Abstract
Background
The relationship between the presence of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the risk of subsequent reinfection remains unclear.
Methods
We investigated the incidence of SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR) in seropositive and seronegative health care workers attending testing of asymptomatic and symptomatic staff at Oxford University Hospitals in the United Kingdom. Baseline antibody status was determined by anti-spike (primary analysis) and anti-nucleocapsid IgG assays, and staff members were followed for up to 31 weeks. We estimated the relative incidence of PCR-positive test results and new symptomatic infection according to antibody status, adjusting for age, participant-reported gender, and changes in incidence over time.
Results
A total of 12,541 health care workers participated and had anti-spike IgG measured; 11,364 were followed up after negative antibody results and 1265 after positive results, including 88 in whom seroconversion occurred during follow-up. A total of 223 anti-spike–seronegative health care workers had a positive PCR test (1.09 per 10,000 days at risk), 100 during screening while they were asymptomatic and 123 while symptomatic, whereas 2 anti-spike–seropositive health care workers had a positive PCR test (0.13 per 10,000 days at risk), and both workers were asymptomatic when tested (adjusted incidence rate ratio, 0.11; 95% confidence interval, 0.03 to 0.44; P=0.002). There were no symptomatic infections in workers with anti-spike antibodies. Rate ratios were similar when the anti-nucleocapsid IgG assay was used alone or in combination with the anti-spike IgG assay to determine baseline status.
Conclusions
The presence of anti-spike or anti-nucleocapsid IgG antibodies was associated with a substantially reduced risk of SARS-CoV-2 reinfection in the ensuing 6 months. (Funded by the U.K. Government Department of Health and Social Care and others.)
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection produces detectable immune responses in most cases reported to date; however, the extent to which previously infected people are protected from a second infection is uncertain. Understanding whether postinfection immunity exists, how long it lasts, and the degree to which it may prevent symptomatic reinfection or reduce its severity has major implications for the SARS-CoV-2 pandemic.
Postinfection immunity may be conferred by humoral and cell-mediated immune responses. Key considerations when investigating postinfection immunity include identifying functional correlates of protection, identifying measurable surrogate markers, and defining end points, such as prevention of disease, hospitalization, death, or onward transmission.1
The assay-dependent antibody dynamics of SARS-CoV-2 anti-spike and anti-nucleocapsid antibodies are being defined.2-6 Neutralizing antibodies against the spike protein receptor-binding domain may provide some postinfection immunity. However, the association between antibody titers and plasma neutralizing activity is assay- and time-dependent.7-10
Evidence for postinfection immunity is emerging. Despite more than 76 million people infected worldwide and widespread ongoing transmission, reported reinfections with SARS-CoV-2 have been rare, occurring mostly after mild or asymptomatic primary infection,11-20 which suggests that SARS-CoV-2 infection provides some immunity against reinfection in most people. In addition, small-scale reports suggest that neutralizing antibodies may be associated with protection against infection.21 We performed a prospective longitudinal cohort study of health care workers to assess the relative incidence of subsequent positive SARS-CoV-2 polymerase-chain-reaction (PCR) tests and symptomatic infections in health care workers who were seropositive for SARS-CoV-2 antibodies and in those who were seronegative.
Methods
Cohort
Oxford University Hospitals offer SARS-CoV-2 testing to all symptomatic and asymptomatic staff working at four teaching hospitals in Oxfordshire, United Kingdom. SARS-CoV-2 PCR testing of combined nasal and oropharyngeal swab specimens for symptomatic staff (those with new persistent cough, temperature ≥37.8°C, or anosmia or ageusia) was offered beginning on March 27, 2020. Asymptomatic health care workers were invited to participate in voluntary nasal and oropharyngeal swab PCR testing every 2 weeks and serologic testing every 2 months (with some participating more frequently for related studies) beginning on April 23, 2020, as previously described.5,22 Staff were followed until November 30, 2020. Deidentified data were obtained from the Infections in Oxfordshire Research Database, which has generic research ethics committee, Health Research Authority, and Confidentiality Advisory Group approvals.
Laboratory Assays
Serologic investigations were performed with use of an anti-trimeric spike IgG enzyme-linked immunosorbent assay (ELISA), developed by the University of Oxford,23,24 and an anti-nucleocapsid IgG assay (Abbott). See the Supplementary Appendix, available with the full text of this article at NEJM.org, for details on the assays and PCR tests.
Statistical Analysis
We classified health care workers according to their baseline antibody status. Those with only negative antibody assays were considered to be at risk for infection from their first antibody assay until either the end of the study or their first PCR-positive test, whichever occurred earlier. Those with a positive antibody assay were considered to be at risk for infection (or reinfection) from 60 days after their first positive antibody result to either the end of the study or their next PCR-positive test, whichever occurred earlier, irrespective of subsequent seroreversion (i.e., any negative antibody assay occurring later). The 60-day window was prespecified to exclude persistence of PCR-positive RNA after the index infection that led to seroconversion, on the basis of earlier reports of RNA persistence for 6 weeks or more.22,25,26 Similarly, we considered only PCR-positive tests occurring at least 60 days after the previous PCR-positive test.
We used Poisson regression to model the incidence of PCR-positive infection per at-risk day according to baseline antibody status, adjusting for incidence over time, age, and participant-reported gender. Primary analyses used anti-spike IgG assay results, which were expected before the start of the study to be more closely related to neutralizing activity and protection from infection.7,10 We also investigated anti-nucleocapsid antibody assay results and a combined model with three baseline antibody statuses (both assays negative, both positive, or only one positive). Sensitivity analyses investigated the effect of different asymptomatic testing rates according to antibody status and different follow-up windows (see the Supplementary Appendix).
Results
Baseline Anti-Spike IgG Assays and PCR Testing Rates
A total of 12,541 health care workers underwent measurement of baseline anti-spike antibodies; 11,364 (90.6%) were seronegative and 1177 (9.4%) seropositive at their first anti-spike IgG assay, and seroconversion occurred in 88 workers during the study (Table 1, and Fig. S1A in the Supplementary Appendix). Of 1265 seropositive health care workers, 864 (68%) recalled having had symptoms consistent with those of coronavirus disease 2019 (Covid-19), including symptoms that preceded the widespread availability of PCR testing for SARS-CoV-2; 466 (37%) had had a previous PCR-confirmed SARS-CoV-2 infection, of which 262 were symptomatic. Fewer seronegative health care workers (2860 [25% of the 11,364 who were seronegative]) reported prebaseline symptoms, and 24 (all symptomatic, 0.2%) were previously PCR-positive. The median age of seronegative and seropositive health care workers was 38 years (interquartile range, 29 to 49). Health care workers were followed for a median of 200 days (interquartile range, 180 to 207) after a negative antibody test and for 139 days at risk (interquartile range, 117 to 147) after a positive antibody test.
Table 1. Demographic Characteristics and SARS-CoV-2 PCR Testing for 12,541 Health Care Workers According to SARS-CoV-2 Anti-Spike IgG Status.*.
| Characteristic | Anti-Spike Seronegative at Baseline and throughout Follow-Up (N=11,276) |
Anti-Spike Seronegative at Baseline, Converting to Seropositive† (N=88) |
Anti-Spike Seropositive at Baseline (N=1177) |
|---|---|---|---|
| Age — yr | |||
| Median (IQR) | 38 (29–49) | 41 (28–49) | 38 (29–49) |
| Range | 16–86 | 21–67 | 17–69 |
| Gender — no. (%)‡ | |||
| Female | 8360 (74.1) | 68 (77) | 835 (70.9) |
| Male | 2900 (25.7) | 20 (23) | 339 (28.8) |
| Other | 16 (0.1) | 0 | 3 (0.3) |
| Race or ethnic group — no. (%)§ | |||
| White | 8313 (73.7) | 58 (66) | 703 (59.7) |
| Asian | 1719 (15.2) | 20 (23) | 287 (24.4) |
| Black | 425 (3.8) | 4 (5) | 81 (6.9) |
| Chinese | 121 (1.1) | 0 | 9 (0.8) |
| Other | 698 (6.2) | 6 (7) | 97 (8.2) |
| Role — no. (%) | |||
| Nurse or health care assistant | 3930 (34.9) | 43 (49) | 555 (47.2) |
| Physician | 1671 (14.8) | 4 (5) | 184 (15.6) |
| Administrative staff | 1452 (12.9) | 10 (11) | 95 (8.1) |
| Medical or nursing student | 578 (5.1) | 6 (7) | 36 (3.1) |
| Laboratory staff | 413 (3.7) | 3 (3) | 36 (3.1) |
| Physical, occupational or speech therapist | 342 (3.0) | 7 (8) | 37 (3.1) |
| Porter or domestic worker | 319 (2.8) | 0 | 58 (4.9) |
| Security, estates, or catering staff | 245 (2.2) | 3 (3) | 23 (2.0) |
| Other | 2326 (20.6) | 12 (14) | 153 (13.0) |
| Symptoms resembling Covid-19 between February 1, 2020, and baseline serologic assay — no. (%) | 2826 (25.1) | 34 (39)¶ | 810 (68.8) |
| ≥1 PCR test for symptoms before baseline — no. (%) | 857 (7.6) | 10 (11) | 358 (30.4) |
| ≥1 Positive PCR test with symptoms before baseline — no. (%) | 19 (0.2) | 5 (6) | 239 (20.3) |
| Person-days of follow-up | 2,036,358 | 7121 (while seronegative) 5076 (while seropositive) |
152,983 |
| Positive PCR during follow-up — no. | |||
| Total | 197 | 26‖ | 2 |
| Symptomatic | 106 | 17 | 0 |
| Asymptomatic | 91 | 9 | 2 |
Percentages may not total 100 because of rounding. Covid-19 denotes coronavirus disease 2019, IQR interquartile range, and PCR polymerase chain reaction.
Those in whom seroconversion occurred were included in the analysis twice, once while they were at risk for infection and antibody-negative and then subsequently while they were antibody-positive and at risk for reinfection.
Gender was reported by the participants. “Other” includes transgender and nondisclosed gender; the categories were combined owing to small numbers.
Race and ethnic group were reported by the participants.
Twenty additional health care workers in whom seroconversion occurred reported symptoms between baseline testing and seroconversion.
All PCR-positive results in workers with seroconversion occurred while they were in the seronegative follow-up group. A single health care worker in whom seroconversion occurred first tested PCR-positive while asymptomatic, and is recorded in the asymptomatic category, but also had a further PCR-positive result when symptomatic 8 days later.
Rates of symptomatic PCR testing were similar in seronegative and seropositive health care workers: 8.7 and 8.0 tests per 10,000 days at risk, respectively (rate ratio, 0.92; 95% confidence interval [CI], 0.77 to 1.10). A total of 8850 health care workers had at least one postbaseline asymptomatic screening test; seronegative health care workers attended asymptomatic screening more frequently than seropositive health care workers (141 vs. 108 per 10,000 days at risk, respectively; rate ratio, 0.76; 95% CI, 0.73 to 0.80).
Incidence of PCR-Positive Results According to Baseline Anti-Spike IgG Status
Positive baseline anti-spike antibody assays were associated with lower rates of PCR-positive tests. Of 11,364 health care workers with a negative anti-spike IgG assay, 223 had a positive PCR test (1.09 per 10,000 days at risk), 100 during asymptomatic screening and 123 while symptomatic. Of 1265 health care workers with a positive anti-spike IgG assay, 2 had a positive PCR test (0.13 per 10,000 days at risk), and both workers were asymptomatic when tested. The incidence rate ratio for positive PCR tests in seropositive workers was 0.12 (95% CI, 0.03 to 0.47; P=0.002). The incidence of PCR-confirmed symptomatic infection in seronegative health care workers was 0.60 per 10,000 days at risk, whereas there were no confirmed symptomatic infections in seropositive health care workers. No PCR-positive results occurred in 24 seronegative, previously PCR-positive health care workers; seroconversion occurred in 5 of these workers during follow-up.
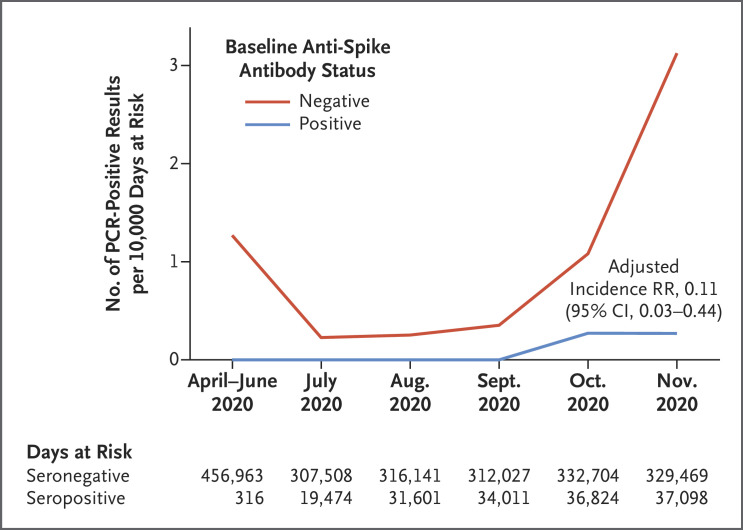
Incidence varied by calendar time (Figure 1), reflecting the first (March through April) and second (October and November) waves of the pandemic in the United Kingdom, and was consistently higher in seronegative health care workers. After adjustment for age, gender, and month of testing (Table S1) or calendar time as a continuous variable (Fig. S2), the incidence rate ratio in seropositive workers was 0.11 (95% CI, 0.03 to 0.44; P=0.002). Results were similar in analyses in which follow-up of both seronegative and seropositive workers began 60 days after baseline serologic assay; with a 90-day window after positive serologic assay or PCR testing; and after random removal of PCR results for seronegative health care workers to match asymptomatic testing rates in seropositive health care workers (Tables S2 through S4). The incidence of positive PCR tests was inversely associated with anti-spike antibody titers, including titers below the positive threshold (P<0.001 for trend) (Fig. S3A).
Figure 1. Observed Incidence of SARS-CoV-2–Positive PCR Results According to Baseline Anti-Spike IgG Antibody Status.
The incidence of polymerase-chain-reaction (PCR) tests that were positive for SARS-CoV-2 infection during the period from April through November 2020 is shown per 10,000 days at risk among health care workers according to their antibody status at baseline. In seronegative health care workers, 1775 PCR tests (8.7 per 10,000 days at risk) were undertaken in symptomatic persons and 28,878 (141 per 10,000 days at risk) in asymptomatic persons; in seropositive health care workers, 126 (8.0 per 10,000 days at risk) were undertaken in symptomatic persons and 1704 (108 per 10,000 days at risk) in asymptomatic persons. RR denotes rate ratio.
Anti-Nucleocapsid IgG Status
With anti-nucleocapsid IgG used as a marker for prior infection in 12,666 health care workers (Fig. S1B and Table S5), 226 of 11,543 (1.10 per 10,000 days at risk) seronegative health care workers tested PCR-positive, as compared with 2 of 1172 (0.13 per 10,000 days at risk) antibody-positive health care workers (incidence rate ratio adjusted for calendar time, age, and gender, 0.11; 95% CI, 0.03 to 0.45; P=0.002) (Table S6). The incidence of PCR-positive results fell with increasing anti-nucleocapsid antibody titers (P<0.001 for trend) (Fig. S3B).
A total of 12,479 health care workers had both anti-spike and anti-nucleocapsid baseline results (Fig. S1C and Tables S7 and S8); 218 of 11,182 workers (1.08 per 10,000 days at risk) with both immunoassays negative had subsequent PCR-positive tests, as compared with 1 of 1021 workers (0.07 per 10,000 days at risk) with both baseline assays positive (incidence rate ratio, 0.06; 95% CI, 0.01 to 0.46) and 2 of 344 workers (0.49 per 10,000 days at risk) with mixed antibody assay results (incidence rate ratio, 0.42; 95% CI, 0.10 to 1.69).
Seropositive Health Care Workers with PCR-Positive Results
Three seropositive health care workers subsequently had PCR-positive tests for SARS-CoV-2 infection (one with anti-spike IgG only, one with anti-nucleocapsid IgG only, and one with both antibodies). The time between initial symptoms or seropositivity and subsequent positive PCR testing ranged from 160 to 199 days. Information on the workers’ clinical histories and on PCR and serologic testing results is shown in Table 2 and Figure S4.
Table 2. Demographic, Clinical, and Laboratory Characteristics of Health Care Workers with Possible SARS-CoV-2 Reinfection.
| Health Care Worker | Baseline Serologic Assay | No. of Days between Episodes* |
Clinical Characteristics | Timing of PCR, Ct Value, and Assay |
Follow-up Serologic Assay |
|---|---|---|---|---|---|
| Worker 1: White female physician, 25–29 yr of age |
|
160 |
|
|
Dual antibody seroconversion with a rise in anti-nucleocapsid IgG titer |
| Worker 2: White female nurse, 55–59 yr of age |
|
190 |
|
|
No rise in antibody titers |
| Worker 3: White female administrator with patient contact, 50–54 yr of age |
|
199 |
|
|
Dual antibody seroconversion, with a rise in anti-spike IgG titer |
The number of days between episodes was calculated from the date of symptom onset if the index infection was symptomatic (as it was for health care Workers 2 and 3) or the date of the first clinic attendance if the presumed first episode was asymptomatic with no PCR performed (as it was for Worker 1). Both baseline serologic assays for Worker 1 were repeated and confirmed. A single positive PCR result for Worker 1 was confirmed by repeat nucleic acid extraction. Abbott PCR assay cycle number (CN) values are approximately equivalent to cycle threshold (Ct) values 10 units higher (e.g., CN 21 is approximately equivalent to Ct 31). See Figure S4 for quantitative antibody results. All PCR tests listed were performed at Oxford University Hospitals.
Only the health care worker with both antibodies had a history of PCR-confirmed symptomatic infection that preceded serologic testing; after five negative PCR tests, this worker had one positive PCR test (low viral load: cycle number, 21 [approximate equivalent cycle threshold, 31]) at day 190 after infection while the worker was asymptomatic, with subsequent negative PCR tests 2 and 4 days later and no subsequent rise in antibody titers. If this worker’s single PCR-positive result was a false positive, the incidence rate ratio for PCR positivity if anti-spike IgG–seropositive would fall to 0.05 (95% CI, 0.01 to 0.39) and if anti-nucleocapsid IgG–seropositive would fall to 0.06 (95% CI, 0.01 to 0.40).
A fourth dual-seropositive health care worker had a PCR-positive test 231 days after the worker’s index symptomatic infection, but retesting of the worker’s sample was negative twice, which suggests a laboratory error in the original PCR result. Subsequent serologic assays showed waning anti-nucleocapsid and stable anti-spike antibodies.
Discussion
In this longitudinal cohort study, the presence of anti-spike antibodies was associated with a substantially reduced risk of PCR-confirmed SARS-CoV-2 infection over 31 weeks of follow-up. No symptomatic infections and only two PCR-positive results in asymptomatic health care workers were seen in those with anti-spike antibodies, which suggests that previous infection resulting in antibodies to SARS-CoV-2 is associated with protection from reinfection for most people for at least 6 months. Evidence of postinfection immunity was also seen when anti-nucleocapsid IgG or the combination of anti-nucleocapsid and anti-spike IgG was used as a marker of previous infection.
The incidence of SARS-CoV-2 infection was inversely associated with baseline anti-spike and anti-nucleocapsid antibody titers, including titers below the positive threshold for both assays, such that workers with high “negative” titers were relatively protected from infection. In addition to the 24 seronegative health care workers with a previous positive PCR test, it is likely that other health care workers with baseline titers below assay thresholds, which were set to ensure high specificity,23 had been previously infected with SARS-CoV-2 and had low peak postinfection titers or rising or waning responses at testing.5
Two of the three seropositive health care workers who had subsequent PCR-positive tests had discordant baseline antibody results, a finding that highlights the imperfect nature of antibody assays as markers of previous infection. Neither worker had a PCR-confirmed primary SARS-CoV-2 infection. Subsequent symptomatic infection developed in one worker, and both workers had subsequent dual antibody seroconversion. It is plausible that one or both had false positive baseline antibody results (e.g., from immunoassay interference27). The health care worker in whom both anti-spike and anti-nucleocapsid antibodies were detected had previously had PCR-confirmed SARS-CoV-2 infection; the subsequent PCR-positive result with a low viral load was not confirmed on repeat testing and was not associated with a change in IgG response. These results could be consistent with a reexposure to SARS-CoV-2 that did not lead to symptoms but could also plausibly have arisen from undetected laboratory error; although contemporaneous retesting of the PCR-positive sample was not undertaken, samples tested 2 and 4 days later were both negative. If the PCR-positive result is incorrect, the incidence rate ratio for PCR positivity if anti-spike IgG–seropositive would fall to 0.05. We detected and did not include in our analysis a presumed false positive PCR test in a fourth seropositive health care worker.
Owing to the low number of reinfections in seropositive health care workers, we cannot say whether past seroconversion or current antibody levels determine protection from infection or define which characteristics are associated with reinfection. Similarly, we cannot say whether protection is conferred through the antibodies we measured or through T-cell immunity, which we did not assess. It was not possible to use sequencing to compare primary and subsequent infections, since only one of the three seropositive health care workers with a subsequent PCR-positive test had PCR-confirmed primary infection and that worker’s original sample was not stored. Our study was relatively short, with up to 31 weeks of follow-up. Ongoing follow-up is needed in this and other cohorts, including the use of markers of both humoral and cellular immunity to SARS-CoV-2, to assess the magnitude and duration of protection from reinfection, symptomatic disease, and hospitalization or death and the effect of protection on transmission.
Health care workers were enrolled in a voluntary testing program with a flexible follow-up schedule, which led to different attendance frequencies. Although health care workers were offered asymptomatic PCR testing every 2 weeks, the workers attended less frequently than that (mean, once every 10 to 13 weeks). Therefore, asymptomatic infection is likely to have been underascertained. In addition, as staff were told their antibody results, “outcome ascertainment bias” occurred, with seropositive staff attending asymptomatic screening less frequently. However, a sensitivity analysis suggests that the differing attendance rates did not substantially alter our findings. Staff were told to follow guidance on social distancing and use of personal protective equipment and to attend testing if Covid-19 symptoms developed, even if the worker had been previously PCR- or antibody-positive. This is reflected in the similar rates of testing of symptomatic seropositive and seronegative health care workers.
Some health care workers were lost to follow-up after terminating employment at our hospitals; this was likely to have occurred at similar rates in seropositive and seronegative staff. Not all PCR-positive results from government symptomatic testing sites were communicated to the hospital. This is a study of predominantly healthy adult health care workers 65 years of age or younger; further studies are needed to assess postinfection immunity in other populations, including children, older adults, and persons with coexisting conditions, including immunosuppression.
In this study, we found a substantially lower risk of reinfection with SARS-CoV-2 in the short term among health care workers with anti-spike antibodies and those with anti-nucleocapsid antibodies than among those who were seronegative.
Acknowledgments
We thank all Oxford University Hospitals personnel who participated in the staff testing program and the staff and medical students who ran the program. This study uses data provided by health care workers and collected by the U.K. National Health Service as part of their care and support. We thank additional members of the Infections in Oxfordshire Research Database team: L. Butcher, H. Boseley, C. Crichton, O. Freeman, J. Gearing (community), R. Harrington, M. Landray, A. Pal, T.P. Quan, J. Robinson (community), J. Sellors, B. Shine, and D. Waller; and the patient and public panel: G. Blower, C. Mancey, P. McLoughlin, and B. Nichols.
Supplementary Appendix
Disclosure Forms
The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, the Department of Health, or Public Health England.
This article was published on December 23, 2020, at NEJM.org.
Footnotes
Supported by the U.K. Government Department of Health and Social Care; the National Institute for Health Research (NIHR) Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Oxford University, in partnership with Public Health England (NIHR200915); the NIHR Biomedical Research Centre (BRC), Oxford; and benefactions from the Huo Family Foundation and Andrew Spokes. This study is affiliated with Public Health England’s SARS-CoV-2 Immunity and Reinfection Evaluation (SIREN) study. Dr. Eyre is a Robertson Foundation Fellow and an NIHR Oxford BRC Senior Fellow; Dr. Lumley is a Wellcome Trust Clinical Research Fellow; Prof. Stuart’s work is supported by the Medical Research Council (MR/N00065X/1); Dr. Matthews holds a Wellcome Intermediate Fellowship (110110/Z/15/Z); Dr. Marsden’s work is supported by the Kennedy Trust for Rheumatology Research and by the SGC, a registered charity (no. 1097737) that receives funds from AbbVie, Bayer Pharma, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada through Ontario Genomics Institute (OGI-055), Innovative Medicines Initiative (EU/EFPIA) (ULTRA-DD grant no. 115766), Janssen, Merck, Darmstadt, Germany, MSD, Novartis Pharma, Pfizer, São Paulo Research Foundation (FAPESP), Takeda, and Wellcome. Prof. Screaton is a Wellcome Trust Senior Investigator with funding from the Schmidt Foundation; Dr. Timothy Walker is a Wellcome Trust Clinical Career Development Fellow (214560/Z/18/Z); and Prof. A. Sarah Walker is an NIHR Senior Investigator.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008;47:401-409. [DOI] [PubMed] [Google Scholar]
- 2.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020;5:1598-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020;584:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020;383:1724-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumley SF, Wei J, O’Donnell D, et al. The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. November 4, 2020. (https://www.medrxiv.org/content/10.1101/2020.11.02.20224824v1). preprint. [DOI] [PMC free article] [PubMed]
- 6.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020;26:1200-1204. [DOI] [PubMed] [Google Scholar]
- 7.Muecksch F, Wise H, Batchelor B, et al. Longitudinal analysis of serology and neutralizing antibody levels in COVID19 convalescents. J Infect Dis 2020. November 3 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020;20:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GeurtsvanKessel CH, Okba NMA, Igloi Z, et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun 2020;11:3436-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020;370:1227-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tillett RL, Sevinsky JR, Hartley PD, et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis 2020. October 12 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Elslande J, Vermeersch P, Vandervoort K, et al. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clin Infect Dis 2020. September 5 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prado-Vivar B, Becerra-Wong B, Guadalupe JJ, et al. COVID-19 re-infection by a phylogenetically distinct SARS-CoV-2 variant, first confirmed event in South America. 2020. (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3686174). [Google Scholar]
- 14.To KK-W, Hung IF-N, Ip JD, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis 2020. August 25 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta V, Bhoyar RC, Jain A, et al. Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2. Clin Infect Dis 2020. September 23 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker AS, Pritchard E, House T, et al. Viral load in community SARS-CoV-2 cases varies widely and temporally October 27, 2020. (https://www.medrxiv.org/content/10.1101/2020.10.25.20219048v1). preprint.
- 17.Mumoli N, Vitale J, Mazzone A. Clinical immunity in discharged medical patients with COVID-19. Int J Infect Dis 2020;99:229-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang K, Yiu-Nam Lau J, Yang L, Ma Z. SARS-CoV-2 reinfection in two patients who have recovered from COVID-19. Prec Clin Med 2020. (https://academic.oup.com/pcm/advance-article/doi/10.1093/pcmedi/pbaa031/5901533). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect 2020;81:816-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Centre for Disease Prevention and Control. Reinfection with SARS-CoV-2: considerations for public health response. September 21, 2020. (https://www.ecdc.europa.eu/sites/default/files/documents/Re-infection-and-viral-shedding-threat-assessment-brief.pdf).
- 21.Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol 2020;58(11):e02107-20-e02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyre DW, Lumley SF, O’Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife 2020;9:e60675-e60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 2020;20:1390-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams ER, Ainsworth M, Anand R, et al. Antibody testing for COVID-19: a report from the National COVID Scientific Advisory Panel. Wellcome Open Research. June 11, 2020. (https://wellcomeopenresearch.org/articles/5-139/v1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cento V, Colagrossi L, Nava A, et al. Persistent positivity and fluctuations of SARS-CoV-2 RNA in clinically-recovered COVID-19 patients. J Infect 2020;81(3):e90-e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu CM, Leung WS, Cheng VCC, et al. Duration of RT-PCR positivity in severe acute respiratory syndrome. Eur Respir J 2005;25:12-14. [DOI] [PubMed] [Google Scholar]
- 27.Ward G, Simpson A, Boscato L, Hickman PE. The investigation of interferences in immunoassay. Clin Biochem 2017;50:1306-1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.