The use of electronic cigarettes (e-cigarettes, or electronic nicotine delivery systems [ENDS]), otherwise known as “vaping,” has been on the rise worldwide (1). The proponents of the e-cigarette have touted it as a “healthier” alternative, or simply a replacement, in traditional cigarette smoking cessation attempts (1). However, the short- and long-term effects of e-cigarettes remain poorly understood and understudied. Importantly, with the rapid rise of its use, especially among younger generations, and also spurred by mental stresses during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, studies of its effects on human health are ever more crucial in ensuring that users are not in fact using a more harmful alternative (2).
As e-cigarette usage is a relatively new practice, studies to assess its effects, especially long-term ones, on human respiratory health remains a challenge. Early reports had shown that the use of e-cigarettes contributes to respiratory epithelium cell death and damage, immune cell death, and altered inflammatory responses (3–5) (Figure 1). In addition, the e-cigarette liquid has also been shown to increase inflammation and susceptibility to viral infection in primary human airway cells (6). Currently, in the midst of the SARS-CoV-2 pandemic, there is renewed interest in the relationship between tobacco products and susceptibility to respiratory viruses, especially those that can cause severe diseases such as influenza viruses and SARS-CoV-2 (7). Recent findings suggested that e-cigarette usage may also contribute to an increase in susceptibility to viral infection, as it alters the antiviral immune response (8). The study by Wu and colleagues found increased susceptibility of primary human airway cells to viruses (6), but that work was done using the liquid of e-cigarettes, and direct studies assessing the association between e-cigarette use and viral susceptibility has been lacking.
Figure 1.
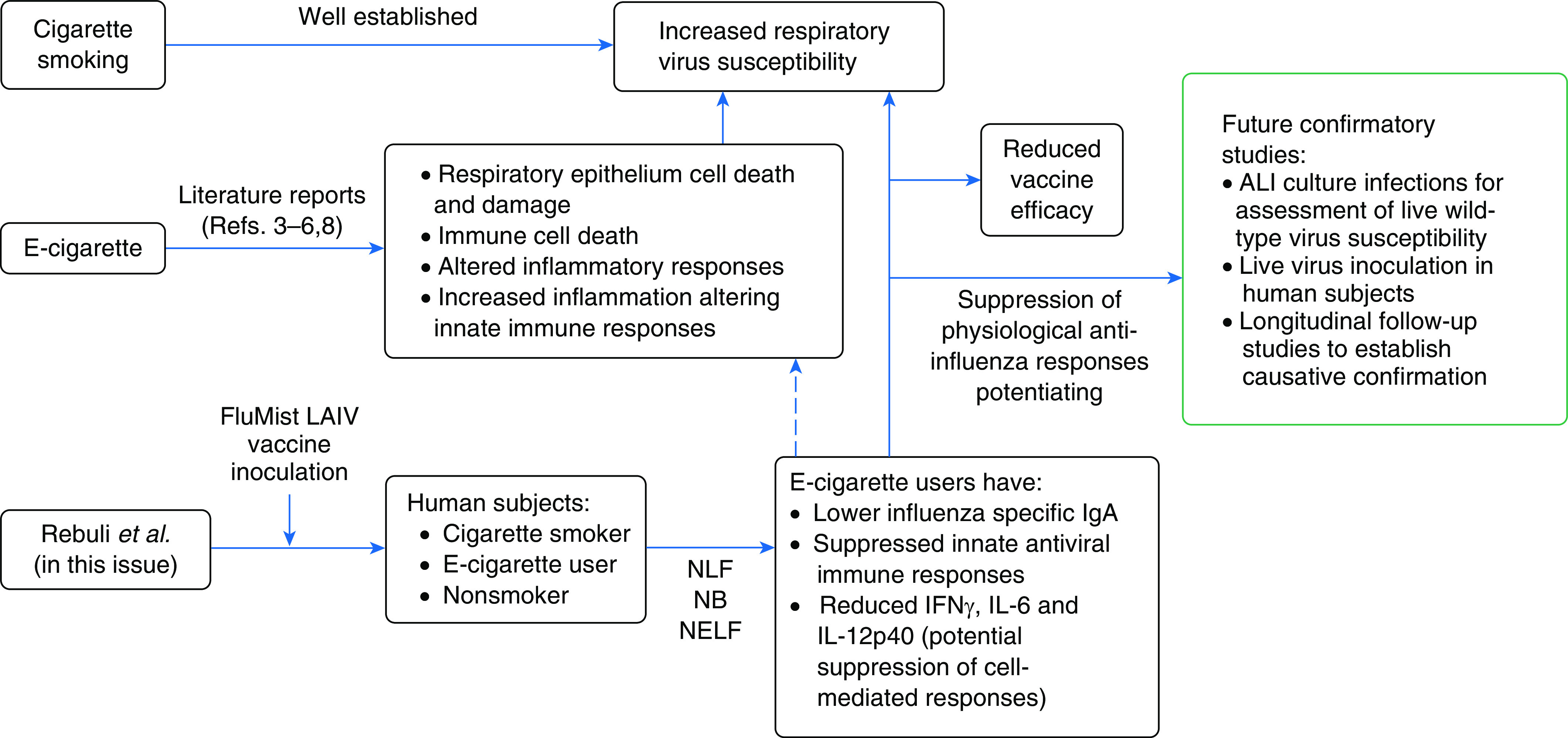
Effects of electronic cigarettes on potentiating susceptibility toward respiratory viruses. ALI = air–liquid interface; e-cigarette = electronic cigarette; LAIV = live attenuated influenza virus; NB = nasal biopsy; NELF = nasal epithelial lining fluid; NLF = nasal lavage fluid.
In this issue of the Journal, Rebuli and colleagues (pp. 126–137) begin to address this by reporting on a series of experiments comparing cigarette and e-cigarette users with nonsmokers in terms of their responses to live attenuated influenza virus (LAIV) vaccine, a surrogate for viral infection (9). To assess the systemic effects of e-cigarette use and antiviral responses, the group recruited volunteers and inoculated them with the FluMist LAIV vaccine (Figure 1). They then followed up by collecting nasal lavage fluid (NLF), nasal biopsy samples, and nasal epithelial lining fluid for comparison of responses between the groups. Following inoculation, they found no change in NLF viral loads between groups, a result that differed from a previous study possibly because of the lower average number of cigarettes smoked (10). However, the mucosal IgA levels in NLF values did differ significantly, such that influenza-specific IgA levels were lower in both cigarette and e-cigarette groups, an indication of impaired humoral immunity against the virus and/or vaccine.
The group then assessed the nasal epithelial gene expression changes following LAIV inoculation between the groups using their nasal biopsy samples. The assessment allowed the comparison of how the e-cigarette usage group fared in terms of their antiviral responses. The time points of Day 1 and Day 8 postinoculation allowed assessment of both early innate and late adaptive changes in response to the LAIV vaccine. Interestingly, the e-cigarette group was found to fare worst among the groups. Although both the cigarette and e-cigarette groups exhibited suppressed response genes, the e-cigarette group experienced a greater suppression. In addition, the e-cigarette group also showed consistent suppression in their cytokine profile, in which key antiviral cytokines including IFNγ, IL-6, and IL-12p40 were markedly reduced. The general finding from the gene expression and cytokine profiles suggested that innate epithelial responses, initial cross-talk between epithelium and innate immune cells, and potentially the cell-mediated and humoral immune responses were all suppressed by e-cigarette usage. With a blanket suppression of the antiviral responses following LAIV inoculation, it is hence not surprising that the accompanying covariate analysis identified tobacco products, specifically e-cigarettes in this study, to interact and influence host antiviral defenses against LAIV. Overall, the findings from this study suggest that e-cigarette use suppresses physiological anti-influenza responses. As responses to viral pathogens tend to be similar, it is possible that e-cigarettes might also increase susceptibility to other respiratory viruses, but that would need to be confirmed by further study. Nevertheless, this study convincingly shows that protection against influenza conferred by the LAIV vaccine was markedly reduced, as manifested by the lowered IgA production in the e-cigarette group. This finding is important as it may also affect efficacy of other vaccines such as the SARS-CoV-2 vaccines currently in development and testing pipelines.
In summary, this study provides novel information regarding the effects of e-cigarette use on antiviral responses, which is important in the context of the current SARS-CoV-2 pandemic. Findings from this study set the baseline of e-cigarette usage and respiratory viral infections, which may be relevant to the development of public health policies regarding e-cigarette usage. Future studies can build upon this to assess viral susceptibility using live virus infection, as although LAIV retains the ability to replicate, it does not possess certain virulence factors that may alter the course of infection. Live infections can be tested in physiologically relevant models, such as the air–liquid interface human airway epithelial cells (hAECs), which is an established model for respiratory viral infections (11–13). The hAECs model subjected to e-cigarette vapor followed by infection could be suitable for comparing viral load after infection, providing a more accurate assessment of susceptibility toward respiratory viruses. That assessment can also be extended to include a comparison of epithelial-immune cross-talk via immune cell coculture models of hAECs (14). Susceptibility to other viruses that cause milder infection, such as rhinoviruses (15), can be assessed using live virus inoculation in human subjects. Finally, the authors rightly point out the need for causative verification via longitudinal studies in e-cigarette users to compare their frequency and severity of viral infections with nonsmokers. These future studies will likely confirm the detrimental effects of e-cigarettes identified in this study, thereby underscoring the public health threat they may pose.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2020-0501ED on November 20, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Knapp S. Vaping: cell damage at the receiving ENDS. Am J Respir Cell Mol Biol. 2020;63:271–272. doi: 10.1165/rcmb.2020-0244ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pearce K, Gray N, Gaur P, Jeon J, Suarez A, Shannahan J, et al. Toxicological analysis of aerosols derived from three electronic nicotine delivery systems using normal human bronchial epithelial cells. Toxicol In Vitro. 2020;69:104997. doi: 10.1016/j.tiv.2020.104997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larcombe AN, Janka MA, Mullins BJ, Berry LJ, Bredin A, Franklin PJ. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Am J Physiol Lung Cell Mol Physiol. 2017;313:L67–L79. doi: 10.1152/ajplung.00203.2016. [DOI] [PubMed] [Google Scholar]
- 4. Serpa GL, Renton ND, Lee N, Crane MJ, Jamieson AM. Electronic nicotine delivery system aerosol-induced cell death and dysfunction in macrophages and lung epithelial cells. Am J Respir Cell Mol Biol. 2020;63:306–316. doi: 10.1165/rcmb.2019-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197:492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9:e108342. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alla F, Berlin I, Nguyen-Thanh V, Guignard R, Pasquereau A, Quelet S, et al. Tobacco and COVID-19: a crisis within a crisis? Can J Public Health. doi: 10.17269/s41997-020-00427-x. [online ahead of print] 14 Oct 2020; DOI: 10.17269/s41997-020-00427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2016;311:L135–L144. doi: 10.1152/ajplung.00170.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rebuli ME, Glista-Baker E, Hoffman JR, Duffney PF, Robinette C, Speen AM, et al. Electronic-cigarette use alters nasal mucosal immune response to live-attenuated influenza virus: a clinical trial. Am J Respir Cell Mol Biol. 2021;64:126–137. doi: 10.1165/rcmb.2020-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noah TL, Zhou H, Monaco J, Horvath K, Herbst M, Jaspers I. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ Health Perspect. 2011;119:78–83. doi: 10.1289/ehp.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan KS, Ong HH, Yan Y, Liu J, Li C, Ong YK, et al. In vitro model of fully differentiated human nasal epithelial cells infected with rhinovirus reveals epithelium-initiated immune responses. J Infect Dis. 2018;217:906–915. doi: 10.1093/infdis/jix640. [DOI] [PubMed] [Google Scholar]
- 12. Yan Y, Tan KS, Li C, Tran T, Chao SS, Sugrue RJ, et al. Human nasal epithelial cells derived from multiple subjects exhibit differential responses to H3N2 influenza virus infection in vitro. J Allergy Clin Immunol. 2016;138:276–281, e15. doi: 10.1016/j.jaci.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 13. Essaidi-Laziosi M, Brito F, Benaoudia S, Royston L, Cagno V, Fernandes-Rocha M, et al. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J Allergy Clin Immunol. 2018;141:2074–2084. doi: 10.1016/j.jaci.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luukkainen A, Puan KJ, Yusof N, Lee B, Tan KS, Liu J, et al. A co-culture model of PBMC and stem cell derived human nasal epithelium reveals rapid activation of NK and innate T cells upon Influenza A virus infection of the nasal epithelium. Front Immunol. 2018;9:2514. doi: 10.3389/fimmu.2018.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ravi A, Chang M, van de Pol M, Yang S, Aliprantis A, Thornton B, et al. U-BIOPRED Study Group. Rhinovirus-16 induced temporal interferon responses in nasal epithelium links with viral clearance and symptoms. Clin Exp Allergy. 2019;49:1587–1597. doi: 10.1111/cea.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


