GPCRs (G protein–coupled receptors) are the largest gene family in the human genome and are the direct or indirect targets of most prescribed drugs. In obstructive lung diseases such as asthma and chronic obstructive pulmonary disease, two GPCRs have been historically targeted for therapeutic control of the exaggerated contraction of airway smooth muscle (ASM) and the associated resistance to breathing: the β2AR (β-2 adrenoceptor) and the m3 mAChR (m3 muscarinic acetylcholine receptor) (1). The m3 mAChR mediates the procontractile effects of acetylcholine on ASM, which are enhanced in asthma by inflammatory actions in the airway. The β2AR mediates prorelaxant signaling in ASM, effectively counteracting the procontractile signaling of the m3 mAChR.
Other well-known GPCRs with procontractile and prorelaxant signaling capabilities have been identified and characterized in ASM, making them putative therapeutic targets (reviewed in References 1–3). Recently, a curious collection of GPCRs, including orphan receptors, as well as other GPCRs known better for their actions in tissues and cells outside the lung, have also been found in ASM. These include members of the bitter taste receptor family (4), odorant receptors (5), calcium sensing receptors (6), and proton-sensing receptors (7). Each has been shown to be expressed, signal, and, when activated, either contract or relax ASM cells and airways ex vivo or promote or reverse airway resistance in vivo. In this issue of the Journal, Wu and colleagues (pp. 59–68) advance the photoreceptive GPCR OPN3 (Opsin 3) receptor as a novel ASM GPCR capable of regulating the ASM contractile state (8).
OPN3 is member of the Opsin family of GPCRs more commonly known for their role in vision. OPN2 (rhodopsin) is a well-characterized GPCR expressed in the retina, mediating vision signal transduction in response to photons. Primordial opsins, found in single-cell organisms, react with external light in the visible spectrum and modulate intracellular processes during times of dark and light. Although primitive opsin receptors are mostly ion channels and ion pumps, vertebrate opsins rely on G protein second messengers to transduce cellular signals (Figure 1). As evolution progressed from single-cell organisms to vertebrates, regions of specialized cells retained a high sensitivity to light (i.e., the eye). However, the expression of opsins in nonvisual tissues was retained despite no known direct connection to the central nervous system. Opsin 3 in particular is expressed and functional in an array of different organs (9–14). But what is the use of light-sensitive proteins in areas that have dramatically less light, if any light at all? Perhaps evolution retained these primordial adaptations of our predecessors, such that somatic opsins modulate peripheral functions with light through circadian rhythm (9).
Figure 1.
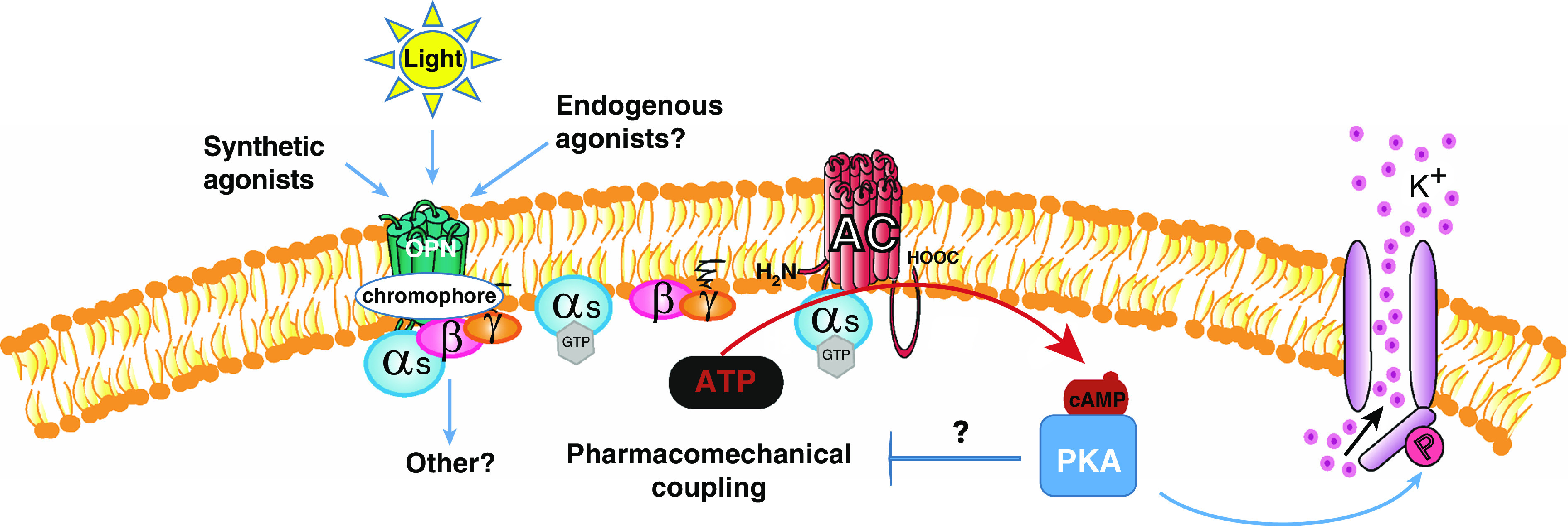
Putative Opsin (OPN)-mediated signaling promotes relaxation of airway smooth muscle (ASM). Light, synthetic, or endogenous agonist(s) activate the OPN receptor, which initiates a signaling cascade causing activation of AC via Gαs leading to an increase in cAMP, followed by activation of PKA, which can promote downstream pathways of ASM relaxation. AC = adenylyl cyclase; HOOC = carboxyl group; K+ = potassium; P = phosphate; PKA = protein kinase A.
OPN3 and other extraocular opsins respond to specific wavelengths of light in the presence of a chromophore. In their 2019 paper, Yim and colleagues first noted the expression of OPN3 in both mouse and human ASM cells, and demonstrated photorelaxation of ex vivo contracted airways from both human and mouse, with blue light having the greatest effect (15). Moreover, this relaxation could be enhanced by the addition of the OPN chromophore 9 cisretinal, whereas pharmacological blockade of the large conductance calcium-activated potassium channel (BK) significantly attenuated the relaxation. In the current study, Wu and colleagues demonstrate that Opn3 gene ablation significantly reduces the relaxation of contracted tracheal rings by blue light.
The effect of BK channel inhibition notwithstanding, the prorelaxant signaling and mechanisms mediating blue light–induced photorelaxation of ASM remained largely undetermined. Whereas the signaling mechanisms by photon-activated rhodopsin enabling vision have been exhaustively delineated, the signaling mechanisms by which extraocular opsin regulates cellular function are poorly understood. Wu and colleagues undertook a systematic analysis of OPN3 downstream signaling elements in ASM, with the goal of linking these elements to the regulation of ASM contraction. In human ASM cells, Gαs was shown to coimmunoprecipitate with OPN3 and could also be visualized in close proximity to OPN3 in cellular fluorescence assays. Consistent with these observations, blue light was able to induce cAMP accumulation in tracheal ring preparations. Of note, BK channel conductance is known to be enhanced by the (cAMP-activated) cAMP-dependent PKA (protein kinase A).
The critical role of OPN3/Gαs/cAMP/PKA signaling in OPN3-mediated photorelaxation was confirmed by demonstrating that the pharmacological inhibition of PKA with Rp-cAMPS effectively reversed the relaxation of contracted tracheal rings by blue light.
Collectively, these findings suggest that OPN3 uses canonical GPCR signaling to PKA to relax ASM, similar to the mechanisms of relaxation effected by β2AR agonism, albeit with a seemingly greater dependence on BK channels as PKA effectors. In addition, the present study reveals that OPN3 appears subject to similar mechanisms of desensitization known to affect the β2AR. GRK2 (GPCR kinase 2), which phosphorylates the β2AR (and other GPCRs) to diminish its coupling to G protein, is shown to colocalize with OPN3, and to coimmunoprecipitate with OPN3 as well. Consistent with GRK2-mediated desensitization, inhibition of GRK2 also significantly increases the relaxant effect of blue light.
Finally, the retention of some measure of photorelaxation in tracheal rings from OPN3 null mice suggests the possibility of other photoreceptive opsins in ASM. Indeed, the original description of opsin receptors in ASM by this group (12) described the expression of OPN4 in murine ASM as well.
Owing to the low penetration of blue wavelengths of light through tissues, it seems likely that ligands that activate OPN3 in the absence of light will be the only practical way of harnessing prorelaxant signaling capabilities of OPN3 in ASM. An obvious teleological assumption is “if these receptors are in the airway, there must be an endogenous ligand that is not light.” Whether or not an endogenous ligand can be found, the potential exists for synthetic ligand development of OPN3 agonists, based on structure–activity relationship studies, receptor modeling, and other creative strategies, such as those recently used to discover agonists for the proton-sensing receptor GPR68 (16), whose cognate ligand is believed to be the proton.
And although we can likely bank on the increasing capabilities of synthetic chemistry to deliver a small-molecule OPN3 agonist, it should be noted that (therapeutic) photorelaxation of ASM is still on the table. Wu and colleagues present data that ASM transduced to express an isoform of opsin sensitive to more tissue-penetrant wavelengths of light (OPN1LW), increased cyclic AMP in response to longer wavelengths of light. This raises the question as to whether ASM and other peripheral tissues expressed isoforms of opsins that indeed respond to tissue-penetrating wavelengths of light.
These findings describe yet another family of receptors that are expressed in ASM that were previously thought to be only expressed in sensory tissues, joining the families of odorant and olfactory receptors in this regard. Future research will need to identify endogenous or synthetic ligands, and sensitizing chromophores, of the opsin family of receptors to fully realize their prorelaxant potential in ASM.
Supplementary Material
Footnotes
Supported by U.S. National Institutes of Health grants R01 HL131626, R01 HL151467, R01 HL141462-01A1, and U01 HL131022 (V.P.K.), and R01 HL58506, R01 AI110007, R01 HL136209, R01 AI161296, R01 HL145392, and P01 HL114471 (R.B.P.).
Originally Published in Press as DOI: 10.1165/rcmb.2020-0468ED on November 9, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal. 2006;18:2105–2120. doi: 10.1016/j.cellsig.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2. Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- 3. Pera T, Penn RB. Bronchoprotection and bronchorelaxation in asthma: new targets, and new ways to target the old ones. Pharmacol Ther. 2016;164:82–96. doi: 10.1016/j.pharmthera.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deshpande DA, Wang WCH, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aisenberg WH, Huang J, Zhu W, Rajkumar P, Cruz R, Santhanam L, et al. Defining an olfactory receptor function in airway smooth muscle cells. Sci Rep. 2016;6:38231. doi: 10.1038/srep38231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yarova PL, Stewart AL, Sathish V, Jr, RDB, Thompson MA, Lowe APP, et al. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med. 2015;7:284ra60. doi: 10.1126/scitranslmed.aaa0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saxena H, Deshpande DA, Tiegs BC, Yan H, Battafarano RJ, Burrows WM, et al. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol. 2012;166:981–990. doi: 10.1111/j.1476-5381.2011.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu AD, Dan W, Zhang Y, Vemaraju S, Upton BA, Lang RA, et al. Opsin 3–Gαs promotes airway smooth muscle relaxation modulated by G protein receptor kinase 2. Am J Respir Cell Mol Biol. 2021;64:59–68. doi: 10.1165/rcmb.2020-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nayak G, Zhang KX, Vemaraju S, Odaka Y, Buhr ED, Holt-Jones A, et al. Adaptive thermogenesis in mice is enhanced by opsin 3-dependent adipocyte light sensing. Cell Rep. 2020;30:672–686, e8. doi: 10.1016/j.celrep.2019.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barreto Ortiz S, Hori D, Nomura Y, Yun X, Jiang H, Yong H, et al. Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition. Am J Physiol Lung Cell Mol Physiol. 2018;314:L93–L106. doi: 10.1152/ajplung.00091.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Assis LVM, Mendes D, Silva MM, Kinker GS, Pereira-Lima I, Moraes MN, et al. Melanopsin mediates UVA-dependent modulation of proliferation, pigmentation, apoptosis, and molecular clock in normal and malignant melanocytes. Biochim Biophys Acta Mol Cell Res. 2020;1867:118789. doi: 10.1016/j.bbamcr.2020.118789. [DOI] [PubMed] [Google Scholar]
- 12. Kasper G, Taudien S, Staub E, Mennerich D, Rieder M, Hinzmann B, et al. Different structural organization of the encephalopsin gene in man and mouse. Gene. 2002;295:27–32. doi: 10.1016/s0378-1119(02)00799-0. [DOI] [PubMed] [Google Scholar]
- 13. Sikka G, Hussmann GP, Pandey D, Cao S, Hori D, Park JT, et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc Natl Acad Sci USA. 2014;111:17977–17982. doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White JH, Chiano M, Wigglesworth M, Geske R, Riley J, White N, et al. GAIN investigators. Identification of a novel asthma susceptibility gene on chromosome 1qter and its functional evaluation. Hum Mol Genet. 2008;17:1890–1903. doi: 10.1093/hmg/ddn087. [DOI] [PubMed] [Google Scholar]
- 15. Yim PD, Gallos G, Perez-Zoghbi JF, Zhang Y, Xu D, Wu A, et al. Airway smooth muscle photorelaxation via opsin receptor activation. Am J Physiol Lung Cell Mol Physiol. 2019;316:L82–L93. doi: 10.1152/ajplung.00135.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foster SR, Hauser AS, Vedel L, Strachan RT, Huang X-P, Gavin AC, et al. Discovery of human signaling systems: pairing peptides to G protein-coupled receptors. Cell. 2019;179:895–908, e21. doi: 10.1016/j.cell.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


