Abstract
Purpose
We evaluated the dose-responsiveness, efficacy, and safety of low-dose triple antihypertensive combination therapies in patients with mild-to-moderate hypertension.
Patients and Methods
After a 1 to 2-week placebo run-in period, 248 patients were randomized to the half-dose triple combination (amlodipine 2.5 mg + losartan 25 mg + chlorthalidone 6.25 mg), third-dose triple combination (amlodipine 1.67 mg + losartan 16.67 mg + chlorthalidone 4.17 mg), quarter-dose triple combination (amlodipine 1.25 mg + losartan 12.5 mg + chlorthalidone 3.13mg), amlodipine 10mg, amlodipine 5mg, losartan 100mg, and placebo groups for 8 weeks. The primary outcome was the mean change in systolic blood pressure (SBP) from baseline to week 8.
Results
The placebo-corrected SBP reductions of the half-dose, third-dose, quarter-dose combination, amlodipine 10 mg, amlodipine 5 mg and losartan 100 mg treatments were −17.2, −19.5, −14.9, −18.5, −11.3 and −9.9 mmHg, respectively. The BP control and response rates were significantly higher in the half-dose, third-dose, and quarter-dose combination groups than in the placebo group (all p < 0.01). Despite no intergroup differences in study drug-related adverse events, ankle circumference increased significantly in the amlodipine group compared to those in the combination treatment groups. The quarter-dose combination, amlodipine 5 mg, and losartan 100 mg groups showed similar SBP reduction and BP response rates. The SBP reduction and BP response rate in the third-dose and half-dose combination groups were not significantly different from those in the amlodipine 10 mg group but superior to those in the losartan 100 mg group.
Conclusion
Low-dose triple combination therapies could be effective as antihypertensive therapies.
Trial Registration
ClinicalTrials.gov identifier NCT03897868.
Keywords: hypertension, blood pressure, combination therapy, low-dose, amlodipine, losartan, chlorthalidone
Introduction
Hypertension is the most prevalent risk factor of cardiovascular disease morbidity and mortality, and large-scale studies have shown the benefits of rapid blood pressure (BP) control for better cardiovascular outcomes.1,2 Although awareness about and treatment of hypertension have considerably improved, the control rate varies substantially across countries and rarely reaches the levels achieved in countries with high-quality regional hypertension programs.3,4 It is difficult to rapidly and effectively reach the target BP by using standard-dose antihypertensive monotherapy with sequential upward dose titration as the initial treatment strategy for mild-to-moderate hypertension because of individual variability in response to antihypertensive drugs,5 modest BP responses despite an increase in adverse drug reactions,6 and lengthening of the time required to control BP.7 Therefore, to reach the target BP, recent guidelines recommend using combinations of different classes of antihypertensive drugs as an initial treatment for hypertension.8,9 However, the combination of antihypertensive drugs at standard doses as an initial treatment for hypertension may be associated with an increase in adverse drug reactions including excessive BP lowering, despite providing better BP control rates.10
There is a growing interest in combination treatment with low-dose antihypertensive drugs. In meta-analyses,10,11 low-dose combinations were reported to show comparable or superior BP-lowering efficacy with fewer side effects than those associated with standard-dose monotherapy. The BP-lowering efficacy seemed to depend on the number of combined low-dose antihypertensive drugs. The results of these meta-analyses10,11 indicate the considerable promise of low-dose combinations of antihypertensive drugs as first-line therapies for mild-to-moderate hypertension to achieve earlier BP control with fewer side effects. However, no studies have evaluated the BP-lowering efficacy and dose-responsiveness of low-dose triple combination therapy. Therefore, the objective of this study was to assess the dose-responsiveness, efficacy, and safety of low-dose triple combinations of antihypertensive drugs as initial therapies for patients with mild-to-moderate hypertension.
Patients and Methods
Study Design
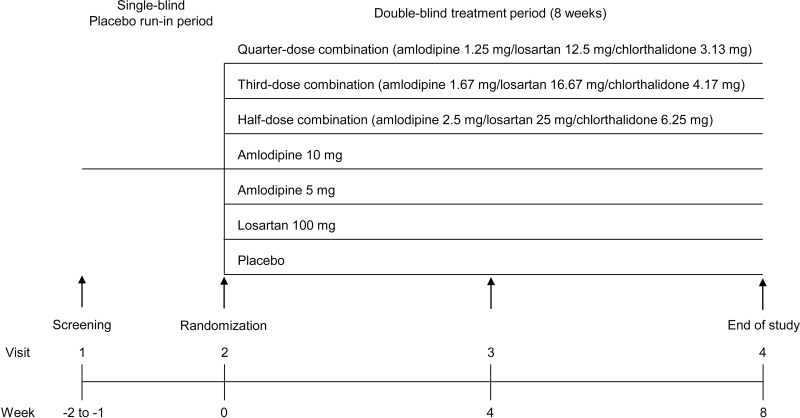
This was a multicenter, randomized, double-blind, parallel-group, phase II study, conducted at 16 hospitals in the Republic of Korea. The study protocol and informed consent form were approved by the regulatory authority of the Korea Ministry of Food and Drug Safety and the institutional review board of each participating institution (Online Supplement pp 27). This study was conducted in accordance with Declaration of Helsinki. Figure 1 shows the study flow. We investigated the dose-responsiveness, efficacy, and safety of the following therapies in comparison with placebo in patients showing mild-to-moderate hypertension: half-dose triple combination (amlodipine 2.5 mg + losartan potassium 25 mg + chlorthalidone 6.25 mg; HCP1803-2.5/25/6.25), third-dose triple combination (amlodipine 1.67 mg + losartan potassium 16.67 mg + chlorthalidone 4.17 mg; HCP1803-1.67/16.67/4.17), and quarter-dose triple combination (amlodipine 1.25 mg + losartan potassium 12.5 mg + chlorthalidone 3.13 mg; HCP1803-1.25/12.5/3.13) therapies; monotherapy with amlodipine (5 and 10 mg; Norvasc®, Pfizer Pharmaceutical Korea Ltd.); and monotherapy with losartan potassium (100 mg; Cozaar®, MSD Korea Ltd.). We selected combinations of amlodipine, losartan potassium, and chlorthalidone because fixed standard doses of single-pill combination drugs have been approved and are already in use in Korea, with proven efficacy and safety in a Phase III study12 and lack of pharmacokinetic drug–drug interaction between component drugs in a Phase I study (unpublished data, NCT02387554).13 At Visit 1, subjects who satisfied the inclusion/exclusion criteria participated in a 1- to 2-week run-in period, during which they received placebo once daily. At the randomization visit, subjects who satisfied the eligibility criteria were randomly allocated to one of the seven treatment groups and were administered the assigned study drug for 8 weeks.
Figure 1.
Study flow.
Study Population
Participants were eligible if they were aged ≥19 years and satisfied the criteria of the mean sitting systolic BP (SBP) of ≥140 to <180 mmHg and mean sitting diastolic BP (DBP) of <110 mmHg at the screening (Visit 1) and randomization (Visit 2) visits. The important exclusion criteria were as follows: a difference greater than 20 mmHg for the mean sitting SBP or 10 mmHg for the mean sitting DBP between two arms; a difference of >15 mmHg in the mean sitting SBP between Visit 1 and Visit 2; use of an antihypertensive drug within 2 weeks of Visit 1 or necessity of taking contraindicated medication during the trial period; serious cardiovascular or ischemic heart disease within 6 months before the trial; severe heart disease (NYHA class III–IV heart failure); clinically significant renal (serum creatinine level: ≥2 mg/dL) or hepatic diseases (aspartate transaminase or alanine transaminase level: ≥3 times the upper limit of normal); a history of hypersensitivity to amlodipine, losartan, chlorthalidone, dihydropyridines, angiotensin II receptor blockers, or thiazide diuretics; and women who were pregnant, breastfeeding, or of childbearing potential without the willingness to practice adequate contraception throughout the study. Patients with the mean sitting SBP or DBP of ≥180 or ≥110 mmHg, respectively, at any visit after randomization, and/or with the mean sitting SBP or DBP of <100 or <60 mmHg, respectively, at any visit during the study were dropped from the study for safety. Other exclusion criteria and withdrawal criteria are provided in the online supplement pp 28–30. All participants provided written informed consent before participation in the study.
Randomization and Masking
Patients were randomly assigned to one of the seven treatment groups in a 1:1:1:1:1:1:1 ratio. Randomization was stratified based on the sitting SBP (<160 or ≥160 mmHg at Visit 2). The randomization list was generated using the PROC PLAN procedure of SAS software. The randomization list contained information about the drug administration group to which each subject was allocated according to the randomization number. Patients were randomly assigned centrally using the interactive web response system to provide and manage the study drug.
Patients, investigators, clinical research pharmacists of each institution, and sponsors were masked to the assigned drugs until the end of the study. The combination drugs and placebo were manufactured as finished pharmaceutical products by Hanmi Pharmaceutical Co., Ltd. To maintain a double-blind status, the study drugs were packaged as gelatin capsules (DB caps®; Capsugel, Greenwood, SC, USA) and the capsules were packaged to be similar in weight. All study drugs were prepared and packaged at a manufacturing facility licensed with a Certificated of Good Manufacturing Practice by the Korea Ministry of Food and Drug Safety.
Study Procedure
During the 1- to 2-week run-in period, one placebo tablet was administered daily to all the subjects in a single-blinded manner. During the 8-week treatment period, all subjects were instructed to take the assigned study drug once daily every morning for the study duration. During the treatment period, each subject visited the clinical trial institution during week 0 (Visit 2), week 4 (Visit 3), and week 8 (Visit 4) for the assessments of efficacy and safety.
BP was measured using an electronic sphygmomanometer (HEM-7080IC, Omron, Tokyo, Japan). At each visit, BP was measured twice with a 2-min interval, and the mean value of the two measurements was used. At the screening visit, the index arm showing the higher mean SBP was determined and all subsequent BP measurements were conducted on the index arm. If the difference between two consecutively obtained readings of sitting SBP was >5 mmHg, the measurement was repeated. SBP values with a difference within 5 mmHg from two consecutive measurements were used to calculate the mean sitting SBP and DBP.
All adverse events (AEs) were assessed at every visit and recorded on the electronic Case Report Form.
Outcomes
The primary outcome was the change in the mean sitting SBP from baseline to week 8. The secondary outcomes were (1) change in the mean sitting SBP from baseline to week 4, (2) change in the mean sitting DBP from baseline to weeks 4 and 8, (3) BP control rate after 4 and 8 weeks (percentage of subjects with sitting SBP of <140 and sitting DBP of <90 mmHg), (4) BP response rate after 4 and 8 weeks (percentage of subjects with a change in sitting SBP of ≥20 and/or sitting DBP of ≥10 mmHg relative to the baseline), and (5) change in mean pulse pressure (mean sitting SBP – mean sitting DBP) from baseline to weeks 4 and 8.
Safety was assessed based on AEs; vital signs; and clinical laboratory test, physical examination, electrocardiography, and ankle edema test findings. As an assessment of peripheral edema, we measured the ankle circumference at Visit 2 (baseline), Visit 3 (week 4), and Visit 4 (week 8). Briefly, the ankle circumference 5 cm proximal to the midpoint of the medial malleolus was measured using a specially designed device, to ensure a consistent measurement location, and tension-controlled measuring tape.
Statistical Analysis
Generally, data are expressed as the mean (standard deviation, SD) values for continuous variables and as number (percentage) of patients for categorical variables. Efficacy data analyses were performed based on the full-analysis set (FAS) and additionally on the per-protocol set (PPS) population. The FAS included all randomized patients who had received the study drug at least once after randomization and had their sitting SBP measured at least once during the treatment period. The PPS included all eligible patients who completed the 8-week regimen according to the clinical trial protocol without serious protocol violations among the patients in the FAS. Additionally, a subgroup analysis was performed in patients whose baseline sitting SBP was lower than 160 mmHg.
For pairwise comparisons of the changes in sitting SBP, sitting DBP, and pulse pressure from baseline to weeks 4 and 8, analysis of covariance (ANCOVA) was performed with the baseline values and stratification variables (except for the variables related to sitting SBP) as covariates. Because the main purpose of this exploratory phase II study was to demonstrate the superiority of each triple combination treatment to placebo treatment in terms of changes in sitting SBP from baseline to week 8, multiple comparison among groups was not considered. To compare the BP control rate and BP response rate after 4 and 8 weeks between each treatment group, respectively, Pearson’s chi-square test or Fisher’s exact test was used. For subjects with missing values, the last-observation-carried-forward (LOCF) approach was applied.
Safety data were analyzed based on the safety analysis set population, which included patients who had taken the study drug at least once after randomization and had undergone the safety assessment at least once during the treatment period. AEs were presented as the number (percentage) of patients and events for AEs occurring after the randomization (treatment-emergent AEs: TEAEs). Moreover, TEAEs were summarized as serious AEs, according to severity, as AEs resulting in withdrawal, and study drug-related AEs. The ankle circumference with the greater absolute value of change from baseline at week 8 was used. The comparison of the changes in ankle circumference between the combination treatment (quarter-dose + third-dose + half-dose) and amlodipine treatment (amlodipine 5 mg + amlodipine 10 mg) groups was conducted based on the FAS. The LOCF method was applied for subjects with missing values. The Wilcoxon rank-sum test was used for between-group comparisons, and the Wilcoxon signed-rank test was used for within-group comparisons. For changes in laboratory parameters after 8 weeks relative to baseline, ANCOVA or the Wilcoxon rank-sum test was used for between-group comparison.
The effects of low-dose triple combinations on BP could not be predicted because there is no previous study. Therefore, considering the exploratory characteristic of phase II clinical trials, the sample size was calculated based on the difference in the BP-lowering effect between the amlodipine 5 mg and placebo groups. A sample size of 210 patients (30 per treatment group) was calculated as sufficient to have 80% power to detect a difference of 10.97 mmHg in change from the baseline sitting SBP between the amlodipine 5 mg and placebo treatment groups with a 0.05 two-sided significance level, assuming an SD of 12.90 mmHg and a 20% dropout rate.14–16
SAS software, version 9.4 (SAS Institute, Cary, North Carolina) was used for statistical analyses. This trial was registered with ClinicalTrials.gov, NCT03897868.
Results
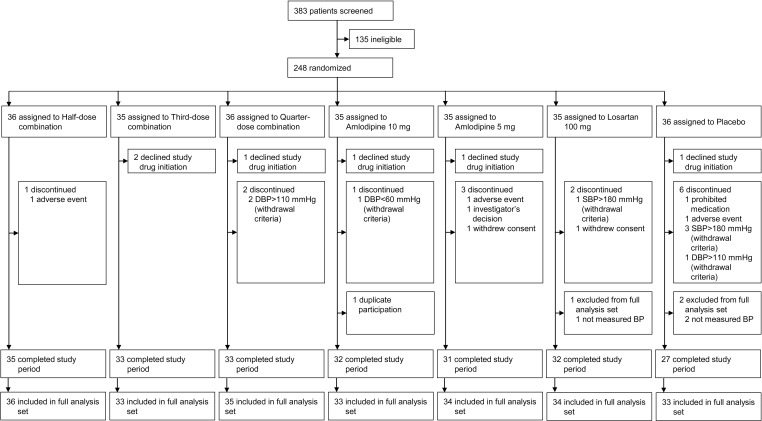
Between March 2019 and January 2020, 383 patients were screened, and 248 patients who were eligible for the trial were randomized among seven groups including the placebo group (Figure 2). Six patients declined study drug initiation after randomization, and 238 patients were included in the FAS. Baseline patient characteristics such as mean age; sex; body mass index; and rates of current smoking, drinking, and diabetes were similar among the seven groups (Table 1). Intergroup differences in baseline SBP and DBP were not significant.
Figure 2.
Patient disposition.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants
| Total | Half-Dose Combination | Third-Dose Combination | Quarter-Dose Combination | Amlodipine 10 mg | Amlodipine 5mg | Losartan 100 mg | Placebo | |
|---|---|---|---|---|---|---|---|---|
| N | 238 | 36 | 33 | 35 | 33 | 34 | 34 | 33 |
| Age (years) | 64 (11) | 64 (8) | 64 (11) | 60 (12) | 65 (10) | 63 (10) | 66 (12) | 62 (14) |
| Sex | ||||||||
| Male | 166 (70%) | 27(75%) | 25 (76%) | 23 (66%) | 22 (67%) | 22 (65%) | 24 (71%) | 23 (70%) |
| Female | 72 (30%) | 9(25%) | 8 (24%) | 12 (34%) | 11 (33%) | 12 (35%) | 10 (29%) | 10 (30%) |
| BMI (kg/m2) | 25.4 (3.2) | 26.8 (3.7) | 25.3 (3.2) | 24.9 (3.3) | 24.5 (3.1) | 25.3 (3.0) | 25.3 (3.0) | 25.5 (3.2) |
| Current smoker | 41 (17%) | 8 (22%) | 9 (27%) | 3 (9%) | 5 (15%) | 6 (18%) | 4 (12%) | 6 (18%) |
| Drinking | 125 (53%) | 22 (61%) | 17 (52%) | 19 (54%) | 19 (58%) | 16 (47%) | 16 (47%) | 16 (48%) |
| Diabetes | 60 (25%) | 12 (33%) | 7 (21%) | 9 (26%) | 7 (21%) | 8 (24%) | 10 (29%) | 7 (21%) |
| Sitting systolic blood pressure (mmHg) | 154.4 (9.3) | 155.3 (8.7) | 153.7 (10.1) | 154.4 (10.3) | 154.3 (7.4) | 154.6 (10.3) | 154.6 (9.7) | 153.6 (8.9) |
| Sitting diastolic blood pressure (mmHg) | 91.6 (8.9) | 92.4 (8.8) | 91.3 (8.7) | 94.1 (8.7) | 90.68 (11.5) | 92.5 (8.3) | 89.3 (7.5) | 90.5 (8.5) |
Notes: Half-dose combination, amlodipine/losartan/chlorthalidone 2.5/25/6.25mg; Third-dose combination, amlodipine/losartan/chlorthalidone 1.67/16.67/4.17mg; Quarter-dose combination, amlodipine/losartan/chlorthalidone 1.25/12.5/3.13mg; Data are mean (SD) or number of patients (%).
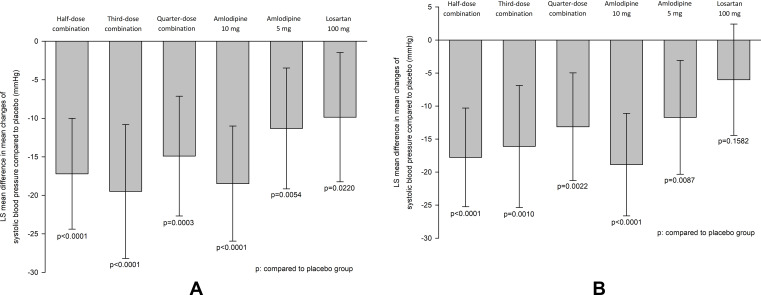
The mean reduction (SD) in SBP after the 8-week treatment period was −18.1 (10.4) mmHg, −20.6 (17.9), −16.1 (13.6), −19.5 (10.7), −12.4 (13.1) and −11.1 (15.9) mmHg in the half-dose combination, third-dose combination, quarter-dose combination, amlodipine 10 mg, amlodipine 5 mg and losartan 100 mg groups, respectively (Supplementary Table S1 and Figure S1). The placebo-corrected difference in SBP reduction (95% confidence interval, 95% CI) was −17.2 mmHg (−24.4 to −10.0) for the half-dose combination, −19.5 mmHg (−28.2 to −10.8) for the third-dose combination, −14.9 mmHg (−22.7 to −7.1) for the quarter-dose combination, −18.5 mmHg (−25.9 to −11.0) for the amlodipine 10 mg, −11.3 mmHg (−19.2 to −3.5) for the amlodipine 5 mg and −9.9 mmHg (−18.2 to −1.5) for the losartan 100 mg group (Figure 3A and Supplementary Table S1). Among the patients with baseline SBP of <160 mmHg (n = 178, 70% of the study population), there seemed to be a trend of a dose-dependent reduction in SBP after the 8-week treatment period among the half-dose, third-dose, and quarter-dose combination groups, although statistical significance was not reached (−17.8, −16.11, and −13.1 mmHg, respectively, p = 0.0843) (Figure 3B and Supplementary Table S11).
Figure 3.
Change of sitting systolic blood pressure from baseline at week 8. (A) Full analysis set, (B) patients with sitting systolic blood pressure < 160 mmHg at baseline.
The mean reduction in DBP (SD) from baseline at 8 weeks in the half-dose, third-dose, and quarter-dose combination groups was −8.7 (6.9), −9.3 (7.8), and −8.0 (8.5) mmHg, respectively. The difference in DBP (95% CI) reduction between the half-dose combination and placebo groups was −9.4 mmHg (−13.5 to −5.2), between the third-dose combination and placebo groups was −10.0 mmHg (−14.3 to −5.7), and between the quarter-dose combination and placebo groups was −8.2 mmHg (−12.7 to −3.6) (Supplementary Table S2 and Figure S2).
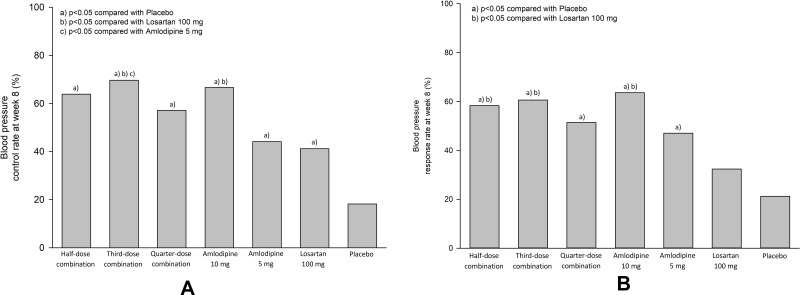
The BP control rates at week 8 in the half-dose, third-dose, and quarter-dose combination groups (63.9%, 69.7%, and 57.1%, respectively) were significantly higher than that in the placebo group (18.2%, p = 0.0001, p < 0.0001, and p = 0.0010, respectively) (Figure 4A and Supplementary Table S5). The BP response rates at week 8 in the half-dose, third-dose, and quarter-dose combination groups (58.3%, 60.6%, and 51.4%, respectively) were significantly higher than that in the placebo group (21.2%, p = 0.0017, p = 0.0011 and p = 0.0098, respectively) (Figure 4B and supplementary Table S6). Changes in the mean SBP and DBP from baseline to week 4 and in pulse pressure from baseline to weeks 4 and 8, and BP control and response rate at week 4 are shown in Supplementary Tables S3 and S4 and S6 and S9 and S10 and Figure S3.
Figure 4.
Control and response rate of blood pressure at week 8. (A) control rate, (B) response rate.
In comparison with each component drugs, the quarter-dose combination resulted in a similar SBP reduction from baseline to week 8 as did amlodipine 5 mg (difference −3.8 mmHg, 95% CI −10.1 to 2.5) and losartan 100 mg (difference −5.1 mmHg, 95% CI −11.9 to 1.6) (Supplementary Table S1). The BP response rate at week 8 in the quarter-dose combination group (51.4%) was not different from that in the amlodipine 5 mg and losartan 100 mg groups (47.1%, p = 0.7166; 32.4%, p = 0.1085, respectively) (Figure 4B and Supplementary Table S6). Of note, the SBP control rate (percentage of subjects with sitting SBP of <140 mmHg) and SBP response rate (percentage of subjects with SBP reduction of ≥20 mmHg) in the quarter-dose combination group at week 4 were significantly greater than the corresponding values in the amlodipine 5 mg group (65.7% vs 41.2%, p = 0.0410; 42.9% vs 17.7%, p = 0.0229, respectively) (Supplementary Tables S7 and S8, Figures S4 and S5). The third-dose combination group showed no difference in SBP reduction at week 8 compared to that in the amlodipine 10 mg group (difference −1.4 mmHg, 95% CI −8.1 to 5.3). However, the SBP reduction was significant compared to the findings in the amlodipine 5 mg and losartan 100 mg group (difference −8.6 mmHg, 95% CI −15.9 to −1.4 and difference −10.1 mmHg, 95% CI −17.6 to −2.5) (Supplementary Table S1). The half-dose combination group showed no difference in SBP reduction at week 8 compared to that in the amlodipine 10 mg group (difference 1.5 mmHg, 95% CI −3.6 to 6.6), an insignificant but marginal difference compared to that in the amlodipine 5 mg group (difference −5.7 mmHg, 95% CI −11.4 to 0.0), and a significant difference compared to that in the losartan 100 mg group (difference −6.8 mmHg, 95% CI −13.2 to −0.5) (Supplementary Table S1). The BP response rates at week 8 in the half-dose combination (58.3%) and third-dose combination (60.6%) groups were significantly higher than that in the losartan 100 mg group (32.4%, p = 0.0292 and 0.0204, respectively), but not different from that in the amlodipine 10 mg group (63.6% and 0.7997, respectively, p = 0.6521) (Figure 4B and supplementary Table S6).
Among the combination groups, the half-dose and third-dose combination groups showed greater SBP and DBP reduction and higher control and response rates compared to those in the quarter-dose combination group. However, the differences were not significant (Supplementary Tables S1 and S2 and S5 and S6).
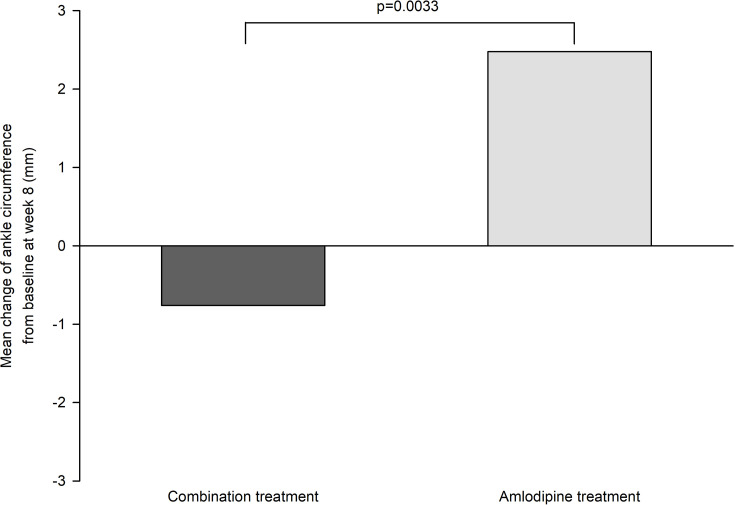
Intergroup differences in study drug-related AEs were not significant (Table 2 and Supplementary Table S13). One participant (2.8%) in the half-dose combination group reported both dizziness and headache, which were judged to be related or possibly related to the study treatment. Moreover, one participant (2.9%) in the amlodipine 5 mg group reported ankle edema during the 8-week treatment period (Table 2). Ankle circumference significantly increased from baseline to week 8 in the amlodipine treatment group but did not change in the combination treatment groups. The difference in ankle circumference changes between the combination treatment and amlodipine groups was significant (−0.76 mm versus 2.48 mm, difference −3.23 mm, 95% CI −5.53 to −0.94) (Figure 5 and Supplementary Table S12). Changes in serum creatinine and sodium levels after the 8-week treatment period in the half-dose, third-dose, and quarter-dose combination groups were not different from those in the placebo group (Table 3). However, the mean change (SD) in serum blood urea nitrogen (BUN) level in the half-dose combination group was 1.94 (3.43) mg/dL and was significantly different from that in the placebo group (−0.37 (3.42) mg/dL, difference 2.31 mg/dL, 95% CI 0.57 to 4.06). The mean change (SD) in serum potassium level in the half-dose combination group was −0.22 (0.35) mmol/L and was significantly different from that in the placebo group (−0.03 (0.28) mmol/L, difference −0.15 mmol/L, 95% CI −0.28 to −0.02) (Table 3). Changes in other laboratory parameters and summary of serious adverse events are shown in Supplementary Tables S14 and S15, respectively.
Table 2.
Study Drug-Related Adverse Events
| Half-Dose Combination | Third-Dose Combination | Quarter-Dose Combination | Amlodipine 10 mg | Amlodipine 5mg | Losartan 100 mg | Placebo | |
|---|---|---|---|---|---|---|---|
| n | 36 | 33 | 35 | 33 | 34 | 35 | 35 |
| Ankle edema | - | - | - | - | 1 (2.9) | - | - |
| Dizziness | 1 (2.8) | - | - | - | - | - | - |
| Headache | 1 (2.8) | - | - | - | - | - | 1 (2.9) |
| Pruritus | - | - | - | - | - | 1 (2.9) | - |
| Skin lesion | - | - | - | - | 1 (2.9) | - | - |
Notes: Half-dose combination, amlodipine/losartan/chlorthalidone 2.5/25/6.25mg; Third-dose combination, amlodipine/losartan/chlorthalidone 1.67/16.67/4.17mg; Quarter-dose combination: amlodipine/losartan/chlorthalidone 1.25/12.5/3.13mg; Data are number of events (%).
Figure 5.
Changes in ankle circumference from baseline at week 8.
Table 3.
Changes of Laboratory Values from Baseline at Week 8
| Half-Dose Combination | Third-Dose Combination | Quarter-Dose Combination | Amlodipine 10 mg | Amlodipine 5mg | Losartan 100 mg | Placebo | |
|---|---|---|---|---|---|---|---|
| n | 36 | 33 | 35 | 33 | 34 | 35 | 35 |
| Creatinine, mg/dL | |||||||
| Mean changes from baseline at week 8 | 0.00 (0.10) | 0.03 (0.08) | −0.00 (0.07) | −0.03 (0.11) | −0.01 (0.07) | 0.04 (0.10) | −0.01 (0.08) |
| Difference of changes compared to placebo | 0.01 (−0.04, 0.06) | 0.04 (−0.01, 0.08) | 0.01 (−0.03, 0.05) | −0.03 (−0.08, 0.02) | −0.00 (−0.04, 0.04) | 0.05 (−0.00, 0.10) | |
| p value | 0.8078† | 0.1065† | 0.8059† | 0.2897† | 0.8088† | 0.1185† | |
| BUN, mg/dL | |||||||
| Mean changes from baseline at week 8 | 1.94 (3.43) | 1.61 (3.86) | 0.52 (3.91) | −0.63 (2.95) | −0.48 (4.10) | 0.06 (3.40) | −0.37 (3.42) |
| Difference of changes compared to placebo | 2.31 (0.57, 4.06) | 1.98 (0.07, 3.88) | 0.46 (−1.24, 2.17) | −0.25 (−1.91, 1.41) | −0.19 (−2.12, 1.73) | 0.28 (−1.50, 2.06) | |
| p value | 0.0225† | 0.0618† | 0.5883* | 0.8242† | 0.8426* | 0.7540* | |
| Serum sodium, mmol/L | |||||||
| Mean changes from baseline at week 8 | −0.58 (1.50) | −0.91 (2.16) | −0.61 (2.01) | −0.41 (2.17) | 0.58 (1.84) | −0.06 (1.88) | −0.15 (1.49) |
| Difference of changes compared to placebo | −0.60 (−1.32, 0.13) | −0.74 (−1.66, 0.19) | −0.46 (−1.39, 0.48) | −0.53 (−1.48, 0.42) | 0.54 (−0.31, 1.38) | 0.08 (−0.80, 0.97) | |
| p value | 0.1035* | 01163* | 0.3731† | 0.2710* | 0.2108* | 0.8502* | |
| Serum potassium, mmol/L | |||||||
| Mean changes from baseline at week 8 | −0.22 (0.35) | −0.12 (0.36) | −0.13 (0.28) | −0.14 (0.40) | −0.14 (0.32) | 0.02 (0.39) | −0.03 (0.28) |
| Difference of changes compared to placebo | −0.15 (−0.28, −0.02) | −0.06 (−0.22, 0.09) | −0.09 (−0.24, 0.05) | −0.10 (−0.25, 0.05) | −0.09 (−0.23, 0.05) | 0.05 (−0.13, 0.23) | |
| p value | 0.0245* | 0.4027* | 0.4030† | 0.1758* | 0.1904* | 0.3803† |
Notes: Half-dose combination, amlodipine/losartan/chlorthalidone 2.5/25/6.25mg; Third-dose combination, amlodipine/losartan/chlorthalidone 1.67/16.67/4.17mg; Quarter-dose combination, amlodipine/losartan/chlorthalidone 1.25/12.5/3.13mg; p value, *ANCOVA and †Wilcoxon rank sum test, compared to placebo group.
Discussion
In the present study, we showed that low-dose triple combination therapies provided effective BP lowering efficacy in patients with mild to moderate hypertension. The placebo-corrected BP lowering was 17.2/9.4, 19.5/10.0, and 14.9/8.2 mmHg for the half-dose triple combination, third-dose triple combination, and quarter-dose triple combination therapies, respectively. The BP control and response rates in the quarter-dose, third-dose, and half-dose triple combination groups were significantly higher than the corresponding rate in the placebo group. After the 8-week treatment period, ankle circumference did not change in the combination therapy groups but increased in the amlodipine monotherapy group. Furthermore, there was a significant difference in changes in ankle circumference between the combination therapy and amlodipine groups. Along with changes in ankle circumference, one case of ankle edema was reported in the amlodipine 5 mg group.
In comparison with each component drug, a further significant reduction of 8.6 mmHg compared to that achieved with amlodipine 5 mg monotherapy was achieved with third-dose triple combination therapy. The point estimates of 3.8 mmHg with the quarter-dose triple combination therapy and 5.7 mmHg with the half-dose triple combination therapy from amlodipine 5 mg monotherapy were not significant; however, differences of this extent would be regarded as clinically relevant. These results suggest possibility of a new therapeutic option for a stepped-care approach involving initial treatment with a quarter-dose triple combination therapy as the first-line treatment and up-titration to third-dose or half-dose triple combination therapy for the management of mild-to-moderate hypertension, similar to the stepped-care therapy starting from amlodipine 5 mg and up-titrating to amlodipine 10 mg.
Combination of low-dose antihypertensive drugs has been suggested as an option for hypertension treatment because of increased efficacy and reduced adverse effects.10 In a meta-analysis, the BP-lowering efficacy of half-standard-dose triple combination therapy was predicted to be 20/11 mmHg.10 Similar to the predicted efficacy, the placebo-corrected BP lowering of the half-dose triple combination was 17.2/9.4 mmHg in our study and comparable to that of amlodipine 10 mg. Only a few studies have evaluated the BP-lowering efficacy of low-dose combinations of three or four antihypertensive drugs. In a double-blind placebo-controlled crossover trial of a Polypill containing half the standard doses of three antihypertensive drugs and simvastatin, the placebo-corrected BP-lowering effect was 18/10 mmHg and similar to the effect predicted by the meta-analysis.17 The BP-lowering efficacy of a quarter-dose quadruple combination was higher than that of the standard dose of each component drug.18 The placebo-corrected BP-lowering efficacy of the quarter-dose quadruple combination was 22/13 mmHg in a randomized, placebo-controlled, crossover trial.19 Based on the results of previous studies,18,19 the BP-lowering efficacy of the quarter-dose triple combination therapy seems to be between that of the quarter-dose quadruple combination and quarter-dose dual combination therapies. The initiation of low-dose dual combination therapy was evaluated, and it provided the benefits of better and earlier BP control than that afforded by the conventional stepped-care up-titration approach for monotherapies.7,20 In an open-label trial, low-dose triple combination treatment showed a higher rate of target BP goal achievement than that achieved with the usual care approach21 and was analyzed to be cost-effective.22 In terms of side effects, the reductions in sodium and potassium levels were greater in the low-dose combination treatment using chlorthalidone 12.5 mg than those associated with the usual care treatment.21 In the present study, the half-dose triple combination therapy significantly lowered the serum potassium level compared to that associated with the placebo treatment, but the quarter- and third-dose triple combination therapies did not. However, the difference in changes between the half-dose triple combination and placebo groups was relatively small, and the mean serum potassium level lowered from 4.43 to 4.21 mmol/L, suggesting a low probability of clinical significance. The changes in serum creatinine and sodium levels were similar to those associated with the placebo treatment.
The strength of our study is that this is the first study, to our knowledge, to evaluate the dose-responsiveness of low-dose triple antihypertensive drug combinations (quarter-dose, third-dose, and half-dose) therapies in comparison to that of placebo. Although our study was not designed to compare the efficacy of low-dose triple combination treatments with each component drug therapy and further trials are needed, the comparable efficacy of the quarter-dose combination therapy and amlodipine 5 mg therapy and that of the third-dose and half-dose combination therapies with amlodipine 10 mg suggests the possibility of a new therapeutic option for stepped-care treatment involving initial treatment with the quarter-dose triple combination drug therapy and up-titration to the third-dose or half-dose triple combination drug therapy for the management of mild-to-moderate hypertension. The better SBP control and response rate with the quarter-dose triple combination therapy compared to those with the amlodipine 5 mg monotherapy at week 4 also suggests the likelihood of rapid achievement of the target BP goal by initiating treatment with quarter-dose triple combination therapy in patients with mild-to-moderate hypertension.
Because of the small sample size and short study duration, in our study, we measured the ankle circumference to assess the development of peripheral edema. The low-dose triple combination therapies were not associated with an increase in ankle circumference, but amlodipine treatment was associated with an increase in ankle circumference. However, it is unclear whether an increase in ankle circumference in our study is clinically relevant and whether it would progress to apparent peripheral edema after long-term exposure.8 The introduction of low-dose triple combination drug therapies as first-line therapies in the management of hypertension is expected to reduce treatment inertia among patients and physicians because these therapies could help achieve the target BP more rapidly with fewer side effects than those associated with the conventional stepped-care approach starting with a standard-dose monotherapy.
The limitations of our study are the short study duration and small sample size. An 8-week treatment period is not enough to evaluate the sustained BP-lowering effect and AEs that occur after long-term treatment. This study was an exploratory phase II study and primarily designed to determine the optimal low-dose triple combinations by evaluating superiority of BP lowering efficacy compared to placebo treatment. Therefore, whether low-dose triple combination therapies had efficacy and tolerability comparable to that of each component drug monotherapy could not be confirmed. Whether the initiation of low-dose combination therapies may provide better protection against cardiovascular diseases through early BP control should be evaluated in long-term follow-up studies with large study populations. In addition, pharmacokinetic and pharmacodynamics studies of low-dose combination drugs is needed because they may provide an insight into the mechanism of hypotensive effect of low-dose combination drugs.
Conclusion
In conclusion, the placebo-corrected BP-lowering effect of low-dose triple combination therapies demonstrated in our study indicates that these therapies could be effective as antihypertensive therapies. The favorable effect on ankle edema could be an additional advantage of low-dose triple combination drug therapies when compared to conventional amlodipine monotherapies for hypertension management.
Acknowledgments
HM_APOLLO investigators: Moo-Yong Rhee (Dongguk University Ilsan Hospital, College of Medicine, Dongguk University) Soon Jun Hong (Korea University Anam Hospital, Korea University College of Medicine), Ki-Chul Sung (Gangbuk Samsung Hospital, Sungkyunkwan University College of Medicine), Sang-Wook Lim (CHA Bundang Medical Center, CHA university), Seok-Yeon Kim (Seoul Medical Center), Weon Kim (Kyung Hee University Medical Center, Kyung Hee University), Jinho Shin (Hanyang University College of Medicine), Sungha Park (Severance Hospital, Yonsei University College of Medicine), Taek-Jong Hong (Pusan National University Hospital, Pusan National University Pusan, Republic of Korea), Chang-Gyu Park, Prof. (Korea University Guro Hospital, Korea University, Seoul, Republic of Korea), Sang-Hyun Ihm, Prof. (Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Republic of Korea), Hae-Young Lee, Prof. (Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea), Sang-Hyun Kim, Prof. (Seoul Boramae Hospital, College of Medicine, Seoul National University, Seoul, Republic of Korea), Kwang-Il Kim, Prof. (Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Republic of Korea), Jin-Ok Jeong, Prof. (Chungnam National University Hospital, Chungnam National University, Daejeon, Republic of Korea), and Jin-A Jung and Jong-Soo Woo (Hanmi Pharm.Co., Ltd., Seoul, Republic of Korea). We thank Hyoeun Kang for statistical assistance; Yirang Lim and Minsook Jung for writing assistance; and Donghwan Lee, Jihyun Kwon, and Mijung Jang for study monitoring. Soon Jun Hong and Ki-Chul Sung are co-first authors for this study.
Funding Statement
Funding was provided by Hanmi Pharmaceutical Co. Ltd., Seoul, Republic of Korea.
Abbreviations
AEs, adverse events; ANCOVA, analysis of covariance; BP, blood pressure; DBP, diastolic blood pressure; FAS, full-analysis set; LOCF, last-observation-carried-forward; PPS, per-protocol set; SBP, systolic blood pressure; TEAEs, treatment-emergent adverse events.
Data Sharing Statement
We are not planning to share data besides what is included in the manuscript.
Author Contributions
All authors made substantial contributions to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
M.Y. Rhee has received lecture honoraria from Pfizer Inc., LG Life Sciences Ltd., Boehringer Ingelheim Pharma GmbH & Co. KG., Hanmi Pharm. Co. Ltd., Yuhan Co. Ltd., and Boryung Pharmaceutical Co. Ltd.; consulting fees from Hanmi Pharm. Co. Ltd. and Shin Poong Pharma. Co. Ltd.; and research grants from Boryung Pharmaceutical Co. Ltd. and Dong-A Pharmaceutical Co. Ltd. J. Shin has received lecture honoraria from Pfizer Inc., Hanmi Pharm. Co. Ltd., Yuhan Co. Ltd., and Boryung Pharmaceutical Co. Ltd.; consulting fees from Hanmi Pharm. Co. Ltd.; and research grants from Sanofi Pharm. and Hanmi Pharm. Co. Ltd. S. Park has received lecture honoraria from Pfizer Inc., Hanmi Pharm. Co. Ltd, Boryung Pharmaceutical Co. Ltd, Daewoong Pharmaceutical Co. Ltd, Sankyo Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Dong-A Pharmaceutical Co. Ltd. and Servier Pharmaceutical Co. Ltd., and a research grant from Sankyo Pharmaceutical Co. Ltd. The other authors have indicated that they have no conflicts of interest with regard to the content of this article.
References
- 1.Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. doi: 10.1016/S0140-6736(04)16456-8 [DOI] [PubMed] [Google Scholar]
- 2.Gradman AH, Parise H, Lefebvre P, Falvey H, Lafeuille MH, Duh MS. Initial combination therapy reduces the risk of cardiovascular events in hypertensive patients: a matched cohort study. Hypertension. 2013;61:309–318. doi: 10.1161/HYPERTENSIONAHA.112.201566 [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration (NCD-RisC). Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet. 2019;394:639–651. doi: 10.1016/S0140-6736(19)31145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Materson BJ. Variability in response to antihypertensive drugs. Am J Med. 2007;120:S10–20. doi: 10.1016/j.amjmed.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038 [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Asmar R, Amodeo C, et al. Comparison of single-pill strategies first line in hypertension: perindopril/amlodipine versus valsartan/amlodipine. J Hypertens. 2015;33:401–411. doi: 10.1097/HJH.0000000000000409 [DOI] [PubMed] [Google Scholar]
- 8.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 9.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 10.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett A, Chow CK, Chou M, et al. Efficacy and safety of quarter-dose blood pressure-lowering agents: a systematic review and meta-analysis of randomized controlled trials. Hypertension. 2017;70:85–93. doi: 10.1161/HYPERTENSIONAHA.117.09202 [DOI] [PubMed] [Google Scholar]
- 12.Hong SJ, Jeong HS, Han SH, et al. Comparison of fixed-dose combinations of amlodipine/losartan potassium/chlorthalidone and amlodipine/losartan potassium in patients with stage 2 hypertension inadequately controlled with amlodipine/losartan potassium: a randomized, double-blind, multicenter, phase III study. Clin Ther. 2017;39:2049–2060. doi: 10.1016/j.clinthera.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 13.Hanmi Pharmaceutical Company Ltd. and Seoul National University Bundang Hospital. Pharmacokinetic interaction between HGP0904, HGP0608 and HGP1405. October, 2014. Available from: https://ClinicalTrials.gov/show/NCT02387554. Accessed December24, 2020.
- 14.Park CG, Youn HJ, Chae SC, et al. Evaluation of the dose-response relationship of amlodipine and losartan combination in patients with essential hypertension: an 8-week, randomized, double-blind, factorial, phase II, multicenter study. Am J Cardiovasc Drugs. 2012;12:35–47. doi: 10.2165/11597170-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 15.Littlejohn TW, Jones SW, Zhang J, Hsu H, Keefe DL. Efficacy and safety of aliskiren and amlodipine combination therapy in patients with hypertension: a randomized, double-blind, multifactorial study. J Hum Hypertens. 2013;27:321–327. doi: 10.1038/jhh.2012.42 [DOI] [PubMed] [Google Scholar]
- 16.Boehringer Ingelheim. Telmisartan (Micardis) and Amlodipine (Norvasc) - Factorial Design Study for the Treatment of Hypertension. March, 2007. Availble from: https://ClinicalTrials.gov/show/NCT00281580. Accessed December24, 2020.
- 17.Wald DS, Morris JK, Wald NJ. Randomized Polypill crossover trial in people aged 50 and over. PLoS One. 2012;7:e41297. doi: 10.1371/journal.pone.0041297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmud A, Feely J. Low-dose quadruple antihypertensive combination: more efficacious than individual agents–a preliminary report. Hypertension. 2007;49:272–275. doi: 10.1161/01.HYP.0000254479.66645.a3 [DOI] [PubMed] [Google Scholar]
- 19.Chow CK, Thakkar J, Bennett A, et al. Quarter-dose quadruple combination therapy for initial treatment of hypertension: placebo-controlled, crossover, randomised trial and systematic review. Lancet. 2017;389:1035–1042. doi: 10.1016/S0140-6736(17)30260-X [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Mancia G, Poulter N. Perindopril 3.5 mg/amlodipine 2.5 mg versus renin-angiotensin system inhibitor monotherapy as first-line treatment in hypertension: a combined analysis. J Hypertens. 2018;36:1915–1920. doi: 10.1097/HJH.0000000000001766 [DOI] [PubMed] [Google Scholar]
- 21.Webster R, Salam A, de Silva HA, et al. Fixed low-dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA. 2018;320:566–579. doi: 10.1001/jama.2018.10359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lung T, Jan S, de Silva HA, et al. Fixed-combination, low-dose, triple-pill antihypertensive medication versus usual care in patients with mild-to-moderate hypertension in Sri Lanka: a within-trial and modelled economic evaluation of the TRIUMPH trial. Lancet Glob Health. 2019;7:e1359–e1366. doi: 10.1016/S2214-109X(19)30343-2 [DOI] [PubMed] [Google Scholar]