Abstract
Emerging evidence shows that after injury or infection, the mesenteric lymph acts as a conduit for gut-derived toxic factors to enter the blood circulation, causing systemic inflammation and acute lung injury. Neither the cellular and molecular identity of lymph factors nor their mechanisms of action have been well understood and thus have become a timely topic of investigation. This review will first provide a summary of background knowledge on gut barrier and mesenteric lymphatics, followed by a discussion focusing on the current understanding of potential injurious factors in the lymph and their mechanistic contributions to lung injury. We also examine lymph factors with antiinflammatory properties as well as the bidirectional nature of the gut–lung axis in inflammation.
Keywords: acute lung injury, gut-lung axis, inflammation, mesenteric lymph, permeability
It has long been recognized that sepsis, severe infection (e.g., pneumonia), intestinal ischemia/reperfusion (I/R), trauma, burn, and hemorrhagic shock (HS) can cause systemic inflammation, acute respiratory distress syndrome (ARDS), and multiple organ failure (1). The gut is considered the motor of critical illness (2), in which a common feature is splanchnic hypoperfusion and I/R injury. Intestinal I/R leads to epithelial mucosal injury, barrier dysfunction, and translocation of gut bacteria or toxic factors into blood circulation, causing systemic inflammation and ARDS (1, 2). Conventionally, the hepatic portal vein is thought to be the delivery route of intestinal toxins. However, this theory cannot explain why the lung, rather than the liver, is usually the first organ affected. Moreover, failure to detect bacteria or their products in the portal vein of trauma patients casts doubt on this hypothesis (3).
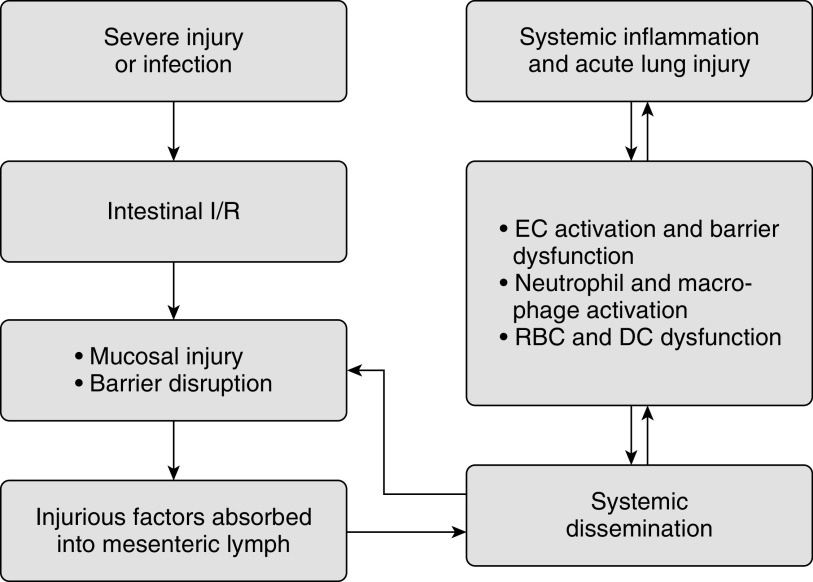
Recently, a body of evidence demonstrated that the mesenteric lymphatics, instead of portal venous system, are the major conduit of the gut-derived injurious factors triggering ARDS (4, 5). This is supported by the fact that the lung is the first organ exposed to the mesenteric lymph and that the lung is exposed to a high concentration of lymph contents before they get diluted by the systemic blood volume. It is plausible that gut I/R or infection results in mucosal injury and the production of injurious factors or inflammatory mediators capable of disrupting the gut barrier. These factors are absorbed into the mesenteric lymph duct and conveyed to the blood circulation, where they activate endothelial cells (ECs) and immune cells, ultimately causing acute lung injury (Figure 1). However, the identity of injurious factors and the mechanisms underlying their effects are incompletely understood.
Figure 1.
Mesenteric lymph as a conduit in the gut–lung axis. Severe injury or infection induces intestinal ischemia/reperfusion injury, resulting in mucosal injury, gut barrier disruption, and the production of injurious factors. These factors are transported through the mesenteric lymph into blood circulation, where they trigger endothelial cell activation and barrier dysfunction, activate neutrophils and macrophages, and induce red blood cell and dendritic cell dysfunction, ultimately causing systemic inflammation and acute lung injury. Vice versa, lung disease can also cause gut mucosal injury and barrier impairment through systemic dissemination. DC = dendritic cell; EC = endothelial cell; I/R = ischemia/reperfusion; RBC = red blood cell.
Inflammatory bowel diseases (IBDs), including ulcerative colitis and Crohn’s disease, are chronic inflammatory conditions caused by an inappropriate immune response to commensal enteric bacteria (6). Emerging evidence has revealed that more than half of patients with IBD present respiratory symptoms indicative of pulmonary dysfunction (7–9). Animal models of colitis also display lung inflammatory characteristics, such as elevated neutrophils and cytokines in lung tissues (10, 11). IBD causes gut–epithelial barrier dysfunction and elevated mucosal cytokine concentrations (e.g., IL-6), which can prime circulatory neutrophils for extravasation (10, 12). Because the pulmonary circulation provides one of the largest margination sites for neutrophils, lung tissues are highly susceptible to neutrophil-mediated damage. In addition to neutrophils, aberrant lymphocyte homing and commensal microbiota dysbiosis have been shown to participate in IBD-driven lung injury (12). Both human and experimental IBD models also exhibit substantial lymphatic alterations, including intestinal lymphatic vessel obstruction, lymphoedema, and lymphangiogenesis (13, 14), supporting the involvement of the lymphatic system in gut inflammation.
Intestinal Barrier and Vasculature
The intestinal barrier plays an essential role in metabolic homeostasis by regulating the absorption of water, electrolytes, and nutrients while preventing gut luminal bacteria or harmful substances from entering the circulation. The gut barrier is composed of the following three layers: mucus, epithelium, and endothelium (Figure 2) (15). The mucus layer, lining the gut lumen, is a mesh network of fibers mainly composed of highly glycosylated proteins and mucins produced by epithelial goblet cells. It also contains antimicrobial peptides secreted by Paneth cells and secretory IgA released by plasma B cells (15). Mucus property varies from the small intestine to the large intestine. The small intestine has a single layer of loose and discontinuous mucus, whereas the colon has two layers of mucus (16). The majority of commensal microbiota exists in the colon outer layer, and the inner layer is dense and firmly attached to the epithelium (15). The epithelial layer is formed by a monolayer of enterocytes, goblet, Paneth, and other enteroendocrine cells (e.g., L cells), which are connected through tight junctions (TJs), adherens junctions (AJs), desmosomes, and gap junctions (15). The TJs, which are composed of occludins and claudins, are the predominant structures that determine epithelial barrier property. The gap junction is generally not considered a barrier structure because it is formed primarily by connexin hemichannels and pannexin channels, which conduct ion flow and the exchange of small hydrophilic substances between cells (17, 18). The endothelium is composed of ECs connected via different types of junctions similar to those in the epithelium, with the AJs playing a relatively more important role in the peripheral microvascular exchange of fluid and solutes such as plasma proteins (19). Pericytes and enteric glial cells are in close proximity to the endothelium; both contribute to microvascular permeability (20).
Figure 2.
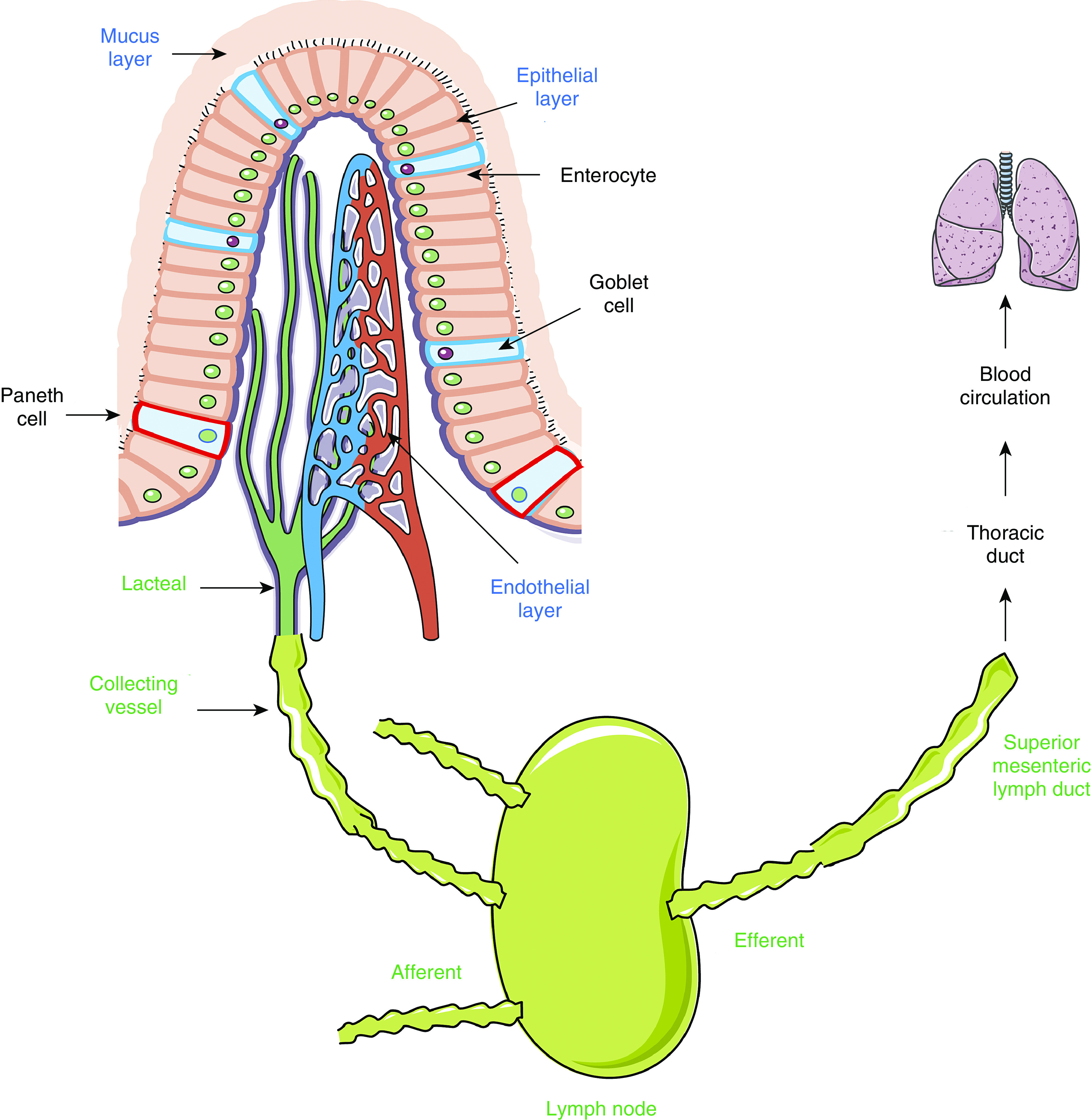
Diagram of gut barrier and mesenteric lymphatic system. The gut barrier is composed of three physical layers (mucus, epithelial, and endothelial layers). The mesenteric lymphatic vasculature is organized into a network of lymphatic capillaries (lacteals), collecting vessels, and lymph nodes that transports lymph from the gut to the superior mesenteric lymph duct, thoracic duct, and eventually blood circulation. The close anatomical proximity of epithelial villus, blood microvasculature, and lymphatic terminals facilitates the dissemination of gut luminal bacteria, toxins, and tissue products across the leaky barrier during inflammation. Images of tissues and organs are adapted from Servier Medical ART, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
Impairment of the gut barrier, also termed “leaky gut,” is commonly observed in critical illness and IBD (21, 22). Preserving gut barrier function via enteral feeding decreases the incidence of multiorgan failure in patients with sepsis or trauma patients (23, 24), indicating that the intestinal barrier disruption plays a causative role. A number of inflammatory mediators, such as IFN-γ, IL-6, and TNF-α, are able to disrupt TJs and increase epithelial permeability (25).
The superior mesenteric artery supplies the blood from duodenum to part of the transverse colon, and the inferior mesenteric artery supplies the rest of colon and rectum. In response to stress, the enteric vasculature constricts in order to deliver more blood to vital organs; this compensatory response renders intestinal hypoperfusion. Because the intestinal villus is supplied by a single arterial vessel and is highly sensitive to I/R injury, short periods of ischemia can elicit severe injury to local tissues, resulting in epithelial apoptosis and barrier leakage (22). The close anatomical proximity of epithelial villus, blood microvasculature, and lymphatic terminals facilitates the dissemination of gut luminal bacteria, toxins, and tissue products across the leaky barrier (Figure 2).
Mesenteric Lymphatics
The lymphatic system consists of a large network of vessels that run in parallel to the blood vasculature. Unlike blood vessels, most lymphatic vessels (except capillaries) contain valves that ensure a one-way flow from terminal tissue to blood circulation (26). A major function of lymphatics is to drain fluid and other substances that leak from the blood microvessels. The gut has two sets of drainage systems, as follows: the portal venous system and mesenteric lymphatics. The portal venous system transports blood from the gastrointestinal (GI) tract, spleen, pancreas, and gallbladder to the liver. Nutrients and metabolites absorbed in the small intestine travel first to the liver for further processing before reaching the whole body. The portal vein is also involved in the transportation of short and medium chain length non–lipoprotein-associated lipids absorbed by blood capillaries.
The gut lymphatic system plays a key role in interstitial fluid transport, lipid absorption, and immunosurveillance. The mesenteric lymphatic vasculature is organized into a network of lymphatic capillaries (lacteals) and collecting vessels that transports lymph from the gut to blood circulation (26). Interstitial fluid extravasated from gut microvessels drains into blind-ended, permeable lacteals to form lymph (27). Lymph flows via a network of lymphatic vessels and nodes to the superior mesenteric lymph duct, thoracic duct, and eventually back to blood circulation via the subclavian vein (Figure 2).
Lacteals are formed by a mix of discontinuous button-like and continuous zipper-like junctions of ECs that form flap valves, which are directly attached to the extracellular matrix (ECM) by anchoring filaments (28). This highly permeable feature allows one way transport of fluid and cells into lacteals (27). Lacteals are in close proximity to villous smooth muscle cells (SMCs), which provide growth factors and pump lymph by constricting lacteals (27, 29). In contrast, collecting lymphatics are much less permeable because their wall displays continuous zipper-like junctions of ECs, which are covered by SMCs with contractility (27). In addition, lymphatic collecting vessels have abundant intraluminal lymphatic valves that prevent lymph backflow (28). Gut tissue contains only lacteals, whereas collecting lymphatic vessels are in the mesentery (27). Although the portal vein was conventionally believed to be the route of transporting gut-derived factors, recent studies indicate that lymphatics may be an important conduit in systemic dissemination.
By forming lymphatic microvessel wall and the sinuses of lymph nodes, lymphatic ECs (LECs) play key roles in maintaining fluid balance as well as in inflammation and tissue repair. Junctions formed by LECs determine the permeability of lymphatic capillaries. LECs also regulate lymphatic vasomotor function in conjunction with SMCs (30). With respect to the immune response, LECs participate in the sorting of lymph-derived antigens, serve as antigen-presenting cells, and promote peripheral tolerance through programmed death-ligand 1 to eliminate tissue-specific responses (31, 32). They secrete chemokines that recruit immune cells to lymph nodes after an inflammatory stimulus (32). Moreover, LECs interact with subcapsular sinus macrophages and regulate the organogenesis of lymph nodes (31). Whether LECs are involved in the generation of lymph bioactive substances after injury or infection remains to be elucidated.
Mesenteric Lymph as a Conduit of Splanchnic Factors to Cause Acute Lung Injury
Lung Endothelial Barrier
Acute lung inflammation is characterized by increased alveolar–capillary membrane permeability, neutrophil infiltration, and macrophage activation, culminating in lung edema and ARDS (33–35). Recent studies by us and others have emphasized the critical contributions of pulmonary microvascular endothelial barrier dysfunction to acute lung injury under either septic or sterile inflammatory conditions, in which the barrier is activated by an array of hyperpermeability factors that come in contact with the endothelial surface or ECM as well as by tissue factors and catalytically active enzymes (19, 36, 37). The pulmonary microvascular barrier is composed of ECs that are connected to each other via AJs and TJs. The luminal surface of this barrier is protected by a meshwork of glycocalyx containing glycosaminoglycans, proteoglycans, and glycoproteins, and its basolateral side is anchored to the ECM in the basement membrane via focal adhesions. Altering the structural composition or conformational dynamics of these components affects the permeability function of endothelial barrier. For example, AJ/TJ protein degradation or phosphorylation-triggered dissociation increases paracellular permeability (19, 37). Junction opening can be facilitated during actomyosin cytoskeleton contraction or stress fiber formation, which generates tension that pulls neighboring cells apart. Moreover, glycocalyx shedding exposes the endothelium to activated cells or harmful agents in the blood; its shedding products act as autocrine-signaling molecules in hyperpermeability (38, 39). Finally, although focal adhesions are crucial for maintaining endothelial barrier integrity under normal conditions, their activation during inflammation increases permeability (40, 41). An array of humoral factors have been found to possess barrier disruptive capability, including histamine, thrombin, bradykinin, platelet-activating factor, cytokines, and nitric oxide. The signaling pathways underlying their effects involve the activation of phospholipases (e.g., PLC and PLA), protein kinases (e.g., PKC, PKG, and MAPK), and nonreceptor tyrosine kinases (e.g., c-Src) (36, 42). We have recently reported the detection of extracellular vesicles (EVs) in the blood of animals subjected to cecal ligation and puncture-induced sepsis as well as in cell-culture media after inflammatory stimulation; these EVs carry Src-rich cargos that are capable of increasing endothelial paracellular permeability via tyrosine phosphorylation of AJs (43). Cleavage of syndecan-3/4, a key component of glycocalyx, generates ectodomain fragments that lead to AJ disorganization, stress fiber formation, and hyperpermeability (38). In addition, ADAM15 (a disintegrin and metalloproteinase 15) has been found to be upregulated in pneumonia or sepsis and mediates endothelial barrier injury and neutrophil infiltration by shedding glycocalyx CD44 and triggering AJ dissociation (44, 45).
Effects of Postinjury Mesenteric Lymph on Lung Injury
Preventing lymph flow from reaching the blood circulation abrogates acute lung injury secondary to gut I/R, peritoneal infection, trauma/hemorrhagic shock (T/HS), and burns (5, 46, 47). Moreover, the infusion of postinjury lymph into naive animals elicits acute lung injury (48), confirming the causative role of mesenteric lymph. In vitro, postinjury lymph is able to upregulate adhesion molecules and IL-6 expression on ECs, induce EC death, increase microvascular permeability, increase neutrophil surface CD11b expression, and suppress red blood cell deformability (Figure 3) (5). These effects are not seen when cells are treated with sham lymph or postinjury portal vein plasma, confirming that it is the mesenteric lymphatics that carry harmful factors that cause lung damage. The lymph toxicity seems to peak in the first few hours after injury and is blunted over the next several hours (5, 49).
Figure 3.
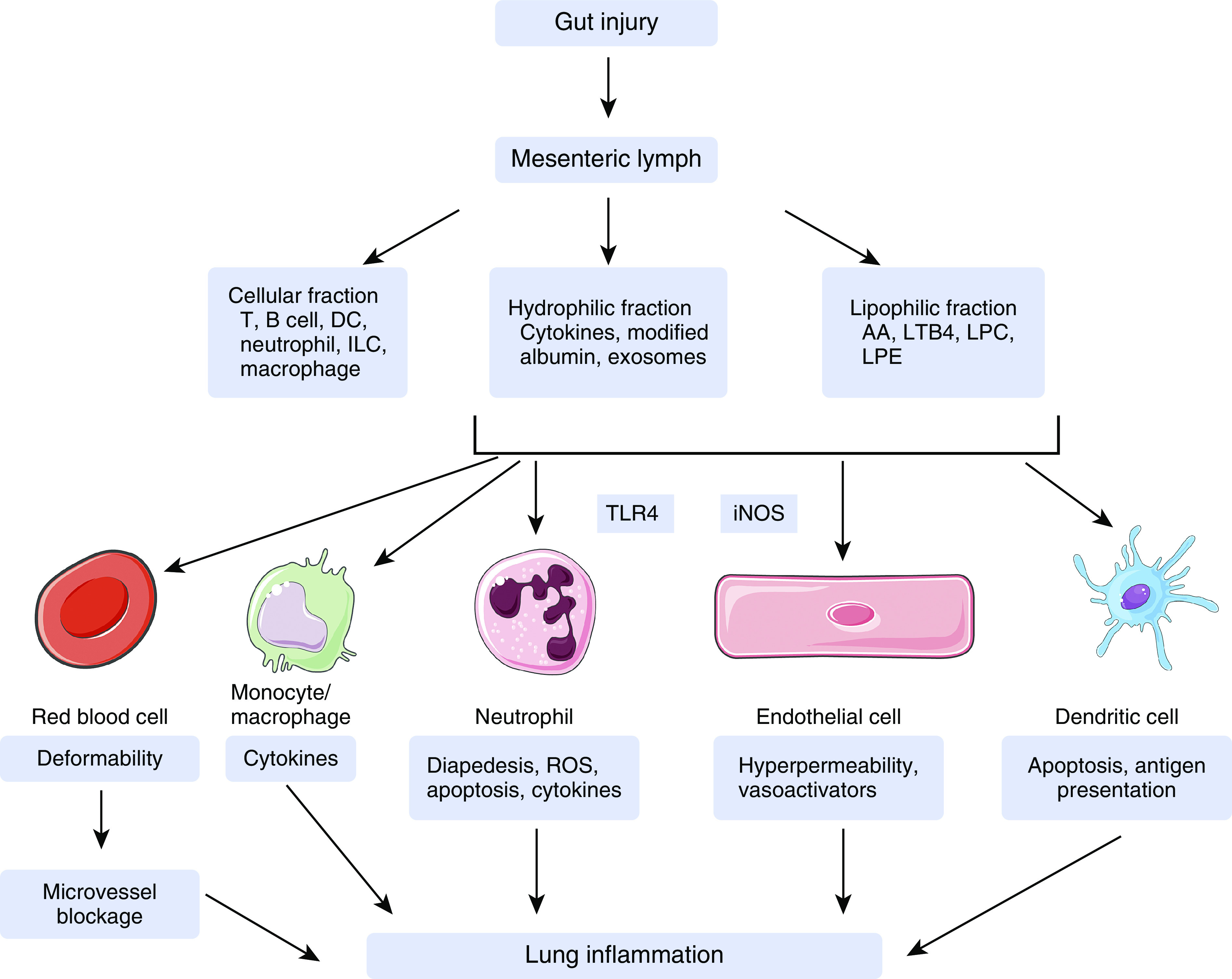
Mechanistic diagram of mesenteric lymph inducing lung injury. After gut injury, mesenteric lymph hydrophilic and lipophilic components can activate endothelial cells, neutrophils, and monocytes/macrophages; induce endothelial cell barrier dysfunction; delay neutrophil apoptosis; and suppress red blood cell deformability and dendritic cell function, all of which contribute to systemic inflammation dissemination and acute lung injury. The underlying signaling mechanisms involve toll-like receptor 4 and inducible nitric oxide synthase. Although the majority of lymph cells are lymphocytes during homeostasis, postinjury lymph also transports neutrophils, macrophages, dendritic cells, and innate lymphoid cells. The role of lymph cells in mediating acute lung injury, however, has not been examined. Images of cells are from Servier Medical ART, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com). AA = arachidonic acid; ILC = innate lymphoid cell; iNOS = inducible nitric oxide synthase; LPC = lysophosphatidyl-choline; LPE = lysophosphatidyl-ethanolamine; LTB4 = leukotriene B4; ROS = reactive oxygen species; TLR4 = Toll-like receptor 4.
On the contrary, some studies suggest that lymph under unstimulated conditions is protective. For example, an in vitro study showed that normal mesenteric lymph attenuated ICAM-1 (intercellular adhesion molecule 1) expression on pulmonary ECs in response to LPS treatment, an effect abrogated when lipids and lipoproteins were removed (50). This indicates that lipids and lipoproteins in normal lymph are beneficial. In another study, the administration of normal mesenteric lymph protected mice from LPS-induced renal injury (51). Whether lipids or lipoproteins in normal lymph bind LPS to inhibit its pathogenicity needs further investigation.
Lymph Components and Toxicity
Lymph is not a simple ultrafiltrate of plasma. Proteomic analysis reveals that lymph proteins are quantitatively and qualitatively different from plasma proteins and that some proteins are rather unique to the lymph under particular physiological and pathophysiological conditions (52, 53). Mesenteric lymph can be fractioned into the following three phases: the hydrophilic phase containing fluid, gut hormones, cytokines, metabolic mediators, antigens, tolerogens, and exosomes; the lipophilic fraction composed of lipids, lipophilic molecules, and apo-proteins; and the cellular fraction containing mainly lymphocytes (54–56).
Although it has been more than 20 years since the first report about lymph mediating acute lung injury (57), the molecular identity and mechanisms of action of lymph components responsible for its injurious effects remain mysterious. Both lymph hydrophilic and lipophilic factors are implicated in this process. In the following sections, we will discuss the likely components in each phase that contribute to lung injury.
Lymph hydrophilic fraction
Lacteals are more permeable than blood microvessels because of the presence of loose cell–cell junctions (28). This might partially explain why the lymphatics, not the blood vessels, are the main conduit of injurious factors. During trauma or infection, bacteria translocation into the blood or lymphatic circulation was originally believed to be a culprit behind the development of ARDS and systemic inflammation. However, emerging studies have shown that postinjury mesenteric lymph is sterile (free of bacteria or its components) (58, 59), which argues against the importance of the presence of bacteria per se in lymph bioactivity. Moreover, the vast majority of critically ill patients show no detectable bacteremia (3), implying that host-derived nonbacterial factors are the major inducers of organ failure.
Changes of inflammatory mediators and cytokines in the lymph vary in different disease models. For example, during intraperitoneal infection, the lymph concentrations of TNF-α, IL-6, IL-4, ICAM-1, and monocyte chemoattractant protein-1 are increased (47), whereas after T/HS, the concentrations of TNF-α, IL-1, granulocyte macrophage colony–stimulating factor, and transforming growth factor-β1 are not altered (60). In particular, TNF-α concentrations are higher in the lymph after gut I/R but are barely detectable in the serum of I/R animals with ligated lymph duct. Blocking TNF-α activity decreases pulmonary myeloperoxidase activity (46), indicating that TNF-α in the lymph plays a role in mediating neutrophil infiltration into the lung. In addition, IL-6 is upregulated in mesenteric lymph after gut I/R and mediates I/R lymph-induced EC detachment (61). Collectively, it appears that cytokines in the lymph play an important role in local infection or I/R, but they do not seem to be essential in systemic T/HS. The relative importance of lymph cytokines in other pathological conditions, such as sepsis, pneumonia, and burns, needs to be further investigated.
Another potentially important constituent of lymph protein phase is digestive enzymes. Under normal conditions, pancreatic enzymes are released into the proximal intestine, where they break down proteins into amino acids, which can be easily absorbed by gut epithelial cells. In certain pathological settings, these enzymes become a source of gut autodigestion and production of toxic factors, leading to mucus and epithelial barrier impairment (62, 63). After intestinal I/R, pancreatic enzymes contribute to the generation of active matrix metalloproteinase-9 and inflammatory mediators (64, 65). Enteral administration of pancreatic enzyme inhibitors attenuates lung injury associated with shock (66). In addition, serine proteases of pancreatic origin are involved in the generation of biologically active mesenteric lymph. Intestinal administration of a serine protease inhibitor (nafamostat) abrogates the biological activity of mesenteric lymph and prevents lung injury after T/HS (67).
Proteomics analysis of T/HS lymph indicates that lymph toxicity is associated with modified forms of serum albumin (68), for example, oxidized or glycosylated albumin. Because post-translational modification is a rapid way to convey bioactivity, it supports the observation that lymph toxicity peaks in the first several hours after injury (5, 49). Moreover, albumin is the most abundant protein in lymph and is thus readily available as a substrate for modification. Indeed, there is evidence demonstrating that serum albumin modified by neutrophil-derived oxidants can activate neutrophils (69). However, another study shows that glycosylated albumin exhibited no effect on EC toxicity (68); thus, glycosylation may not represent a functionally important modification in the lymph. Whether and how other forms of protein modification contribute to lymph toxicity remain largely unknown.
Exosomes are membranous vesicles of 30 to 120 nm in diameter present in various body fluids, belonging to the family of EVs. They are released by an array of cell types, including immune cells, blood and vascular cells, epithelial cells, and tumor cells (70). Exosomes mediate intercellular communication by transferring proteins, lipids, mRNAs, and microRNAs (71). Recent studies have identified the existence of exosomes in mesenteric lymph based on their size, morphology, and expression of exosome markers CD63 and heat shock protein 70 (56). Further investigation reveals that T/HS lymph exosomes express epithelial cell adhesion marker (72), indicating the possibility that they derive from injured or activated gut epithelial cells. Importantly, exosomes, not supernatants of T/HS lymph, induce NF-κB expression and TNF-α production in monocytes and macrophages (Figure 3) (56). Functionally, T/HS lymph exosomes can trigger dendritic cell apoptosis, decrease their antigen-presenting capacity, and suppress the costimulatory molecules CD80 and CD86 induced by LPS (Figure 3) (72), leading to immunosuppression. In line with the above findings from cell experiments, the infusion of postinjury lymph exosomes results in acute lung injury, inflammatory cell recruitment, and elevated vascular permeability in vivo (73). These data suggest that postinjury mesenteric lymph is proinflammatory and immunosuppressive, both of which are detrimental in the development of ARDS.
Although many studies emphasize the proinflammatory effects of EVs, there is a body of evidence supporting their antiinflammatory and proresolution roles. For example, gut epithelial cells are shown to release annexin A1–containing EVs that facilitate mucosal wound closure in vitro (74). Consistently, in vivo administration of annexin A1 mimetic peptide accelerates epithelial barrier repair in a murine model of colitis induced by dextran sulfate sodium (74). Neutrophil-derived microparticles containing annexin A1, resolvin D1, and lipoxin A4 are also shown to promote inflammation resolution and tissue repair (75, 76).
Lymph lipophilic fraction
Gut lymphatics participate in the digestion and absorption of dietary lipids, and therefore mesenteric lymph has high concentrations of lipids. Lipid fraction extracted from HS lymph is able to prime neutrophils for activation and inhibit neutrophil apoptosis (Figure 3) (77). Interestingly, these effects are not abolished by heating or polymyxin B treatment, thus excluding the involvement of proteins and LPS. Of the lymph lipids, (PLA2) (phospholipase A2) is involved in the generation of nonpolar arachidonic acids (AAs) and polar lysophospholipids by hydrolyzing cell membrane (4). PLA2 is activated after intestinal I/R, and inhibiting PLA2 attenuates I/R-induced neutrophil priming and acute lung injury (78). Neutral lipids isolated from HS mesenteric lymph augment neutrophil respiratory burst (Figure 3), which is abrogated by PLA2 inhibition (77, 79).
Arachidonic acid is a precursor of proinflammatory lipid leukotrienes and stimulates LTB4 production from neutrophils. AA is increased in postshock mesenteric lymph (80). More importantly, neutrophil priming by either T/HS lymph or AA is attenuated during LTB4 receptor blockage (80). LTB4 is one of the most potent chemoattractants and neutrophil primers that can mediate lung inflammation after gut I/R (81). Collectively, we propose that gut I/R injury triggers intestinal PLA2 activation, which catalyzes AA metabolism. The metabolites are absorbed into the mesenteric lymph and transported to the lung, where they further stimulate LTB4 production, neutrophil activation, and infiltration into the lungs.
A mass spectrometric study demonstrated that linoleoyl, arachidonoyl as well as docosahexaenoyl lysophosphatidylcholine (LPC) and lysophosphatidyl-ethanolamine (LPE) were significantly higher in T/HS mesenteric lymph compared with sham lymph (82). More importantly, in vitro linoleoyl and arachidonoyl LPCs and LPEs were able to prime neutrophils for superoxide production (82). Neutrophil degranulation was also induced by linoleoyl and arachidonoyl LPCs. These findings indicate that LPCs and LPEs in mesenteric lymph are involved in the mechanisms of neutrophil activation after T/HS (Figure 3).
Although the above-mentioned lipids are deleterious, some are considered beneficial. Oxidized phospholipids and sphingosine-1-phosphate have been shown to prevent endothelial barrier dysfunction and lung injury under pathological settings (83–85). Whether they exist in the lymph and how their concentrations change after injury remain elusive.
Immune cells in the lymph
Within lymph nodes, T and B cells are educated and become mature in response to endogenous or exogenous antigens. In the mesentery, afferent (prenodal) and efferent (postnodal) lymph have different cell types (Figure 2). Afferent lymph cells contain T lymphocytes (80–90%), dendritic cells, and B lymphocytes (86). Most of the T cells are antigen-exposed effector memory cells, and only a small fraction are naive T cells (87). In contrast, cells in the efferent lymph are mainly naive T and B cells. It should be mentioned that all of the above described studies about lymph and lung injury were carried out using cell-free efferent lymph.
Lymph cell numbers and types can change in pathological conditions. During acute inflammation, neutrophils recruited to inflamed tissues can migrate into the lymphatics and subsequently drain into lymph nodes (88, 89), although most neutrophils reach lymph nodes from the bloodstream by crossing high endothelial venules (90). Dendritic cells are released into lymph from the lamina propria of the small intestine after intravenous endotoxin injection (91). Intraperitoneal infection leads to increased neutrophil percentage and decreased macrophagocyte and lymphocyte percentage in mesenteric lymph (47). Although the current literature is largely focused on the biological activities of cell-free lymph, the role of lymph cells has not been well studied. It is highly possible that lymph cells are involved in the production of soluble injurious factors by direct secretion or via indirect immune response. It is also possible that lymph cells are activated by other components of the postinjury lymph, which further activate blood immune cells and ECs. Thus, more in-depth studies are warranted to examine how lymph cells affect the development of systemic inflammation and lung injury.
Innate lymphoid cells (ILCs) are tissue resident innate counterparts of T lymphocytes, composed of ILC1, ILC2, and ILC3. Upon stimulation with IL-25 or helminth, ILC2s residing in intestinal lamina propria migrate to the lung through mesenteric lymphatics and promote antihelminth and tissue repair in the lung (92). This further confirms mesenteric lymphatics as a key route of delivering gut-derived factors to remote organs.
In summary, both hydrophilic and lipophilic fractions contribute to the biological activity of postinjury lymph. Interactions between proteins and lipids may affect lymph bioactivity. For example, cytokine–leukotriene crosstalk can promote inflammation (93). Albumin binding to LPS or other harmful factors can neutralize their toxicity (94). Protective proteins, including albumin, high-density lipoproteins, and proteinase inhibitors, are significantly higher in preshock versus postshock lymph (50, 95). Therefore, both upregulation of deleterious factors and downregulation of beneficial factors in postinjury lymph may contribute to its injurious capacity.
Signaling Mechanisms of Mesenteric Lymph–mediated Lung Injury
Toll-like receptors
Toll-like receptors (TLRs) belong to the pattern recognition receptor family, including 10 members (TLR1–10) in humans and 12 members (TLR1–9 and TLR11–13) in mice. TLRs act as sensors of infection and tissue injury by recognizing pathogen-associated molecular patterns and damage associated molecular patterns (DAMPs). Cell surface TLRs, including TLR1, TLR2, TLR4, TLR5, TLR6, and probably TLR10, TLR11, and TLR12, generally recognize lipids, proteins, and lipoproteins; in contrast, intracellular TLRs, such as TLR3, TLR7, TLR8, TLR9, and TLR13, mainly recognize nucleic acids (96). TLR activation induces innate immune response by producing inflammatory mediators. In particular, TLR4 is typically involved in the initiation of acute inflammatory response, which causes secondary injury in multiple organs (97, 98). TLR4 mutation attenuates gut and lung injury induced by intestinal I/R (99). The infusion of T/HS lymph into normal wild-type mice increases pulmonary permeability and myeloperoxidase activity, which is completely rescued in TLR4−/− mice (100). In vitro, pharmacological inhibition or genetic deletion of TLR4 abolishes macrophage production of cytokines induced by T/HS lymph exosomes (73). Interestingly, the T/HS lymph is sterile and devoid of pathogens (100). It is likely that nonmicrobial DAMPs, rather than pathogen-associated molecular patterns, are produced in T/HS lymph in response to the stressed gut, where they activate TLR4 signaling and trigger distant lung injury (Figure 3). Further investigation is warranted to define the identity of DAMPs in the lymph.
Interestingly, our data showed that post-I/R mesenteric lymph promotes neutrophil production of proinflammatory factors via a mechanism involving NF-κB, independent of TLR4 (unpublished data), indicating that the generation of injurious lymph is context dependent. The NLRP3 inflammasome mediates host immune responses through the activation of caspase-1, IL-1β, and IL-18. NLRP3 inflammasome is involved in colitis-induced pulmonary inflammation (11). LPS can activate TLR4-TRIF and downstream signaling to activate NLRP3 (101). Therefore, further studies are required to determine whether NLRP3 inflammasome acts as downstream signaling of TLR4 in T/HS and IBD and mediates lung injury induced by mesenteric lymph.
The nitric oxide pathway
Nitric oxide (NO) functions in vasomotor control, signal transduction, and inflammation. It is generated by three isoforms of NO synthase (NOS). Neuronal NOS and endothelial NOS are constitutively expressed mainly by neurons and ECs, respectively, whereas inducible NOS (iNOS) is expressed in cells, such as leukocytes, in response to infection or inflammation (102, 103). The role of NO in lymph-induced remote organ dysfunction is supported by the finding that T/HS lymph infusion elevated plasma nitrite/nitrate concentrations (a measure of NO concentrations), which positively correlated with lung injury (48). In agreement with the above finding, pharmacological inhibition of iNOS prevents lung injury in rats injected with T/HS lymph, and iNOS−/− mice are resistant to T/HS lymph–induced lung injury (48). These data indicate that the iNOS–NO pathway accounts, at least in part, for the toxicity of postinjury mesenteric lymph (Figure 3). Regarding the cell source of iNOS activity, a recent study demonstrates that alveolar macrophages and infiltrated neutrophils are the primary cells that generate iNOS (48); however, the precise mechanisms by which lymph activates iNOS remain unclear. Exhaled NO has been shown to correlate with airway inflammation (104) and is being used as standard clinical practice to diagnose asthma. One study reported that patients with IBD exhibited increased concentrations of alveolar NO as a possible indicator of lung inflammation (105). Thus, the activation of iNOS-NO pathway seems to be a common feature in T/HS and IBD.
The Impact of Lung Diseases on the Gut
Although this review focuses on the role of mesenteric lymph as a conduit to deliver gut-derived factors to lungs based on the anatomical structure of large lymphatic vessels that favor one-way flow, the impact of lung diseases on the gut could not be ignored. There is growing evidence showing secondary organ manifestations, particularly in the GI tract, in patients with lung diseases such as chronic obstructive pulmonary disease (COPD) and asthma. A population-based cohort study reports that patients with COPD have a significant higher risk of developing IBD than healthy subjects (106). Compared with healthy control subjects, patients with COPD exhibit enterocyte damage and leaky gut (107). Children with asthma also show a higher frequency of GI symptoms, particularly diarrhea, vomiting, and abdominal pain (108). An animal study demonstrates that pneumonia induced by Pseudomonas aeruginosa disrupts the gut–epithelial barrier and increases permeability (109). Thus, it has been hypothesized that intestinal hypoperfusion-induced gut barrier dysfunction and dysbiosis represent the underlying mechanisms of lung disease–associated GI damage (Figure 1) (110, 111). Whether the lung-draining lymphatics mediate GI injury as mesenteric lymphatics mediate lung injury would be an interesting topic for future investigation.
Conclusions
Under basal conditions, mesenteric lymph exhibits antiinflammatory and barrier-protective properties because of the existence of abundant albumins, high-density lipoproteins, and proteinase inhibitors. After intestinal injury during local or systemic stress, mesenteric lymph participates in the gut–lung axis of inflammatory response by transporting gut-derived cytotoxic/inflammatory agents to the pulmonary circulation and inducing acute lung injury. The cellular/molecular identity of lymph factors and their specific effects on distant organ/tissue dysfunction remain to be established. Given the complex composition of lymph fluid, comprehensive analyses that integrate proteomics, lipidomics, and nanotechnology may help accelerate our understanding of the mechanisms by which lymph circulation contributes to systemic inflammatory response to injury or infection. It is likely that multiple factors or pathways are involved in the disruption of homeostasis maintained by proinflammatory/antiinflammatory factors as well as different subtypes of immune cells in the lymph. Future studies, therefore, need to consider interfering more than one candidate target.
Supplementary Material
Footnotes
Supported by the U.S. National Institutes of Health grants GM097270 (S.Y.Y.), HL150732 (S.Y.Y.), and HL120954 (M.H.W.), and the Department of Veterans Affairs IK6BX004210 (M.H.W.).
Author Contributions: Y.M. performed the literature search, drafted the manuscript, and prepared the figures. X.Y., V.C., M.H.W., and S.Y.Y. edited and revised the manuscript. S.Y.Y. initiated, directed, and sponsored the work throughout all levels of development.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0196TR on September 2, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Assimakopoulos SF, Triantos C, Thomopoulos K, Fligou F, Maroulis I, Marangos M, et al. Gut-origin sepsis in the critically ill patient: pathophysiology and treatment. Infection. 2018;46:751–760. doi: 10.1007/s15010-018-1178-5. [DOI] [PubMed] [Google Scholar]
- 2.Otani S, Coopersmith CM. Gut integrity in critical illness. J Intensive Care. 2019;7:17. doi: 10.1186/s40560-019-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA, Moore EE, Poggetti R, McAnena OJ, Peterson VM, Abernathy CM, et al. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma J Trauma 199131629–636.[Discussion, pp. 636–638]. [DOI] [PubMed] [Google Scholar]
- 4.Moore EE. Claude H. Organ, Jr. memorial lecture: splanchnic hypoperfusion provokes acute lung injury via a 5-lipoxygenase-dependent mechanism. Am J Surg. 2010;200:681–689. doi: 10.1016/j.amjsurg.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207:E103–E111. doi: 10.1111/j.1749-6632.2010.05713.x. [DOI] [PubMed] [Google Scholar]
- 6.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 7.Songür N, Songür Y, Tüzün M, Doğan I, Tüzün D, Ensari A, et al. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol. 2003;37:292–298. doi: 10.1097/00004836-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Wang J, Liu Z, Lin H, Shi Y, Sun X. Pulmonary dysfunction in 114 patients with inflammatory bowel disease. Medicine (Baltimore) 2017;96:e6808. doi: 10.1097/MD.0000000000006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–532. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 10.Mateer SW, Mathe A, Bruce J, Liu G, Maltby S, Fricker M, et al. Il-6 drives neutrophil-mediated pulmonary inflammation associated with bacteremia in murine models of colitis. Am J Pathol. 2018;188:1625–1639. doi: 10.1016/j.ajpath.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Mateer SW, Hsu A, Goggins BJ, Tay H, Mathe A, et al. Platelet activating factor receptor regulates colitis-induced pulmonary inflammation through the NLRP3 inflammasome. Mucosal Immunol. 2019;12:862–873. doi: 10.1038/s41385-019-0163-3. [DOI] [PubMed] [Google Scholar]
- 12.Mateer SW, Maltby S, Marks E, Foster PS, Horvat JC, Hansbro PM, et al. Potential mechanisms regulating pulmonary pathology in inflammatory bowel disease. J Leukoc Biol. 2015;98:727–737. doi: 10.1189/jlb.3RU1114-563R. [DOI] [PubMed] [Google Scholar]
- 13.Pedica F, Ligorio C, Tonelli P, Bartolini S, Baccarini P. Lymphangiogenesis in Crohn’s disease: an immunohistochemical study using monoclonal antibody D2-40. Virchows Arch. 2008;452:57–63. doi: 10.1007/s00428-007-0540-2. [DOI] [PubMed] [Google Scholar]
- 14.Alexander JS, Chaitanya GV, Grisham MB, Boktor M. Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci. 2010;1207:E75–E85. doi: 10.1111/j.1749-6632.2010.05757.x. [DOI] [PubMed] [Google Scholar]
- 15.Julio-Pieper M, Bravo JA. Intestinal barrier and behavior. Int Rev Neurobiol. 2016;131:127–141. doi: 10.1016/bs.irn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maes M, Cogliati B, Crespo Yanguas S, Willebrords J, Vinken M. Roles of connexins and pannexins in digestive homeostasis. Cell Mol Life Sci. 2015;72:2809–2821. doi: 10.1007/s00018-015-1961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes M, Crespo Yanguas S, Willebrords J, Cogliati B, Vinken M. Connexin and pannexin signaling in gastrointestinal and liver disease. Transl Res. 2015;166:332–343. doi: 10.1016/j.trsl.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan SY, Rigor RR.Regulation of endothelial barrier function.San Rafael, CA: Morgan & Claypool Life Sciences; 2010 [PubMed] [Google Scholar]
- 20.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 21.Alsaigh T, Chang M, Richter M, Mazor R, Kistler EB. In vivo analysis of intestinal permeability following hemorrhagic shock. World J Crit Care Med. 2015;4:287–295. doi: 10.5492/wjccm.v4.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sertaridou E, Papaioannou V, Kolios G, Pneumatikos I. Gut failure in critical care: old school versus new school. Ann Gastroenterol. 2015;28:309–322. [PMC free article] [PubMed] [Google Scholar]
- 23.Petrov MS, van Santvoort HC, Besselink MG, van der Heijden GJ, Windsor JA, Gooszen HG. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acute pancreatitis: a meta-analysis of randomized trials. Arch Surg. 2008;143:1111–1117. doi: 10.1001/archsurg.143.11.1111. [DOI] [PubMed] [Google Scholar]
- 24.Kompan L, Kremzar B, Gadzijev E, Prosek M. Effects of early enteral nutrition on intestinal permeability and the development of multiple organ failure after multiple injury. Intensive Care Med. 1999;25:157–161. doi: 10.1007/s001340050809. [DOI] [PubMed] [Google Scholar]
- 25.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic vessel network structure and physiology. Compr Physiol. 2018;9:207–299. doi: 10.1002/cphy.c180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernier-Latmani J, Petrova TV. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol. 2017;14:510–526. doi: 10.1038/nrgastro.2017.79. [DOI] [PubMed] [Google Scholar]
- 28.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choe K, Jang JY, Park I, Kim Y, Ahn S, Park DY, et al. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest. 2015;125:4042–4052. doi: 10.1172/JCI76509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol. 2016;594:5749–5768. doi: 10.1113/JP272088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jalkanen S, Salmi M. Lymphatic endothelial cells of the lymph node. Nat Rev Immunol. 2020;20:566–578. doi: 10.1038/s41577-020-0281-x. [DOI] [PubMed] [Google Scholar]
- 32.Lucas ED, Tamburini BAJ. Lymph node lymphatic endothelial cell expansion and contraction and the programming of the immune response. Front Immunol. 2019;10:36. doi: 10.3389/fimmu.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coates BM, Staricha KL, Koch CM, Cheng Y, Shumaker DK, Budinger GRS, et al. Inflammatory monocytes drive influenza a virus-mediated lung injury in juvenile mice. J Immunol. 2018;200:2391–2404. doi: 10.4049/jimmunol.1701543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haeger SM, Liu X, Han X, McNeil JB, Oshima K, McMurtry SA, et al. Epithelial heparan sulfate contributes to alveolar barrier function and is shed during lung injury. Am J Respir Cell Mol Biol. 2018;59:363–374. doi: 10.1165/rcmb.2017-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu G, Christman JW. Editorial: alveolar macrophages in lung inflammation and resolution. Front Immunol. 2019;10:2275. doi: 10.3389/fimmu.2019.02275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan SY, Shen Q, Rigor RR, Wu MH. Neutrophil transmigration, focal adhesion kinase and endothelial barrier function. Microvasc Res. 2012;83:82–88. doi: 10.1016/j.mvr.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komarova YA, Kruse K, Mehta D, Malik AB. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017;120:179–206. doi: 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jannaway M, Yang X, Meegan JE, Coleman DC, Yuan SY. Thrombin-cleaved syndecan-3/-4 ectodomain fragments mediate endothelial barrier dysfunction. PLoS One. 2019;14:e0214737. doi: 10.1371/journal.pone.0214737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hippensteel JA, Anderson BJ, Orfila JE, McMurtry SA, Dietz RM, Su G, et al. Circulating heparan sulfate fragments mediate septic cognitive dysfunction. J Clin Invest. 2019;129:1779–1784. doi: 10.1172/JCI124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu MH. Endothelial focal adhesions and barrier function. J Physiol. 2005;569:359–366. doi: 10.1113/jphysiol.2005.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu MH, Guo M, Yuan SY, Granger HJ. Focal adhesion kinase mediates porcine venular hyperpermeability elicited by vascular endothelial growth factor. J Physiol. 2003;552:691–699. doi: 10.1113/jphysiol.2003.048405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee V, Yang X, Ma Y, Cha B, Meegan JE, Wu M, et al. Endothelial microvesicles carrying src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis. Cardiovasc Res. 2019 doi: 10.1093/cvr/cvz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Meegan JE, Jannaway M, Coleman DC, Yuan SY. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc Res. 2018;114:1752–1763. doi: 10.1093/cvr/cvy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun C, Beard RS, Jr, McLean DL, Rigor RR, Konia T, Wu MH, et al. ADAM15 deficiency attenuates pulmonary hyperpermeability and acute lung injury in lipopolysaccharide-treated mice. Am J Physiol Lung Cell Mol Physiol. 2013;304:L135–L142. doi: 10.1152/ajplung.00133.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavriani G, Domingos HV, Soares AL, Trezena AG, Ligeiro-Oliveira AP, Oliveira-Filho RM, et al. Lymphatic system as a path underlying the spread of lung and gut injury after intestinal ischemia/reperfusion in rats. Shock. 2005;23:330–336. doi: 10.1097/01.shk.0000157303.76749.9b. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Zhang S, Tsui N. Mesenteric lymph duct drainage attenuates acute lung injury in rats with severe intraperitoneal infection. Inflammation. 2015;38:1239–1249. doi: 10.1007/s10753-014-0091-z. [DOI] [PubMed] [Google Scholar]
- 48.Senthil M, Watkins A, Barlos D, Xu DZ, Lu Q, Abungu B, et al. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 2007;246:822–830. doi: 10.1097/SLA.0b013e3180caa3af. [DOI] [PubMed] [Google Scholar]
- 49.Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129:39–47. doi: 10.1067/msy.2001.109119. [DOI] [PubMed] [Google Scholar]
- 50.Cheng AM, Moore EE, Masuno T, Escobar GA, Sarin EL, Johnson JL, et al. Normal mesenteric lymph blunts the pulmonary inflammatory response to endotoxin. J Surg Res. 2006;136:166–171. doi: 10.1016/j.jss.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Zhao ZG, Zhang LM, Song W, Du HB, Cui H, Niu CY. Normal mesenteric lymph ameliorates acute kidney injury following lipopolysaccharide challenge in mice. Ren Fail. 2014;36:1304–1309. doi: 10.3109/0886022X.2014.938585. [DOI] [PubMed] [Google Scholar]
- 52.Leak LV, Liotta LA, Krutzsch H, Jones M, Fusaro VA, Ross SJ, et al. Proteomic analysis of lymph Proteomics 20044753–765.[Published erratum appears in Proteomics 4:1519.] [DOI] [PubMed] [Google Scholar]
- 53.Dzieciatkowska M, D’Alessandro A, Moore EE, Wohlauer M, Banerjee A, Silliman CC, et al. Lymph is not a plasma ultrafiltrate: a proteomic analysis of injured patients. Shock. 2014;42:485–498. doi: 10.1097/SHK.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trevaskis NL, Hu L, Caliph SM, Han S, Porter CJ. The mesenteric lymph duct cannulated rat model: application to the assessment of intestinal lymphatic drug transport. J Vis Exp. 2015:5239. doi: 10.3791/52389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohan A, Yoder S, Tso P. Lymphatics in intestinal transport of nutrients and gastrointestinal hormones. Ann N Y Acad Sci. 2010;1207:E44–E51. doi: 10.1111/j.1749-6632.2010.05753.x. [DOI] [PubMed] [Google Scholar]
- 56.Kojima M, Gimenes-Junior JA, Langness S, Morishita K, Lavoie-Gagne O, Eliceiri B, et al. Exosomes, not protein or lipids, in mesenteric lymph activate inflammation: unlocking the mystery of post-shock multiple organ failure. J Trauma Acute Care Surg. 2017;82:42–50. doi: 10.1097/TA.0000000000001296. [DOI] [PubMed] [Google Scholar]
- 57.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams CA, Jr, Xu DZ, Lu Q, Deitch EA. Factors larger than 100 kd in post-hemorrhagic shock mesenteric lymph are toxic for endothelial cells. Surgery. 2001;129:351–363. doi: 10.1067/msy.2001.111698. [DOI] [PubMed] [Google Scholar]
- 59.Deitch EA, Shi HP, Lu Q, Feketeova E, Skurnick J, Xu DZ. Mesenteric lymph from burned rats induces endothelial cell injury and activates neutrophils. Crit Care Med. 2004;32:533–538. doi: 10.1097/01.CCM.0000109773.00644.F4. [DOI] [PubMed] [Google Scholar]
- 60.Davidson MT, Deitch EA, Lu Q, Osband A, Feketeova E, Németh ZH, et al. A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines. Surgery. 2004;136:32–41. doi: 10.1016/j.surg.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Breithaupt-Faloppa AC, Vitoretti LB, Coelho FR, dos Santos Franco AL, Domingos HV, Sudo-Hayashi LS, et al. Nitric oxide mediates lung vascular permeability and lymph-borne IL-6 after an intestinal ischemic insult. Shock. 2009;32:55–61. doi: 10.1097/SHK.0b013e31818bb7a1. [DOI] [PubMed] [Google Scholar]
- 62.Altshuler AE, Kistler EB, Schmid-Schönbein GW. Autodigestion: proteolytic degradation and multiple organ failure in shock. Shock. 2016;45:483–489. doi: 10.1097/SHK.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang M, Kistler EB, Schmid-Schönbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock. 2012;37:297–305. doi: 10.1097/SHK.0b013e318240b59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosário HS, Waldo SW, Becker SA, Schmid-Schönbein GW. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am J Pathol. 2004;164:1707–1716. doi: 10.1016/S0002-9440(10)63729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci USA. 2000;97:1772–1777. doi: 10.1073/pnas.97.4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeLano FA, Hoyt DB, Schmid-Schönbein GW. Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock. Sci Transl Med. 2013;5:169ra11. doi: 10.1126/scitranslmed.3005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 68.Kaiser VL, Sifri ZC, Dikdan GS, Berezina T, Zaets S, Lu Q, et al. Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity. Shock. 2005;23:417–425. doi: 10.1097/01.shk.0000160524.14235.6c. [DOI] [PubMed] [Google Scholar]
- 69.Körmöczi GF, Wölfel UM, Rosenkranz AR, Hörl WH, Oberbauer R, Zlabinger GJ. Serum proteins modified by neutrophil-derived oxidants as mediators of neutrophil stimulation. J Immunol. 2001;167:451–460. doi: 10.4049/jimmunol.167.1.451. [DOI] [PubMed] [Google Scholar]
- 70.Ma Y, Yang X, Chatterjee V, Meegan JE, Beard RS, Jr, Yuan SY. Role of neutrophil extracellular traps and vesicles in regulating vascular endothelial permeability. Front Immunol. 2019;10:1037. doi: 10.3389/fimmu.2019.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato K, Kennedy L, Liangpunsakul S, Kusumanchi P, Yang Z, Meng F, et al. Intercellular communication between hepatic cells in liver diseases. Int J Mol Sci. 2019;20:E2180. doi: 10.3390/ijms20092180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kojima M, Costantini TW, Eliceiri BP, Chan TW, Baird A, Coimbra R. Gut epithelial cell-derived exosomes trigger posttrauma immune dysfunction. J Trauma Acute Care Surg. 2018;84:257–264. doi: 10.1097/TA.0000000000001748. [DOI] [PubMed] [Google Scholar]
- 73.Kojima M, Gimenes-Junior JA, Chan TW, Eliceiri BP, Baird A, Costantini TW, et al. Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 2018;32:97–110. doi: 10.1096/fj.201700488R. [DOI] [PubMed] [Google Scholar]
- 74.Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125:1215–1227. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 76.Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez RJ, Moore EE, Biffl WL, Ciesla DJ, Silliman CC. The lipid fraction of post-hemorrhagic shock mesenteric lymph (PHSML) inhibits neutrophil apoptosis and enhances cytotoxic potential. Shock. 2000;14:404–408. doi: 10.1097/00024382-200014030-00028. [DOI] [PubMed] [Google Scholar]
- 78.Koike K, Moore EE, Moore FA, Kim FJ, Carl VS, Banerjee A. Gut phospholipase A2 mediates neutrophil priming and lung injury after mesenteric ischemia-reperfusion. Am J Physiol. 1995;268:G397–G403. doi: 10.1152/ajpgi.1995.268.3.G397. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez RJ, Moore EE, Ciesla DJ, Biffl WL, Offner PJ, Silliman CC. Phospholipase A(2): derived neutral lipids from posthemorrhagic shock mesenteric lymph prime the neutrophil oxidative burst. Surgery. 2001;130:198–203. doi: 10.1067/msy.2001.115824. [DOI] [PubMed] [Google Scholar]
- 80.Jordan JR, Moore EE, Sarin EL, Damle SS, Kashuk SB, Silliman CC, et al. Arachidonic acid in postshock mesenteric lymph induces pulmonary synthesis of leukotriene b4. J Appl Physiol (1985) 2008;104:1161–1166. doi: 10.1152/japplphysiol.00022.2007. [DOI] [PubMed] [Google Scholar]
- 81.Souza DG, Coutinho SF, Silveira MR, Cara DC, Teixeira MM. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur J Pharmacol. 2000;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- 82.Morishita K, Aiboshi J, Kobayashi T, Mikami S, Yokoyama Y, Ogawa K, et al. Lipidomics analysis of mesenteric lymph after trauma and hemorrhagic shock. J Trauma Acute Care Surg. 2012;72:1541–1547. doi: 10.1097/TA.0b013e318256df15. [DOI] [PubMed] [Google Scholar]
- 83.Meliton AY, Meng F, Tian Y, Sarich N, Mutlu GM, Birukova AA, et al. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol. 2015;308:L550–L562. doi: 10.1152/ajplung.00248.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am J Respir Cell Mol Biol. 2013;49:6–17. doi: 10.1165/rcmb.2012-0411TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karki P, Birukov KG. Lipid mediators in the regulation of endothelial barriers. Tissue Barriers. 2018;6:e1385573. doi: 10.1080/21688370.2017.1385573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson DG. Leucocyte trafficking via the lymphatic vasculature- mechanisms and consequences. Front Immunol. 2019;10:471. doi: 10.3389/fimmu.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013;190:970–976. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arokiasamy S, Zakian C, Dilliway J, Wang W, Nourshargh S, Voisin MB. Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation. Sci Rep. 2017;7:44189. doi: 10.1038/srep44189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorlino CV, Ranocchia RP, Harman MF, García IA, Crespo MI, Morón G, et al. Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes. J Immunol. 2014;193:1966–1974. doi: 10.4049/jimmunol.1301791. [DOI] [PubMed] [Google Scholar]
- 90.Hampton HR, Chtanova T. Lymphatic migration of immune cells. Front Immunol. 2019;10:1168. doi: 10.3389/fimmu.2019.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.MacPherson GG, Jenkins CD, Stein MJ, Edwards C. Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J Immunol. 1995;154:1317–1322. [PubMed] [Google Scholar]
- 92.Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359:114–119. doi: 10.1126/science.aam5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Harris HW, Johnson JA, Wigmore SJ. Endogenous lipoproteins impact the response to endotoxin in humans. Crit Care Med. 2002;30:23–31. doi: 10.1097/00003246-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Peltz ED, Moore EE, Zurawel AA, Jordan JR, Damle SS, Redzic JS, et al. Proteome and system ontology of hemorrhagic shock: exploring early constitutive changes in postshock mesenteric lymph. Surgery. 2009;146:347–357. doi: 10.1016/j.surg.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Ma Y, Zhang X, Bao H, Mi S, Cai W, Yan H, et al. Toll-like receptor (TLR) 2 and TLR4 differentially regulate doxorubicin induced cardiomyopathy in mice. PLoS One. 2012;7:e40763. doi: 10.1371/journal.pone.0040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res. 2016;110:51–61. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Q, He G, Wang J, Wang Y, Chen W, Guo T. Down-regulation of toll-like receptor 4 alleviates intestinal ischemia reperfusion injury and acute lung injury in mice. Oncotarget. 2017;8:13678–13689. doi: 10.18632/oncotarget.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reino DC, Pisarenko V, Palange D, Doucet D, Bonitz RP, Lu Q, et al. Trauma hemorrhagic shock-induced lung injury involves a gut-lymph-induced TLR4 pathway in mice. PLoS One. 2011;6:e14829. doi: 10.1371/journal.pone.0014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He Y, Hara H, Núñez G. Mechanism and regulation of nlrp3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837, 837a-837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Durán WN, Beuve AV, Sánchez FA. Nitric oxide, S-nitrosation, and endothelial permeability. IUBMB Life. 2013;65:819–826. doi: 10.1002/iub.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paraskakis E, Brindicci C, Fleming L, Krol R, Kharitonov SA, Wilson NM, et al. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am J Respir Crit Care Med. 2006;174:260–267. doi: 10.1164/rccm.200506-962OC. [DOI] [PubMed] [Google Scholar]
- 105.Protopapas AA, Vradelis S, Karampitsakos T, Steiropoulos P, Chatzimichael A, Paraskakis E. Elevated levels of alveolar nitric oxide may indicate presence of small airway inflammation in patients with inflammatory bowel disease. Lung. 2019;197:663–670. doi: 10.1007/s00408-019-00253-0. [DOI] [PubMed] [Google Scholar]
- 106.Ekbom A, Brandt L, Granath F, Löfdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung. 2008;186:167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 107.Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145:245–252. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 108.Caffarelli C, Deriu FM, Terzi V, Perrone F, De Angelis G, Atherton DJ. Gastrointestinal symptoms in patients with asthma. Arch Dis Child. 2000;82:131–135. doi: 10.1136/adc.82.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, et al. Mechanisms of intestinal barrier dysfunction in sepsis. Shock. 2016;46:52–59. doi: 10.1097/SHK.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keely S, Hansbro PM. Lung-gut cross talk: a potential mechanism for intestinal dysfunction in patients with COPD. Chest. 2014;145:199–200. doi: 10.1378/chest.13-2077. [DOI] [PubMed] [Google Scholar]
- 111.Vaughan A, Frazer ZA, Hansbro PM, Yang IA. COPD and the gut-lung axis: the therapeutic potential of fibre. J Thorac Dis. 2019;11:S2173–S2180. doi: 10.21037/jtd.2019.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.