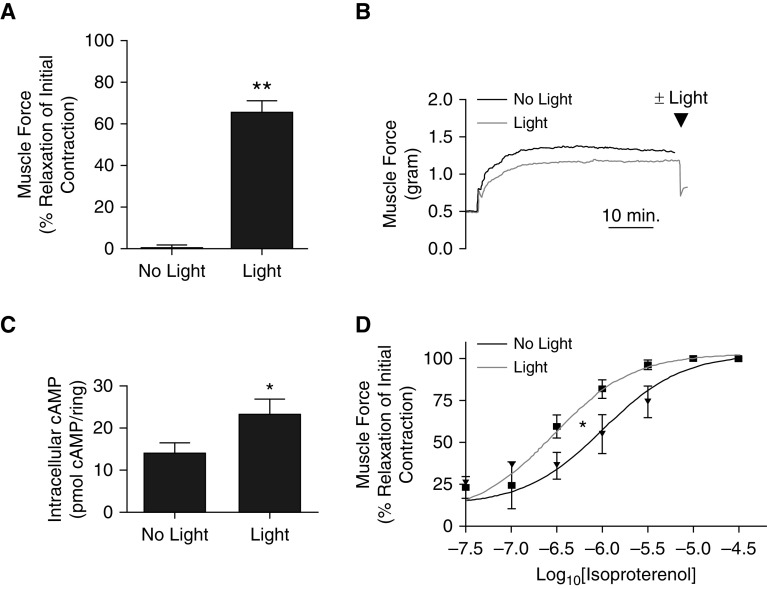
Upon confirming the role of OPN3 in blue light photorelaxation, we sought to determine whether these prorelaxant signaling events involved the classic mediator cAMP. Thus, we assayed cAMP levels in tracheal rings removed from organ baths after blue light–mediated relaxation and compared them with trachea from dark-treated controls. All tracheal rings were contracted with EC50 acetylcholine and pretreated with GRK2i and 9-cis retinal. Then, rings were treated with or without blue light. Those treated with light exhibited a 65 ± 5.8% relaxation normalized to maximum contraction, whereas those left in the dark had a 0.16 ± 1.7% change in muscle force. Tracheas treated with light exhibited significantly more relaxation (**P < 0.001) (n = 11) (Figures 2A and 2B). Rings exposed to blue light contained 23 ± 3.6 pmol cAMP/ring, whereas the tracheas that were not exposed to light had significantly less cAMP, at 14 ± 2.6 pmol cAMP/ring (*P < 0.05) (n = 14) (Figure 2C). Figure 2D demonstrates increased relaxation to isoproterenol when concurrently treated with blue light in comparison with isoproterenol alone (*P < 0.05) (n = 3). These findings suggest a role for cAMP in blue light–mediated relaxation.
Figure 2.
Blue light photorelaxation of an acetylcholine-induced contraction increased cAMP in mouse trachea. (A) Muscle force in wire myography demonstrating increased relaxation in tracheal rings treated with blue light compared with tracheas kept in the dark (65 ± 5.8% vs. 0.16 ± 1.7% relaxation of initial contraction). **P < 0.001. n = 11. (B) Representative muscle force tracing of tracheal rings with (gray) and without blue light (black). Arrowhead indicates the time point where light was administered. (C) Tracheal rings exposed to blue light contained significantly increased levels of cAMP compared with tracheal rings kept in the dark (23 ± 3.6 vs. 14 ± 2.6 pmol cAMP/ring). *P < 0.05. n = 14. (D) Addition of blue light (gray) significantly left-shifts the isoproterenol dose response curve to enhance smooth muscle relaxation of an acetylcholine-induced contraction compared with tracheal rings kept in the dark (black). *P < 0.05. n = 3.
PKA Inhibition Attenuates Photorelaxation Response
To confirm the involvement of PKA, a downstream target of cAMP, in blue light–mediated photorelaxation, we sought to assess the photorelaxation response with and without pretreatment with Rp-cAMPS, a competitive PKA inhibitor, in the presence of GRK2i, 9-cis retinal, and EC50 acetylcholine. Inclusion of Rp-cAMPS during pretreatment significantly attenuated relaxation induced by an intensity-dependent blue light exposure (**P < 0.0001) (n = 3–4) (Figures 3A and 3B). As a positive control, Rp-cAMPS pretreatment successfully attenuated a 1-μM forskolin-induced relaxation response (*P < 0.005) (n = 3–4) (Figures 3C and 3D). A time control demonstrates no significant change in muscle tension over time in the presence or absence of Rp-cAMPS (P > 0.05) (n = 3) (Figures 3E and 3F). These findings implicate a role for PKA in blue light–mediated relaxation.
Figure 3.
PKA inhibition with Rp-cAMPS attenuates photorelaxation of an acetylcholine-induced contraction in mouse trachea. (A) Muscle force in wire myography demonstrating decreased blue light photorelaxation (dose response of 1–5%, 20%, 27%, and 100% light intensities) in tracheal rings pretreated with Rp-cAMPS (circle) compared with control rings (square). **P < 0.0001. n = 3–4. Gray lines indicate SEM. (B) Representative muscle force tracings of tracheal rings with (gray) and without Rp-cAMPS pretreatment upon a dose response of blue light (1–5%, 20%, 27%, and 100%) (black). (C) Muscle force in wire myography demonstrating decreased forskolin-mediated relaxation in tracheal rings pretreated with Rp-cAMPS (circle) compared with control rings (square). *P < 0.005. n = 3–4. Gray lines indicate SEM. (D) Representative wire myography tracing of tracheal rings with (gray) and without Rp-cAMPS pretreatment upon administration of forskolin (arrowhead) (black). (E) Muscle force in wire myography demonstrating no significant change in muscle force over time in tracheal rings pretreated with Rp-cAMPS (circle) compared with control rings (square). n = 3. Gray lines indicate SEM. (F) Representative wire myography tracing of tracheal rings with (gray) and without Rp-cAMPS pretreatment over time (black). PKA = protein kinase A.
Pretreatment with β2 Adrenergic Receptor Agonist Promotes Desensitization of the Photorelaxation Response
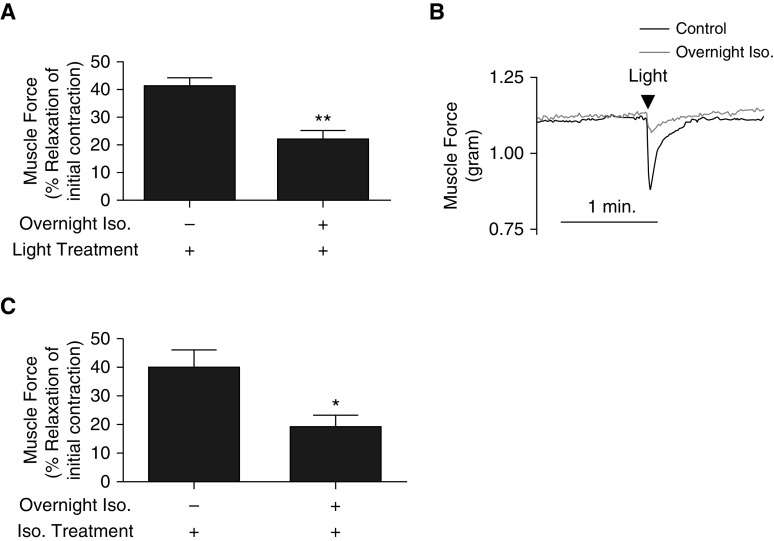
Our group previously demonstrated that GRK2 inhibition significantly enhanced blue light–mediated relaxation in ASM (3). This effect of GRK2 inhibition on photorelaxation has also been previously observed in the vasculature (3–5). It is known that a method of β2AR desensitization is GRK2 activation (11). We questioned whether increased GRK2 activity due to prolonged β2AR agonism would be able to heterologously desensitize opsin-mediated photorelaxation. Tracheal rings were treated with or without the 1 μM isoproterenol overnight in cell culture media. On the day of the experiment, rings were contracted with EC50 acetylcholine. We observed significantly decreased relaxation in response to blue light with 22 ± 3.2% relaxation normalized to maximum contraction in isoproterenol-treated tracheal rings compared with 41 ± 3.1% relaxation in control untreated tracheal rings (**P < 0.0005) (n = 9–10) (Figures 4A and 4B). As a positive control to confirm successful β2AR desensitization from overnight isoproterenol exposure, tracheal rings exposed to chronic isoproterenol demonstrated reduced relaxation in response to an acute isoproterenol treatment compared with control unexposed tracheal rings (40 ± 6.1% vs. 19 ± 4.1% relaxation of initial contraction) (*P < 0.05) (n = 9–10) (Figure 4C). These findings suggest potential β2AR-OPN3 heterologous receptor desensitization mediated by GRK2 activity.
Figure 4.
β2AR desensitization also attenuates photorelaxation in mouse trachea. (A) Muscle force in wire myography demonstrating decreased blue light photorelaxation in tracheal rings pretreated with overnight Iso compared with control rings without overnight Iso (22 ± 3.2% vs. 41 ± 3.1% relaxation of initial acetylcholine-induced contraction). **P < 0.0005. n = 9–10. (B) Representative muscle force tracing of tracheal rings with (gray) and without overnight Iso upon blue light treatment (arrowhead) (black). (C) Muscle force in wire myography demonstrating decreased acute Iso-mediated relaxation of an acetylcholine-induced contraction (confirming β2AR desensitization) in tracheal rings pretreated with overnight Iso compared with rings without overnight Iso (19 ± 4.1% vs. 40 ± 6.1% relaxation of initial contraction). *P < 0.05. n = 9–10. β2AR = β2 adrenoceptor; Iso. = isoproterenol.
Physical Interaction of OPN3 with Gαs and GRK2 in Primary Human ASM Cells
We sought to assess the association between Gαs to OPN3 using primary cultures of human ASM cells. PLAs demonstrated a <40 nm proximity between OPN3 and Gαs (n = 3) (Figure 5A). Control experiments were performed by separately omitting the primary antibodies for OPN3 or Gαs (mouse) to confirm the specificity of the fluorescent signal (Figures 5B and 5C). Next, we sought to assess the interaction between GRK2 and OPN3. PLAs demonstrated a <40 nm proximity between OPN3 and GRK2 (n = 3). GRK2–OPN3 colocalization signaling increased upon overnight desensitization with isoproterenol in the absence of light. Primary antibodies were each separately omitted demonstrating loss of PLA signal (negative controls) (Figures 5C–5F). As a positive control, PLAs demonstrated a <40 nm proximity between β2AR and Gαs (n = 3) (Figure 5G). Again, primary antibodies each incubated alone for β2AR and Gαs (rabbit) along with the respective secondary antibodies resulted in diminished fluorescent signal (Figures 5H and 5I). As a negative control, PLAs demonstrated a >40 nm proximity between OPN3 and MLCK (Figure 5J). To confirm a colocalization between Gαs and OPN3, a co-IP assay was performed, where sample lysate from primary cultures of human ASM cells was subjected to co-IP with a primary antibody directed against Gαs. Subsequent immunoblot analysis of this immunoprecipitate using a primary antibody directed against OPN3 demonstrated the co-IP of Gαs and OPN3, and omission of the OPN3 primary antibody during the Western blot resulted in no immunoreactive bands detected (n = 3) (Figure 5K). To confirm physical interaction between OPN3 and GRK2, co-IP was performed using a primary antibody against GRK2 followed by a Western blot using an OPN3 primary antibody (n = 3). Omission of the OPN3 primary antibody during the Western blot assay resulted in no immunoreactive bands detected (n = 3) (Figure 5L). To note, OPN3 antibody specificity validation was confirmed from the Western blot depicted in Figure 1 with OPN3 null and wild-type mouse tracheas. These data confirm the colocalization and association of Gαs and OPN3 as well as GRK2 and OPN3.
Figure 5.
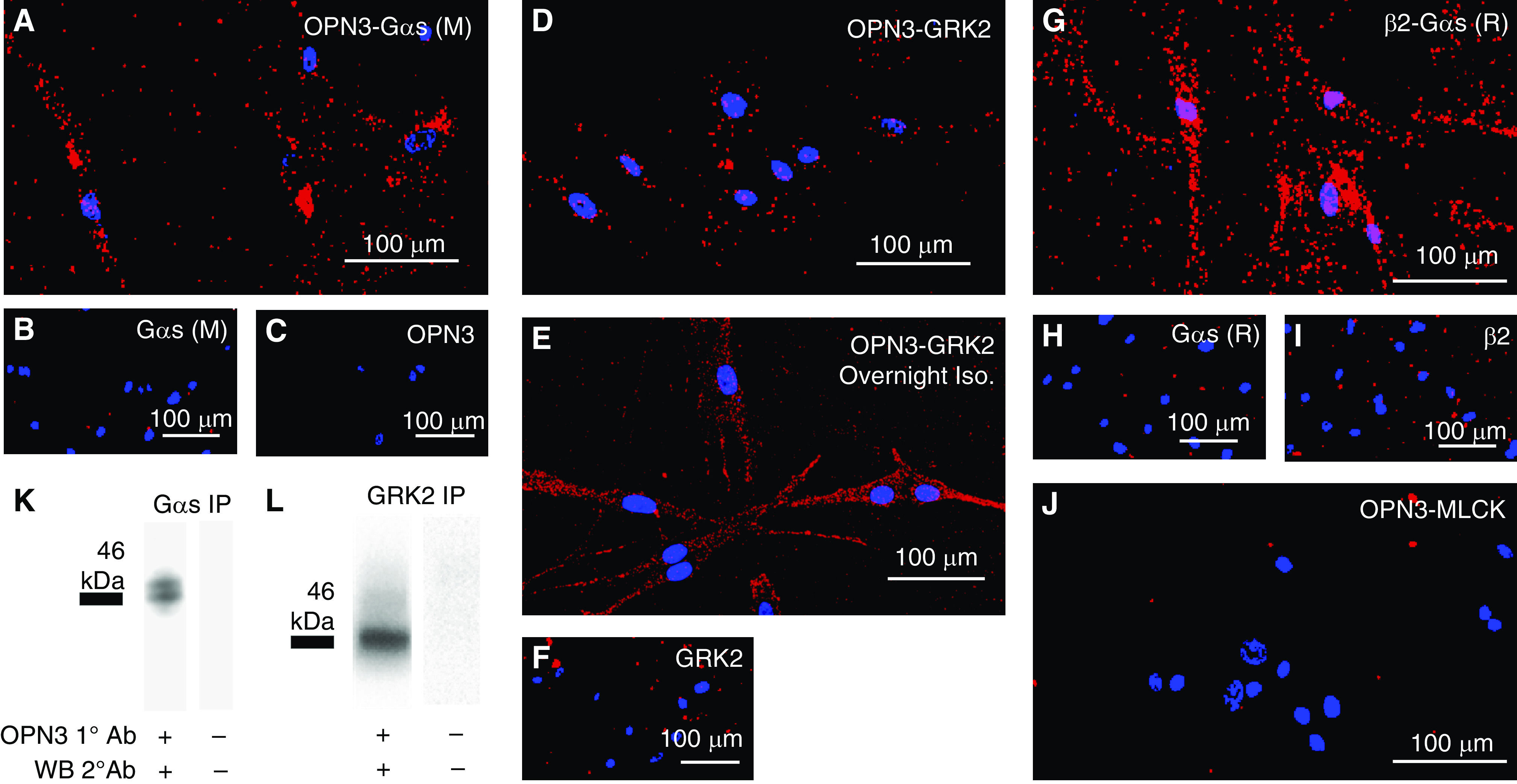
OPN3 colocalizes with Gαs and GRK2 in human ASM cells. (A) PLA of OPN3-Gαs indicating that OPN3 and Gαs are within 40 nm proximity of one another (representative image from three experiments). (B and C) PLA in which one of the primary antibodies within the OPN3–Gαs binding pair were individually omitted demonstrating the absence of red punctate signaling (negative control). (D) PLA of OPN3–GRK2 indicating that OPN3 and GRK2 are within 40 nm proximity of one another (representative image from three experiments). (E) OPN3 and GRK2 40 nm proximity is increased upon desensitization with overnight Iso in the absence of light stimulus. (C and F) PLA in which one of the primary antibodies within the OPN3–GRK2 binding pair was individually omitted demonstrating the absence of red punctate signaling (negative control). (G) PLA of β2AR–Gαs indicating that β2AR and Gαs are within 40 nm proximity of one another (positive control) (representative image from three experiments). (H and I) PLA in which one of the primary antibodies within the β2AR–Gαs binding pair was individually omitted demonstrating the absence of red punctate signaling (negative control). (J) PLA of OPN3–MLCK indicating that OPN3 and MLCK are not within 40 nm proximity of one another (negative control). All scale bars represent 100 μm. (K) Co-IP of Gαs followed by Western blot analysis of OPN3 protein indicating a positive physical association between OPN3 and Gαs upon blue light treatment (representative gel image of three independent experiments). (L) Co-IP of GRK2 followed by Western blot analysis of OPN3 protein indicating a positive physical association between OPN3 and Gαs upon blue light treatment (representative gel image of three independent experiments). Scale bars, 100 μm. 1º = primary; 2º = secondary; Ab = antibody; ASM = airway smooth muscle; GRK2 = G protein receptor kinase; PLA = proximity ligation assay; WB = Western blot.
OPN1LW Overexpression in Immortalized Human ASM Cells Demonstrate Increased cAMP in Response to Red Light
Blue light is a relatively short wavelength of electromagnetic radiation. It is well established that short wavelengths of light are less tissue penetrant than longer wavelengths of electromagnetic radiation, such as wavelengths in the infrared and red ranges. OPN1LW is typically found in the retina and is responsible for “red perception.” Thus, we created an OPN1LW overexpression model in an immortalized ASM cell line to determine whether prorelaxant signaling could be demonstrated. Figure 6A is a fluorescent microscope image of the ASM cells on the first day after transfection with a plasmid coexpressing OPN1LW and GFP, and Figure 6B shows nontransfected cells, which lack green fluorescence. Figure 6C demonstrates green fluorescence from successfully transfected cells after antibiotic selection. RT-PCR of nontransfected cells confirmed the absence of OPN1LW mRNA (data not shown). Figures 6D–6F are the corresponding light microscope images to Figures 6A–6C. Upon exposure to red light, we observed a significant increase in cAMP levels in OPN1LW-transfected cells pretreated with GRK2i and 9-cis retinal (48 ± 13 pmol cAMP/mg protein) compared with nontransfected cells pretreated with GRK2i and 9-cis retinal (13 ± 2.1 pmol cAMP/mg protein) (**P < 0.01) (n = 4). OPN1LW-transfected cells pretreated with GRK2i and 9-cis retinal and exposed to red light also showed a significant increase in cAMP levels (48 ± 13 pmol cAMP/mg protein) compared with OPN1LW-transfected cells pretreated with GRK2i and 9-cis retinal but not exposed to red light (kept in dark) (17 ± 5.2 pmol cAMP/mg protein) (*P < 0.05) (n = 4) (Figure 6G). This red light–mediated increase in cAMP in OPN1LW-transfected cells pretreated with GRK2i and 9-cis retinal and exposed to red light (48 ± 13 pmol cAMP/mg protein) is not significantly different from OPN1LW-transfected cells pretreated with GRK2i and 9-cis retinal and treated with 1 μM forskolin in the absence of light (24 ± 11 pmol cAMP/mg protein) (n = 4).
Figure 6.
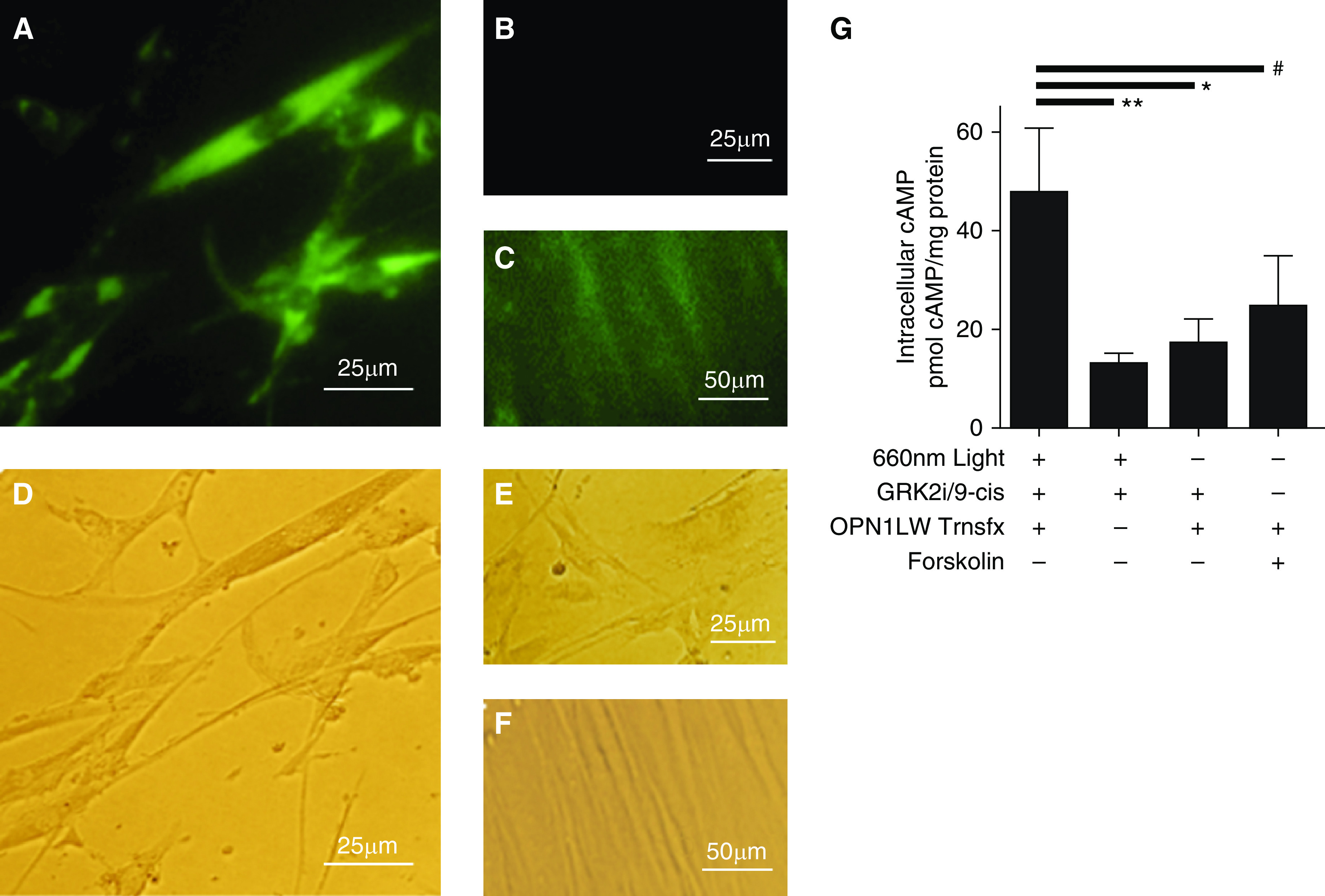
Human ASM cells stably overexpressing OPN1LW demonstrate increased cAMP upon treatment with red light. (A–F) Representative microscopic images of GFP expression (A–C) and corresponding light microscopy images after transfection with OPN1LW plasmid coexpressing GFP and a hygromycin resistance gene for selection of successfully transfected cells. (A and D) ASM cells 1 day after transfection and hygromycin treatment, (B and E) nontransfected control cells at 1 day, and (C and F) transfected cells at 10 days after transfection. (G) Intracellular cAMP concentrations in OPN1LW-transfected cells pretreated with GRK2i and 9-cis retinal and treated with red light exhibited a cAMP level of 48 ± 13 pmol cAMP/mg. n = 4. The transfection control (Trnsfx) (13 ± 2.1 pmol cAMP/mg protein). **P < 0.01, dark control (17 ± 5.2 pmol cAMP/mg protein). *P < 0.05 and forskolin-positive control (#NS) (24 ± 11 pmol cAMP/mg protein) are also shown, respectively. n = 4 independent wells. Scale bars: A, B, D, and E, 25 μm; C and F, 50 μm. GRK2i = G protein receptor kinase inhibitor; ns = not significant; OPN1LW = Opsin 1 long wavelength; Trnsfx = transfection.
Discussion
In the present study, we sought to determine the mechanism behind blue light–mediated photorelaxation in ASM. First, we specifically implicated OPN3 in mediating this effect through demonstrating decreased functional relaxation in an OPN3 null model. We previously determined BK channels to be involved in blue light–mediated photorelaxation in ASM (3). Because BK channels have been previously observed to be regulated by PKA, we suspected a potential Gαs-mediated mechanism (3, 15, 17). In ASM, Gαs-coupled GPCRs typically increase AC activity, thus increasing production of cAMP, which activates PKA. PKA then promotes downstream prorelaxation effectors, such as opening of BK channels. We observed corresponding cAMP increase in ASM upon blue light treatment, confirmed Gαs–OPN3 interaction, and demonstrated the involvement of PKA. Furthermore, we have had previous success in augmenting this photorelaxation effect with GRK2 inhibition in ASM (3). The involvement of GRK2 is additionally suggested by functional heterodesensitization with β-agonist desensitization in conjunction with observations of increased GRK2–OPN3 colocalization upon β-agonist desensitization (3). Furthermore, we also demonstrate potential conservation of relaxation machinery (specifically cAMP) in an OPN1LW overexpression model that is sensitive to red light. In summary, we determine a mechanism of blue light–mediated photorelaxation in ASM to be through OPN3 association to a classic Gαs signaling pathway, potential red-shifting of photorelaxation wavelength sensitivity through OPN1LW transfection, and signal regulation by GRK2.
We established the prorelaxation effect of blue wavelength light on ASM in our previous study. There, we implicated involvement of OPN3 through protein and mRNA expression in human ASM tissue, wavelength sensitivity, and relaxation enhancement upon addition of 9-cis retinal chromophore and GRK2 inhibition (3). Exogenous 9-cis retinal is commonly used as an 11-cis retinal analog to study visual opsins owing to its increased stability resulting from decreased steric hindrance (18, 19). The endogenous chromophore in the ASM molecular environment remains unknown. The chromophores found in retina are unlikely to be found in ASM, because 11-cis retinal is produced by retinal epithelium cells and 11-cis retinal is a highly unstable molecule. GRK2 inhibition was previously shown to enhance OPN4 and 3 activity in vascular smooth muscle (5).
First, to confirm functional involvement of OPN3, we performed studies with OPN3 null mice. We observed significant attenuation of blue light photorelaxation in OPN3 null mouse trachea in comparison with wild-type mouse trachea in organ bath. Notably, the attenuation is not complete in OPN3 null mice; there was still a degree of relaxation that occurred without the presence of OPN3 in ASM. This suggests additional endogenous light-sensitive systems at play, such as OPN4. We have previously demonstrated expression of OPN4 in mouse ASM, but not human ASM, whereas we have observed the presence of OPN3 in both human and mouse ASM (3). We hypothesize potential additional prorelaxant signaling of OPN4 in mouse ASM. However, the relevance of OPN4 in humans remains unknown and unlikely owing to lack of expression in human ASM. Nonetheless, we determine a crucial role in OPN3 signaling for airway relaxation that is likely conserved in humans.
Next, we sought to determine the mechanism by which OPN3 mediates its prorelaxation effect. In our previous study, we were able to extinguish photorelaxation with iberiotoxin, which is an inhibitor of BK channels (3). Activated BK channels hyperpolarize smooth muscle cells, promoting relaxation. It is known that BK channels are phosphorylated and activated by PKA, which in turn is well established to be activated by cAMP in ASM prorelaxant signaling pathways (15–17). Accordingly, we measured cAMP in light-relaxed and control trachea. There was an expected increase in cAMP levels in light-treated trachea. Additional studies using inhibitors against PKA revealed the intermediary role of PKA between cAMP and photorelaxation. The observed increase in cAMP suggested a Gαs-mediated mechanism. This is similar to canonical ASM relaxation pathways, such as that of the βAR receptor, whose activity was enhanced upon addition of blue light. We validated a Gαs-mediated mechanism through two modalities. We first confirmed proximity of OPN3 to Gαs through a proximity assay (PLA). Then, co-IP was used to validate physical association between OPN3 and Gαs. Thus, we conclude a prorelaxation signaling from OPN3 association to Gαs, resulting in increased cAMP, activation of PKA, and hypothesized phosphorylation and activation of BK channels, resulting in hyperpolarization (Figure 7).
Figure 7.
Summary of signaling pathway. A diagram of the signaling pathway of OPN3 through Gαs, which increases cAMP through AC. Increases in cAMP activates PKA, which can promote several downstream pathways of ASM relaxation. GRK2 is activated through PKA, thereby regulating OPN3 signaling. αs = Gαs; AC = adenylyl cyclase.
The opsin family has been known to associate with a variety of G proteins, including Gαt, Gαq, Gαo, Gαs, Gαi, and Gαi/o (23). Canonically, opsin receptors in the eye, such as rhodopsin (OPN2) and cone opsins (OPN1LW, MW, SW), associate with transducin (Gαt). Briefly, transducin activates PDE, which decreases cyclic guanylyl monophosphate levels, thus closing cyclic nucleotide gated cation channels and hyperpolarizing the neuron, which results in visual signal transduction to the brain. OPN3 is relatively less studied compared with other opsins. Despite this, it has been determined to be expressed widely through a diverse number of tissue beds, warranting its nickname of “panopsin.” OPN3 is known to associate with more than one G protein; for example, it has been seen to associate with Gαi in mosquito OPN3, pufferfish teleost multiple tissue opsin, and human melanocytes (7, 24). However, in jellyfish, OPN3 was determined to associate with Gαs (13). OPN3 has also been seen to increase cAMP levels upon stimulation with blue light in murine white adipocytes (22). Our group recently demonstrated OPN3 to physically associate with Gαs in uterine smooth muscle (6). In this present study, we confirm a classic Gαs-mediated relaxation pathway for OPN3 in ASM; however, we cannot rule out the possibility of G protein promiscuity and other pluri-dimensional signaling pathways (25).
G protein receptors kinases are well known to phosphorylate the c-terminus of GPCRs and mediate agonist-bound receptor desensitization through promoting β-arrestin binding and G protein decoupling (26). Effectively, GRKs terminate GPCR signaling pathways. GRK2 is best known to desensitize the β2AR (11). GRK2 inhibition has been shown to greatly enhance photorelaxation in vascular smooth muscle, uterine smooth muscle, and ASM (3–6). To further substantiate potential GRK2 activity on opsin-mediated photorelaxation in ASM, we pretreated tracheal rings with a β2AR agonist (isoproterenol) overnight to increase GRK2 levels within the cell. The tracheas were then treated with light, and we saw a significant reduction in relaxation in comparison with control trachea. We term this a “heterodesensitization” effect. This both suggests GRK2 activity on proteins implicated in photorelaxation in ASM and further implicates the involvement of PKA, which is known to activate GRK2 (27). We similarly assayed proximity between GRK2 and OPN3 through PLA. This was confirmed positive and increased colocalization between GRK2 and OPN3 in the absence of light upon overnight desensitizing treatment with isoproterenol. We also confirmed physical interaction of GRK2 and OPN3 through co-IP. We present multiple layers of evidence that GRK2 likely mediates desensitization of OPN3. However, this does not rule out the possibility of activity of other GRK family proteins, such as the related GRK3. This would warrant further survey of association between OPN3 and proteins of the GRK family.
Longer-wavelength (lower-frequency) electromagnetic radiation is more penetrant than relatively lower wavelength (higher frequencies). Blue light is considered minimally penetrant in biologic tissues, whereas red light is more penetrant, especially within the “water window” of near infrared wavelengths (28). OPN1LW is the red cone opsin, which is maximally sensitive to red wavelength light, with peak absorbances at 559 nm (29). If light were to be therapeutically functional, it would need to be able to penetrate into deep smooth muscle tissues. We sought to determine if we would be able to change the wavelength of ASM relaxation. Thus, we overexpressed OPN1LW in an immortalized ASM cell line. Upon absorption of 660 nm red light, we observed an increase in cAMP. This suggests that OPN1LW in ASM may be able to recruit Gαs to promote a similar Gαs-mediated relaxation response like OPN3. OPN1LW in the eye natively couples to Gαt, which transduces down an entirely different pathway. This suggests possible promiscuous G protein activity and changes in signaling pathway dependent on molecular environment and tissue type. Unexpectedly, we determined 660 nm to increase cAMP the most. This could be due to unexpected interactions between 9-cis retinal and the opsin apoprotein, because the endogenous chromophore within the eye is 11-cis retinal. Chromophores endogenous to the ASM microenvironment are currently unknown. 9-cis has a smaller resonant system than 11-cis, which would result in a lower energy (higher wavelength) of light to be absorbed. Wavelength shift by addition of alternate chromophores has been determined to be important in red-shifting salmon vision (30). The addition of different chromophores and their effects on functional photorelaxation is an area that warrants further investigation.
Conclusions
We propose a potent prorelaxant OPN3–Gαs signaling pathway in ASM in accordance with canonical Gαs signaling, with regulation from GRK2 (Figure 7) (31). Nonetheless, an important biologic limitation to our study to consider is light penetrance into tissue, particularly distal airways, which are important in determining airway resistance. Visual wavelengths of light only exhibit penetrations of less than 5 mm into the skin. This could potentially be addressed by the use of new technologies, such as upconverting nanocrystals, which are nanoparticles that are able to convert long wavelengths of light into shorter wavelengths and have demonstrated promising biocompatibility profiles (32). Alternatively, the presence of a canonically light-sensing receptor in deep tissue beds suggests the presence of endogenous ligands that are able to activate the receptor without light or potential interaction with other receptors to trigger signaling cascades. A technical limitation to consider is the number of protein interactions that can be observed from antibody-based assays. The interactome for OPN3 would be more thoroughly understood with higher-throughput, less “biased” techniques in proteomics. Thus, continued assessment of alternate OPN3 signaling pathways should still be considered.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dingbang Xu for assisting with confirming transfection of OPN1LW.
Footnotes
Supported by funding provided by the Foundation for Anesthesia Education and Research (P.D.Y.), and the U.S. National Institutes of Health grants HD082251 (G.G.), GM065281 (C.W.E.), and HL122340 (C.W.E.).
Author Contributions: A.D.W., W.D., C.W.E., and P.D.Y. designed the research. A.D.W., W.D., Y.Z., and P.D.Y. performed the research. A.D.W., W.D., and P.D.Y. analyzed the data. A.D.W. wrote the paper. A.D.W., W.D., S.V., R.A.L., D.E.B., C.W.E., and P.D.Y. made revisions to the paper. S.V., B.A.U., R.A.L., E.D.B., G.G., and C.W.E. contributed reagents and analytic tools.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0392OC on October 15, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisenberg WH, Huang J, Zhu W, Rajkumar P, Cruz R, Santhanam L, et al. Defining an olfactory receptor function in airway smooth muscle cells. Sci Rep. 2016;6:38231. doi: 10.1038/srep38231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yim PD, Gallos G, Perez-Zoghbi JF, Zhang Y, Xu D, Wu A, et al. Airway smooth muscle photorelaxation via opsin receptor activation. Am J Physiol Lung Cell Mol Physiol. 2019;316:L82–L93. doi: 10.1152/ajplung.00135.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikka G, Hussmann GP, Pandey D, Cao S, Hori D, Park JT, et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc Natl Acad Sci USA. 2014;111:17977–17982. doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreto Ortiz S, Hori D, Nomura Y, Yun X, Jiang H, Yong H, et al. Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition. Am J Physiol Lung Cell Mol Physiol. 2018;314:L93–L106. doi: 10.1152/ajplung.00091.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yim PD, Hyuga S, Wu AD, Dan W, Vink JY, Gallos G. Activation of an endogenous opsin 3 light receptor mediates photo-relaxation of pre-contracting late gestation human uterine smooth muscle ex vivo. Reprod Sci. 2020;27:1791–1801. doi: 10.1007/s43032-020-00180-z. [Published erratum appears in Reprod Sci 27:1802.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozdeslik RN, Olinski LE, Trieu MM, Oprian DD, Oancea E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc Natl Acad Sci USA. 2019;116:11508–11517. doi: 10.1073/pnas.1902825116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackshaw S, Snyder SH. Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19:3681–3690. doi: 10.1523/JNEUROSCI.19-10-03681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalesio NM, Barreto Ortiz SF, Pluznick JL, Berkowitz DE. Olfactory, taste, and photo sensory receptors in non-sensory organs: it just makes sense. Front Physiol. 2018;9:1673. doi: 10.3389/fphys.2018.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz-Gómez A, Mayor F., Jr Beta-adrenergic receptor kinase (GRK2) colocalizes with beta-adrenergic receptors during agonist-induced receptor internalization. J Biol Chem. 1997;272:9601–9604. doi: 10.1074/jbc.272.15.9601. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Woo JA, Geffken E, An SS, Liggett SB. Coupling of airway smooth muscle bitter taste receptors to intracellular signaling and relaxation is via Gαi1,2,3. Am J Respir Cell Mol Biol. 2017;56:762–771. doi: 10.1165/rcmb.2016-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyanagi M, Takano K, Tsukamoto H, Ohtsu K, Tokunaga F, Terakita A. Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc Natl Acad Sci USA. 2008;105:15576–15580. doi: 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1006–G1016. doi: 10.1152/ajpgi.00465.2002. [DOI] [PubMed] [Google Scholar]
- 15.Nara M, Dhulipala PD, Wang YX, Kotlikoff MI. Reconstitution of beta-adrenergic modulation of large conductance, calcium-activated potassium (maxi-K) channels in Xenopus oocytes: identification of the cAMP-dependent protein kinase phosphorylation site. J Biol Chem. 1998;273:14920–14924. doi: 10.1074/jbc.273.24.14920. [DOI] [PubMed] [Google Scholar]
- 16.Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther. 2013;26:112–120. doi: 10.1016/j.pupt.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z-W, Kotlikoff MI. Activation of KCa channels in airway smooth muscle cells by endogenous protein kinase A. Am J Physiol. 1996;271:L100–L105. doi: 10.1152/ajplung.1996.271.1.L100. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard R, Wald G. Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J Gen Physiol. 1952;36:269–315. doi: 10.1085/jgp.36.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamichi H, Okada T. X-ray crystallographic analysis of 9-cis-rhodopsin, a model analogue visual pigment. Photochem Photobiol. 2007;83:232–235. doi: 10.1562/2006-13-RA-920. [DOI] [PubMed] [Google Scholar]
- 20.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol. 1989;256:C329–C335. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- 21.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, et al. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L523–L534. doi: 10.1152/ajplung.00013.2006. [DOI] [PubMed] [Google Scholar]
- 22.Nayak G, Zhang KX, Vemaraju S, Odaka Y, Buhr ED, Holt-Jones A, et al. Adaptive thermogenesis in mice is enhanced by opsin 3-dependent adipocyte light sensing. Cell Rep. 2020;30:672–686, e8. doi: 10.1016/j.celrep.2019.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyanagi M, Terakita A. Diversity of animal opsin-based pigments and their optogenetic potential. Biochim Biophys Acta. 2014;1837:710–716. doi: 10.1016/j.bbabio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Koyanagi M, Takada E, Nagata T, Tsukamoto H, Terakita A. Homologs of vertebrate Opn3 potentially serve as a light sensor in nonphotoreceptive tissue. Proc Natl Acad Sci USA. 2013;110:4998–5003. doi: 10.1073/pnas.1219416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandhu M, Touma AM, Dysthe M, Sadler F, Sivaramakrishnan S, Vaidehi N. Conformational plasticity of the intracellular cavity of GPCR-G-protein complexes leads to G-protein promiscuity and selectivity. Proc Natl Acad Sci USA. 2019;116:11956–11965. doi: 10.1073/pnas.1820944116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurado-Pueyo M, Campos PM, Mayor F, Murga C. GRK2-dependent desensitization downstream of G proteins. J Recept Signal Transduct Res. 2008;28:59–70. doi: 10.1080/10799890801941939. [DOI] [PubMed] [Google Scholar]
- 27.Penela P, Ribas C, Sánchez-Madrid F, Mayor F., Jr G protein-coupled receptor kinase 2 (GRK2) as a multifunctional signaling hub. Cell Mol Life Sci. 2019;76:4423–4446. doi: 10.1007/s00018-019-03274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191–2208. doi: 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neitz J, Neitz M. The genetics of normal and defective color vision. Vision Res. 2011;51:633–651. doi: 10.1016/j.visres.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enright JM, Toomey MB, Sato SY, Temple SE, Allen JR, Fujiwara R, et al. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Curr Biol. 2015;25:3048–3057. doi: 10.1016/j.cub.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kefalov VJ, Carter Cornwall M, Crouch RK. Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors. J Gen Physiol. 1999;113:491–503. doi: 10.1085/jgp.113.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruggiero E, Alonso-de Castro S, Habtemariam A, Salassa L. Upconverting nanoparticles for the near infrared photoactivation of transition metal complexes: new opportunities and challenges in medicinal inorganic photochemistry. Dalton Trans. 2016;45:13012–13020. doi: 10.1039/c6dt01428c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.