Abstract
Background
In this study, we evaluate associations between cumulative antiretroviral adherence/exposure, quantified using tenofovir diphosphate (TFV-DP) in dried blood spots (DBS), and human immunodeficiency virus (HIV)-related aging factors.
Methods
This is a cross-sectional analysis of younger (ages 18–35) and older (ages ≥60) persons with HIV (PWH) taking TFV disoproxil fumarate. Tenofovir diphosphate concentrations were quantified in DBS. Linear and logistic regression models were used to evaluate associations between TFV-DP and bone mineral density (BMD), physical function, frailty, and falls.
Results
Forty-five PWH were enrolled (23 younger, 22 older). Every 500 fmol/punch (equivalent to an increase in ~2 doses/week) increase in TFV-DP was associated with decreased hip BMD (−0.021 g/cm2; 95% confidence interval [CI], −0.040 to −0.002; P = .03). Adjusting for total fat mass, every 500 fmol/punch increase in TFV-DP was associated with higher odds of Short Physical Performance Battery impairment (score ≤10; adjusted odds ratio [OR], 1.6; 95% CI, 1.0–2.5; P = .04). Every 500 fmol/punch increase in TFV-DP was associated with slower 400-meter walk time (14.8 seconds; 95% CI, 3.8–25.8; P = .01) and remained significant after adjusting for age, lean body mass, body mass index (BMI), and fat mass (all P ≤ .01). Every 500 fmol/punch increase in TFV-DP was associated with higher odds of reporting a fall in the prior 6 months (OR, 1.8; 95% CI, 1.1–2.8; P = .02); this remained significant after adjusting for age, lean body mass, BMI, and total fat mass (all P < .05).
Conclusions
Higher TFV-DP levels were associated with lower hip BMD, poorer physical function, and greater risk for falls, a concerning combination for increased fracture risk.
Keywords: aging, bone mineral density, dried blood spots, HIV, tenofovir diphosphate
The associations between tenofovir diphosphate (TFV-DP) in dried blood spots (DBS) and human immunodeficiency virus (HIV)-related aging factors was evaluated. Higher TFV-DP levels were found to be associated with impaired physical function and fracture risk.
Life expectancy for people with human immunodeficiency virus (PWH) has increased due to improved efficacy and tolerability of modern antiretroviral therapy (ART) [1–3]. The increase in life expectancy has been accompanied by an increase in the prevalence of comorbid conditions and age-related syndromes including renal disease, obesity, sarcopenia, osteoporosis, and frailty—conditions that may alter ART exposure and toxicity in PWH [3–5].
Tenofovir disoproxil fumarate (TDF) is a nucleotide reverse-transcriptase inhibitor that has been used to treat human immunodeficiency virus (HIV)-1 infection and is usually used in combination with other antiretroviral medications [6]. Although TDF has been widely used in HIV treatment, it is associated with decreased bone mineral density (BMD) and kidney function [7, 8]. Decreases in BMD occur in a greater proportion of PWH compared with HIV-uninfected individuals regardless of ART, with a greater and mostly reversible decrease seen with use of TDF regimens compared with regimens without TDF [9–13]. Some, but not all, studies have shown that TDF may also increase the risk of fractures associated with increased morbidity and mortality [11, 14, 15].
Tenofovir diphosphate (TFV-DP), the phosphorylated anabolite of TFV, can be measured in dried blood spots (DBS) and is used to determine the cumulative exposure to TFV (derived from both prodrugs TDF and TFV alafenamide [TAF]) over the preceding 2 to 3 months [16]. Very few studies have examined the associations between cumulative ART exposure, as assessed by TFV-DP in DBS, and additional aging related factors that may increase the risk of fractures, such as physical function and fall risk, in PWH. The overall objective of this study was to determine whether TFV-DBS is associated with aging factors in PWH.
METHODS
Study Design
This was a prospective, observational study in PWH conducted at the University of Colorado Anschutz Medical Campus (ClinicalTrials.gov Identifier NCT02304263). Participants were aged either 18–35 or ≥60 years, had a history of consistent ART use with a TDF-based regimen for at least 1 year before enrollment, and were virally suppressed (as evidenced by 2 consecutive visits with HIV-ribonucleic acid [RNA] <48 copies/mL).
Participants fasted overnight before a single study visit. The visit procedures included whole blood collection via venipuncture for DBS; a dual-energy absorptiometry (DXA) scan of the hip and lumber spine for BMD assessment and whole body scan for measurement of lean body mass (LBM), lean mass limited to the extremities (appendicular lean mass), and fat mass; and an iohexol-based glomerular filtration rate (iGFR) procedure for kidney function. For DBS analysis, 25 μL whole blood was spotted onto 903 Protein Saver cards (Whatman/GE Healthcare, Piscataway, NJ), allowed to dry for at least 2 hours (up to overnight), placed in plastic bags with humidity indicators and desiccants, and stored in a sample box at −80°C until analysis [17].
Physical function assessments included the Short Physical Performance Battery (SPPB) [18] and an expanded version [19], consisting of (1) balance test including ability to hold 4 positions of increasing difficulty for 10 and 30 seconds (side-by-side, semitandem, tandem, and 1-leg stand), (2) 4-meter walk at usual pace measured twice, and (3) 10 repeated chair stands, with split times obtained at 5 and 10 chair stands. The SPPB score (from 0-worst to 12-best) was calculated as previously described [18]; time to rise 10 times was used as a continuous outcome and ability to hold the 1-leg stand dichotomized from the modified SPPB. For the 400-meter walk, participants were encouraged to walk as quickly as possible to complete 8 laps of an unobstructed, noncarpeted 25-meter length hallway. Grip strength was measured by the average of 3 dynamometer assessments using the dominant hand. Frailty was measured using the Fried criteria, with modifications as previously described [20, 21]. Participants were classified as nonfrail if they had no components present, prefrail with 1 or 2 components present, and frail with 3 or more components present; frailty and prefrailty were combined for analyses. Participants were also asked to self-report whether they sustained a fall during the previous 6 months [22, 23].
Laboratory Analyses
For measures of DBS, after extraction, TFV-DP concentrations in lysed cellular matrices were assayed with validated liquid chromatography tandem mass spectrometry methods with a lower limit of quantification of 2.5 fmol/sample [17, 24]. Iohexol was measured using a validated method with a reportable range of 10 μg/mL to 1000 μg/mL. Raw iohexol plasma clearance was determined across 5 time points after an initial injection of 5 mL Omnipaque 300 mg/mL at T0 with subsequent blood draws at 120, 150, 180, 210, and 240 minutes. The slope of the plasma clearance, in addition to patient height and weight to determine body surface area (BSA), were used to calculate iGFR (reported as mL/min × 1.73 m2). In addition to iGFR, estimated GFR (eGFR) values were calculated for participants using the Modification of Diet in Renal Disease equation [25, 26].
Statistical Analysis
Frequency and percentage were calculated for categorical variables overall and by categorical age groups. Means and standard deviations were reported for continuous variables overall and by categorical age groups. Baseline characteristics were compared between age groups using χ 2 or Fisher’s exact test (categorical variables) or t test (continuous variables). Linear regression analyses were conducted to determine the associations between TFV-DP concentrations in DBS with continuous measures of renal function, BMD, and physical function outcomes. Logistic regression analyses were conducted to determine the associations between TFV-DP concentrations in DBS and categorical physical function outcomes. A change in 500 fmol/punch was selected for the analyses because it would correspond to a clinically meaningful increase of approximately 2 TDF doses/week [16]. Mean estimates and odds ratios (ORs) with 95% confidence intervals (CIs) were reported from the linear and logistic regression models, respectively. Two-sided tests were reported assuming a 0.05 significance level and all analyses were conducted in SAS v9.4 (Carey, NC).
Patient Consent Statement
The study was approved by the Colorado Multiple Institutional Review Board, and all participants provided written, informed consent before participation.
RESULTS
Of 45 patients enrolled in the study, 23 (51%) were in the younger age group (18–35 years old) and 22 (49%) were in the older age group (≥60 years old). Demographic characteristics of the study population, by age group, are show in Table 1. The majority of the patients were white (73%) and male (91%). Approximately 50% of the overall cohort had osteopenia or osteoporosis. All participants had a suppressed HIV-1 RNA. Older participants had lower iGFR, greater total body fat mass, and LBM. Physical function markers, falls, and frailty indicated greater impairment in the older participants.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | Overall (n = 45)a | Younger (n = 23)b | Older (n = 22)b | P Value |
|---|---|---|---|---|
| Gender | 1.00 | |||
| Male | 41 (91%) | 21 (91%) | 20 (91%) | |
| Race | ||||
| White | 33 (73%) | 17 (74%) | 16 (73%) | .90 |
| Black or African American | 6 (13%) | 2 (9%) | 4 (18%) | |
| Ethnicity | .34 | |||
| Hispanic or Latino | 12 (27%) | 6 (26%) | 6 (27%) | |
| Current smoker | 18 (40%) | 12 (52%) | 6 (27%) | .09 |
| HIV-1 RNA below detection | 45 (100%) | 23 (100%) | 22 (100%) | 1.00 |
| ART Regimen | ||||
| Protease inhibitor | 14 (31%) | 7 (30%) | 7 (32%) | .24 |
| Integrase inhibitor | 16 (36%) | 11 (48%) | 5 (23%) | |
| Nonnucleoside reverse-transcriptase inhibitor | 10 (22%) | 4 (17%) | 6 (27%) | |
| Multiclass | 5 (11%) | 1 (4%) | 4 (18%) | |
| Duration of antiretroviral therapy | 4.3 (1.6) | 3.8 (1.7) | 4.9 (1.2) | .01 |
| iGFR | 78.6 (16.1) | 90.4 (9.9) | 70.4 (14.6) | <.001 |
| eGFRc | 93.7 (20.4) | 101.4 (17.0) | 85.5 (20.8) | .007 |
| TFV-DP in DBS (fmol/punch) | 2234 (874) | 2136 (830) | 2341 (928) | .44 |
| TFV-DP dosing category (fmol/punch) | ||||
| <350 | 1 (2%) | 0 (0%) | 1 (5%) | .82 |
| 350–699 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 700–1249 | 5 (11%) | 3 (13%) | 2 (9%) | |
| 1250–1849 | 10 (22%) | 6 (26%) | 4 (18%) | |
| ≥1850 | 29 (64%) | 14 (61%) | 15 (68%) | |
| Body mass index (kg/m2) | 26.6 (6.2) | 24.3 (5.3) | 29.1 (6.1) | .022 |
| Total body fat mass (kg) | 21.0 (12.0) | 16.9 (10.9) | 25.3 (11.8) | .016 |
| Lean body mass (kg) | 54.2 (9.1) | 51.8 (6.6) | 56.7 (10.8) | .078 |
| Percent body fat | 25.5 (10.1) | 22.2 (10.3) | 29.0 (8.9) | .023 |
| Lumbar bone mineral density (g/cm2) | 0.97 (0.12) | 0.98 (0.13) | 0.96 (0.12) | .62 |
| Normal | 23 (51%) | 13 (56%) | 10 (45%) | |
| Osteopenia | 19 (42%) | 8 (35%) | 11 (50%) | .56 |
| Osteoporosis | 3 (7%) | 2 (9%) | 1 (5%) | |
| Hip Bone Mineral Density (g/cm2) | 0.88 (0.11) | 0.89 (0.10) | 0.86 (0.12) | .41 |
| Normal | 25 (56%) | 13 (57%) | 12 (55%) | |
| Osteopenia | 18 (40%) | 10 (43%) | 8 (36%) | .33 |
| Osteoporosis | 2 (4%) | 0 (0%) | 2 (9%) | |
| 400-meter walk time (seconds) | 272.5 (58.0) | 253.2 (48.0) | 296.1 (61.7) | .018 |
| Time to complete 10 chair rises (seconds) | 25.8 (6.6) | 23.5 (5.6) | 28.3 (6.8) | .016 |
| Grip strength (kg) | 35.9 (7.8) | 37.2 (7.2) | 34.4 (8.3) | .24 |
| Falls in the past 6 months | 11 (24%) | 2 (9%) | 9 (41%) | .012 |
| One leg stand, 30 seconds completed | 38 (84%) | 21 (91%) | 17 (77%) | .29 |
| Short Physical Performance Battery Score | 10.5 (1.7) | 11.1 (1.2) | 9.9 (1.9) | .015 |
| Frailty | ||||
| Nonfrail | 24 (53%) | 16 (70%) | 8 (36%) | .011 |
| Prefrail/Frail | 21 (42%) | 7 (30%) | 14 (64%) |
Abbreviations: ART, antiretroviral therapy; DBS, dried blood spots; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; iGFR, iohexol glomerular filtration rate; RNA, ribonucleic acid; TFV-DP, tenofovir-diphosphate.
aValues presented as frequency (percentage) or mean (standard deviation).
bYounger, 18–35 years old; older, ≥60 years old.
ceGFR by the Modification of Diet in Renal Disease equation.
Tenofovir Diphosphate and Renal Function
As shown in Table 2, renal function as measured by either iGFR or eGFR was not associated with TFV-DP in DBS in either unadjusted or models adjusted singly for age, body mass index (BMI), total fat mass, LBM, appendicular lean mass, or in models adjusted for both age and either BMI or total fat mass.
Table 2.
Unadjusted and Adjusted Linear Regression Models for the Association Between iGFR, eGFR, and TFV-DP-DBS (for Every 500-fmol/Punch)
| Models | TFV-DP-DBS (95% CI) | P Value |
|---|---|---|
| iGFR | ||
| Unadjusted Model | 0.04 (−3.34 to 3.41) | .98 |
| Age Adjusted | 0.08 (−2.63 to 2.79) | .95 |
| BMI Adjusted | −1.23 (−4.33 to 1.86) | .42 |
| Total Fat Mass Adjusted | −1.01 (−3.97 to 1.94) | .49 |
| LBM Adjusted | 0.09 (−3.38 to 3.57) | .96 |
| Appendicular Lean Mass Adjusted | 0.19 (−3.27 to 3.64) | .91 |
| Age and BMI Adjusted | −0.55 (−3.35 to 2.25) | .69 |
| Age and Total Fat Mass Adjusted | −0.52 (−3.21 to 2.16) | .69 |
| eGFRa | ||
| Unadjusted Model | −2.63 (−6.22 to 0.95) | .15 |
| Age Adjusted | −2.10 (−5.47 to 1.27) | .22 |
| BMI Adjusted | −3.06 (−6.78 to 0.67) | .11 |
| Total Fat Mass Adjusted | −2.81 (−6.53 to 0.92) | .14 |
| LBM Adjusted | −2.46 (−6.12 to 1.21) | .18 |
| Appendicular Lean Mass Adjusted | −2.39 (−6.02 to 1.24) | .19 |
| Age and BMI Adjusted | −1.92 (−5.55 to 1.71) | .29 |
| Age and Total Fat Mass Adjusted | −1.74 (−5.30 to 1.82) | .33 |
Abbreviations: BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; DBS, dried blood spots; iGFR, iohexol glomerular filtration rate; LBM, lean body mass; TFV-DP, tenofovir-diphosphate.
aeGFR by the Modification of Diet in Renal Disease equation.
Tenofovir Diphosphate and Bone Mineral Density
For every 500 fmol/punch increase in TFV-DP in DBS, there was a decrease in hip BMD ( = −0.021 g/cm2; P = .03), where is the estimated change in hip BMD for an increase of 500 fmol/punch in TFV-DP in DBS. This association remained similar after adjusting for age ( = −0.020 g/cm2; P = .04), but it was attenuated and no longer reached statistical significance after adjusting for BMI ( = −0.014 g/cm2; P = .14), total fat mass ( = −0.015 g/cm2; P = .10), LBM ( = −0.016 g/cm2; P = .06), appendicular lean mass ( = −0.017 g/cm2; P = .06), or adjusting for both age with either BMI ( = −0.008 g/cm2; P = .37) or total fat mass ( = −0.011 g/cm2; P = .23) (Table 3). Associations between TFV-DP concentrations in DBS and spine BMD were of similar magnitude ( = −0.018 g/cm2), but these did not reach statistical significance (P = .09) (Table 3) and remained statistically nonsignificant after adjusting for age (P = .10), BMI (P = .13), total fat mass (P = .11), LBM (P = .17), appendicular lean mass (P = .16), age with either BMI (P = .17), or total fat mass (P = .13).
Table 3.
Unadjusted and Adjusted Linear Regression Models for the Association Between Hip BMD (g/cm2), Spine BMD (g/cm2), Chair Rise Time (Seconds), 400-Meter Walk (Seconds), Average Grip (kg), and TFV-DP in DBS (for Every 500-fmol/Punch)
| Models | TFV-DP-DBS (95% CI) | P Value |
|---|---|---|
| Hip Bone Mineral Density (g/cm2) | ||
| Unadjusted Model | −0.021 (−0.040 to −0.0019) | .03 |
| Age Adjusted | −0.020 (−0.040 to −0.0009) | .04 |
| BMI Adjusted | −0.014 (−0.031 to 0.0044) | .14 |
| Total Fat Mass Adjusted | −0.015 (−0.033 to 0.0032) | .10 |
| LBM Adjusted | −0.016 (−0.034 to 0.0010) | .06 |
| Appendicular Lean Mass Adjusted | −0.017 (−0.035 to 0.0005) | .06 |
| Age and BMI Adjusted | −0.008 (−0.025 to 0.0095) | .37 |
| Age and Total Fat Mass Adjusted | −0.011 (−0.0285 to 0.0072) | .23 |
| Spine Bone Mineral Density (g/cm2) | ||
| Unadjusted Model | −0.018 (−0.039 to 0.003) | .09 |
| Age Adjusted | −0.018 (−0.039 to 0.004) | .10 |
| BMI Adjusted | −0.017 (−0.039 to 0.005) | .13 |
| Total Fat Mass Adjusted | −0.018 (−0.040 to 0.004) | .11 |
| LBM Adjusted | −0.014 (−0.034 to 0.006) | .17 |
| Appendicular Lean Mass Adjusted | −0.014 (−0.035 to 0.006) | .16 |
| Age and BMI Adjusted | −0.016 (−0.039 to 0.007) | .17 |
| Age and Total Fat Mass Adjusted | −0.018 (−0.040 to 0.005) | .13 |
| Chair Rise Time (Secondsa) | ||
| Unadjusted Model | 1.2 (−0.1 to 2.3) | .06 |
| Age Adjusted | 0.9 (−0.3 to 2.0) | .14 |
| BMI Adjusted | 1.5 (0.3–2.6) | .02 |
| Total Fat Mass Adjusted | 1.5 (0.3–2.6) | .02 |
| LBM Adjusted | 1.1 (−0.1 to 2.3) | .07 |
| Appendicular Lean Mass Adjusted | 1.1 (−0.1 to 2.3) | .07 |
| Age and BMI Adjusted | 1.1 (−0.1 to 2.4) | .06 |
| Age and Total Fat Mass Adjusted | 1.2 (−0.03 to 2.4) | .06 |
| 400-Meter Walk (Secondsa) | ||
| Unadjusted Model | 14.8 (3.8–25.8) | .01 |
| Age Adjusted | 13.5 (3.2–23.7) | .01 |
| BMI Adjusted | 19.5 (9.9–29.1) | <.001 |
| Total Fat Mass Adjusted | 20.2 (11.0–29.5) | <.001 |
| LBM Adjusted | 15.2 (4.0–26.5) | .01 |
| Appendicular Lean Mass Adjusted | 14.6 (3.4–25.9) | .01 |
| Age and BMI Adjusted | 18.3 (8.4–28.1) | <.001 |
| Age and Total Fat Mass Adjusted | 19.1 (9.6–28.7) | <.001 |
| Average Grip (kg) | ||
| Unadjusted Model | −0.23 (−1.63 to 1.16) | .74 |
| Age Adjusted | −0.14 (−1.55 to 1.26) | .84 |
| BMI Adjusted | −0.19 (−1.65 to 1.28) | .80 |
| Total Fat Mass Adjusted | −0.21 (−1.66 to 1.24) | .77 |
| LBM Adjusted | 0.20 (−0.95 to 1.36) | .73 |
| Appendicular Lean Mass Adjusted | 0.17 (−0.97 to 1.30) | .77 |
| Age and BMI Adjusted | 0.07 (−1.43 to 1.57) | .93 |
| Age and Total Fat Mass Adjusted | −0.01 (−1.49 to 1.48) | .99 |
Significant P values are indicated by italics.
Abbreviations: BMD, bone mineral density; BMI, body mass index; CI, confidence interval; DBS, dried blood spots; LBM, lean body mass; TFV-DP, tenofovir diphosphate.
aLower number = faster time to complete.
Tenofovir Diphosphate and Physical Function, Frailty, and Falls
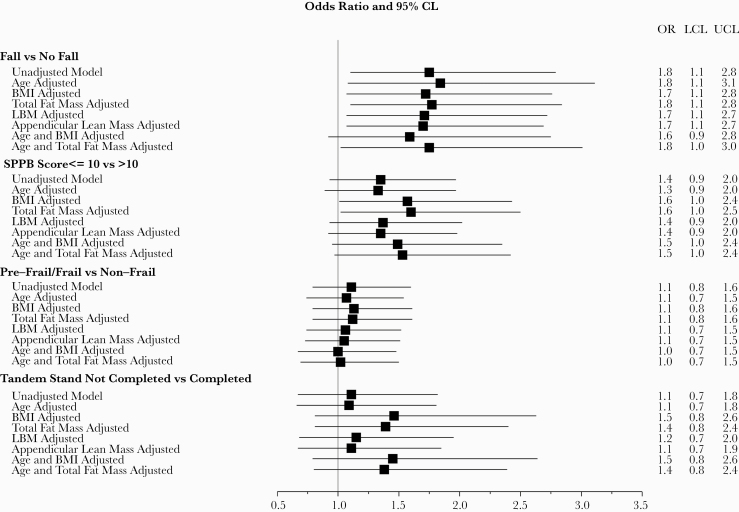
For every 500 fmol/punch increase in TFV-DP in DBS, there was a slower time to complete 10 chair rises in unadjusted models ( = 1.2 seconds), but this did not reach statistical significance (P = .06). The association was strengthened and became significant after adjusting for BMI ( = 1.5 seconds; P = .02) or total fat mass ( = 1.5 seconds; P = .02), but adjusting for age, LBM, appendicular lean mass, and age with either BMI or total fat mass did not strengthen the association (P ≥ .06) (Table 2). Tenofovir-DP in DBS concentrations were also associated with SPPB score after adjusting for BMI (score ≤10: OR = 1.57; 95% CI, 1.01–2.43; P = .04) or total fat mass (score ≤ 10: OR = 1.60; 95% CI, 1.02–2.50; P = .04), but not in unadjusted models (score ≤ 10: OR = 1.35; 95% CI, 0.93–1.97; P = .12) or models adjusting for age, total lean mass, appendicular lean mass, age, and BMI or age and total fat mass (P ≥ .05) (Figure 1). For every 500 fmol/punch increase in TFV-DP in DBS, time to complete the 400-meter walk was 14.8 seconds slower in unadjusted models (P = .01). This association was strengthened when adjusting for fat mass ( = 20.2 seconds slower, P < .001); adjusting for age, BMI, LBM, appendicular lean mass, age, and BMI or age and total fat mass yielded similar effect sizes ( = 13.5–19.5 seconds slower) and remained statistically significant (P < .01). Tenofovir-DP in DBS concentrations were not significantly associated with grip strength (Table 3), frailty, or balance (Figure 1).
Figure 1.
Forest plot showing the associations between falls, Short Physical Performance Battery (SPPB) score, frailty, tandem stand, and tenofovir-diphosphate dried blood spots (TFV-DP-DBS) (for every 500 fmol/punch). Falls were significantly associated with TFV-DP in both unadjusted and adjusted models. Frailty and impaired tandem stand were not significantly associated with TFV-DP in either unadjusted or adjusted models. Low SPPB was only significantly associated with TFV-DP in the model adjusted for body mass index (BMI) or total fat mass. CL, confidence limits; LBM, lean body mass; LCL, lower confidence limits; OR, odds ratio; UCL, upper confidence limits.
The odds of reporting a fall in the past 6 months was significantly associated with greater TFV-DP in DBS levels: for every 500 fmol/punch increase in TFV-DP, the odds of a fall increased by 1.75 (95% CI, 1.1–2.79; P = .02). The effect was similar after adjusting for age, BMI, total fat mass, LBM, and appendicular lean mass or age and total fat mass (all P < .05) (Figure 1). The effect was no longer significant after adjusting for age and BMI (P = .10).
DISCUSSION
Among older adults, greater accumulation of medication due to changes in body composition, renal clearance, liver metabolism, and other factors may contribute to greater toxicity. These effects may be heightened among older adults with HIV, who experience “accelerated aging” [27]. Our study found that greater cumulative ART exposure (ie, as measured by TFV-DP in DBS) was associated with adverse aging outcomes in a cohort of both older and younger PWH. Specifically, higher TFV-DP in DBS was associated with lower hip BMD, decreased physical function, and increased risk for falls. Moreover, many of these associations were strengthened by the inclusion of explanatory variables, primarily that of total fat mass or BMI. Tenofovir-DP in DBS is a highly informative measure of cumulative and overall exposure to ART. Our findings suggest that those with the highest concentrations (presumably due to excellent adherence as well as intrinsically greater drug exposures due to age) may also be those more likely to experience toxicity.
We failed to find an association between TFV-DP in DBS and either iGFR or eGFR, even after bivariable adjustment. The impact of body composition measures (BMI, fat mass, LBM) had a much greater impact on the associations between TFV-DP and aging outcomes. The reasons for this lack of association between iGFR or eGFR and TFV-DP are perplexing but may partially be driven by having a “healthier” older PWH group and/or a “less healthy” younger PWH group in our cohort or other factors (such as body fat) having a greater impact on TFV-DP.
Not surprisingly, we found that higher concentrations of TFV-DP were associated with lower BMD, which has been previously documented in persons taking TDF-based pre-exposure prophylaxis [28]. The association between TDF and BMD is well established, and lower BMD is seen with TDF use across the age spectrum in PWH [29, 30], as demonstrated by 43%–44% of even our younger participants having osteopenia or osteoporosis. It is interesting to note that, in our study, this association was no longer significant after adjusting for body composition (total fat mass, LBM, or appendicular lean mass), suggesting that body composition measures influence both BMD and TFV-DP concentrations.
We have previously shown (1) the impact of fat mass or BMI on physical function performance [31, 32] and (2) a strong association between BMI and TFV-DP in DBS among a large clinical cohort of PWH [33, 34]. Here, higher TFV-DP in DBS were associated with slower chair rise time, slower time to complete a 400-meter walk, and SPPB impairment. These findings were most notable for the association with gait speed. Gait speed incorporates endurance, motor coordination, balance, sensation, motivation, and cognitive status, and it is strongly associated with future health status, including mortality [35]. Thus, gait speed may serve as a particularly strong measure of physiological aging in this population. We also found that after adjusting for total fat mass (or BMI, in most cases), the TFV-DP effect was strengthened (estimate further from the null) with a narrower CI, suggesting that BMI (and specifically the fat mass component of BMI) confounds the relationship between TFV-DP and BMD or functional outcomes.
Finally, we found associations between TFV-DP and greater risk of a fall, independent of age. These findings suggest that higher concentrations of TFV-DP are not merely a marker for drug accumulation (either due to high adherence and/or slower clearance) but may be linked to poorer age-associated outcomes. Whether higher TFV-DP concentrations contributed to the adverse outcome measured or the adverse outcome contributed to a higher ART accumulation cannot be determined from our cross-sectional study. In combination with the association between TFV-DP, lower hip BMD, and impaired physical function, the increased fall risk suggest that high TFV-DP in DBS may be associated with a particularly high risk of fracture. Other antiretrovirals have also been associated with an increased risk of falls, especially efavirenz [36], thus combination regimens with TDF and efavirenz may be an even greater concern for fall and fracture risk.
Strengths of our study include a younger and older “real-world” clinical cohort, the use of an objective ART adherence and exposure measure (TFV-DP in DBS), the inclusion of diverse objective physical function measures, and the use of a gold standard assessment of GFR (iGFR). The main limitations include the small sample size and assessment at a single time point. Longitudinal studies in larger samples are needed to provide further insight into the directionality of the identified associations. Furthermore, participants in this study were taking TDF, and these associations will need to be evaluated in PWH taking TAF, although concentrations in DBS between TDF and TAF are comparable. Tenofovir alafenamide is associated with less bone toxicity, and the associations with function and falls are unknown.
CONCLUSIONS
In conclusion, we demonstrated that TFV-DP concentrations in DBS were associated with multiple HIV-related aging factors that may increase risk of fracture. Our findings provide preliminary evidence that TFV-DP in DBS could serve as a biomarker of TFV cumulative toxicity in aging PWH. Future studies should investigate whether monitoring for TFV-DP by DBS in clinical practice can improve the long-term safety and limit aging-related toxicities of ART.
Acknowledgments
Disclaimer.The contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health (NIH) views.
Financial support. This work was funded by NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards (Grant Number UL1 TR002535) NIH/National Institute on Aging (R01 AG054366) and NIH/National Institute of Allergy and Infectious Diseases (T32 AI7447-22) (to S. M. S.).
Potential conflicts of interest. K. M. E. has received research funding from Gilead Sciences and has consulted for ViiV and Gilead (payment to the University of Colorado). P. L. A. has received personal fees and research funding from Gilead Sciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. van Sighem AI, Gras LA, Reiss P, et al. ; ATHENA National Observational Cohort Study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 2010; 24:1527–35. [DOI] [PubMed] [Google Scholar]
- 2. Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012; 26:335–43. [DOI] [PubMed] [Google Scholar]
- 3. Erlandson KM, Karris MY. HIV and aging: reconsidering the approach to management of comorbidities. Infect Dis Clin North Am 2019; 33:769–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis 2013; 26:17–25. [DOI] [PubMed] [Google Scholar]
- 5. Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection-a trend analysis. J Infect Dis 2017; 216:1525–33. [DOI] [PubMed] [Google Scholar]
- 6. Gallant JE, Deresinski S. Tenofovir disoproxil fumarate. Clin Infect Dis 2003; 37:944–50. [DOI] [PubMed] [Google Scholar]
- 7. Grigsby IF, Pham L, Mansky LM, et al. Tenofovir-associated bone density loss. Ther Clin Risk Manag 2010; 6:41–7. [PMC free article] [PubMed] [Google Scholar]
- 8. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis 2011; 57:773–80. [DOI] [PubMed] [Google Scholar]
- 9. Escota GV, Mondy K, Bush T, et al. High prevalence of low bone mineral density and substantial bone loss over 4 years among HIV-infected persons in the era of modern antiretroviral therapy. AIDS Res Hum Retroviruses 2016; 32:59–67. [DOI] [PubMed] [Google Scholar]
- 10. Goh SSL, Lai PSM, Tan ATB, Ponnampalavanar S. Reduced bone mineral density in human immunodeficiency virus-infected individuals: a meta-analysis of its prevalence and risk factors. Osteoporos Int 2018; 29:595–613. [DOI] [PubMed] [Google Scholar]
- 11. Grant PM, Cotter AG. Tenofovir and bone health. Curr Opin HIV AIDS 2016; 11:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203:1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chisati EM, Constantinou D, Lampiao F. Management of reduced bone mineral density in HIV: pharmacological challenges and the role of exercise. Front Physiol 2018; 9:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adachi JD, Loannidis G, Berger C, et al. ; Canadian Multicentre Osteoporosis Study (CaMos) Research Group. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int 2001; 12:903–8. [DOI] [PubMed] [Google Scholar]
- 15. Szulc P, Feyt C, Chapurlat R. High risk of fall, poor physical function, and low grip strength in men with fracture-the STRAMBO study. J Cachexia Sarcopenia Muscle 2016; 7:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng JH, Rower C, McAllister K, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49:M85–94. [DOI] [PubMed] [Google Scholar]
- 19. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci 2001; 56:M644–9. [DOI] [PubMed] [Google Scholar]
- 20. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–56. [DOI] [PubMed] [Google Scholar]
- 21. Erlandson KM, Wu K, Koletar SL, et al. Association between frailty and components of the frailty phenotype with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017; 215:933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: epidemiology, biology, measurement, interventions, and research needs. Curr HIV/AIDS Rep 2016; 13:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016; 26:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bushman LR, Kiser JJ, Rower JE, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal 2011; 56:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145:247–54. [DOI] [PubMed] [Google Scholar]
- 26. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 2014; 69:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Havens PL, Stephensen CB, Van Loan MD, et al. ; Adolescent Medicine Trials Network for HIV/AIDS Interventions 117 study team. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis 2017; 64:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erlandson KM, Lake JE, Sim M, et al. Bone mineral density declines twice as quickly among HIV-infected women compared with men. J Acquir Immune Defic Syndr 2018; 77:288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erlandson KM, OʼRiordan M, Labbato D, McComsey GA. Relationships between inflammation, immune activation, and bone health among HIV-infected adults on stable antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 65:290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hawkins KL, Zhang L, Ng DK, et al. Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. AIDS 2018; 32:1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erlandson KM, Allshouse AA, Jankowski CM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012; 13:324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coyle RP, Morrow M, Coleman SS, et al. Factors associated with tenofovir diphosphate concentrations in dried blood spots in persons living with HIV. J Antimicrob Chemother 2020; 75:1591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis 2019; 68:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Erlandson KM, Zhang L, Ng DK, et al. Risk factors for falls, falls with injury, and falls with fracture among older men with or at risk of HIV infection. J Acquir Immune Defic Syndr 2019; 81:e117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]