Presentation of Case
Dr. Stephanie M. Shatzman (Medicine): A 24-year-old man was admitted to this hospital in the spring of 2020 because of headache and coronavirus disease 2019 (Covid-19), the illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The patient had been in his usual state of health until 3 weeks before this admission, when fatigue, generalized weakness, and headache developed. He also had myalgias, nausea, and vomiting but no fever, cough, or diarrhea. Ibuprofen and aspirin did not alleviate the symptoms.
Two weeks later, headache and weakness persisted and new shortness of breath, pleuritic pain, and anorexia developed. Five days before this admission, the patient sought evaluation at a local urgent care clinic. The temperature was 36.8°C, and the oxygen saturation 98% while he was breathing ambient air. Physical examination was normal. Laboratory test results are shown in Table 1. A nasopharyngeal swab was obtained to test for SARS-CoV-2 RNA. Intramuscular ketorolac was administered, and the patient was discharged home with recommendations to quarantine.
Table 1. Laboratory Data.*.
| Variable | Reference Range, Urgent Care Clinic | 5 Days before Current Admission, Urgent Care Clinic | Reference Range, This Hospital† | On Current Admission, This Hospital |
|---|---|---|---|---|
| White-cell count (per μl) | 4000–11,000 | 8590 | 4500–11,000 | 7970 |
| Differential count (per μl) | ||||
| Neutrophils | 1800–7000 | 6470 | 1800–7700 | 5940 |
| Lymphocytes | 1000–4800 | 1250 | 1000–4800 | 980 |
| Monocytes | 200–1200 | 730 | 200–1200 | 1050 |
| Hemoglobin (g/dl) | 13.5–17.5 | 12.7 | 13.5–17.5 | 13.1 |
| Hematocrit (%) | 41.0–53.0 | 37.8 | 41.0–53.0 | 38.6 |
| Platelet count (per μl) | 150,000–450,000 | 305,000 | 150,000–400,000 | 353,000 |
| Sodium (mmol/liter) | 136–145 | 134 | 135–145 | 134 |
| Potassium (mmol/liter) | 3.6–5.1 | 3.8 | 3.4–5.0 | 3.4 |
| Chloride (mmol/liter) | 98–107 | 95 | 98–108 | 93 |
| Carbon dioxide (mmol/liter) | 22–32 | 26 | 23–32 | 23 |
| Urea nitrogen (mg/dl) | 6–20 | 6 | 8–25 | 5 |
| Creatinine (mg/dl) | 0.6–1.3 | 0.76 | 0.60–1.50 | 0.74 |
| Glucose (mg/dl) | 65–99 | 113 | 70–110 | 108 |
| Alanine aminotransferase (U/liter) | 10–50 | 19 | 10–55 | 32 |
| Aspartate aminotransferase (U/liter) | 15–41 | 27 | 10–40 | 20 |
| Alkaline phosphatase (U/liter) | 32–100 | 111 | 45–115 | 117 |
| Total protein (g/dl) | 6.1–8.1 | 8.4 | 6.3–8.3 | 8.2 |
| Albumin (g/dl) | 3.5–5.2 | 4.6 | 3.3–5.0 | 4.4 |
| Globulin (g/dl) | 1.9–4.1 | 3.8 | 1.9–4.1 | 3.8 |
| Creatine kinase (U/liter) | 60–400 | 49 | ||
| Lactate dehydrogenase (U/liter) | 110–210 | 166 | ||
| Lactic acid (mmol/liter) | 0.5–2.0 | 1.5 | ||
| Procalcitonin (ng/ml) | 0.00–0.08 | 0.04 | ||
| C-reactive protein (mg/liter) | <8.0 | 0.7 | ||
| Erythrocyte sedimentation rate (mm/hr) | 0–13 | 22 | ||
| Ferritin (μg/liter) | 20–300 | 314 | ||
| d-dimer (ng/ml) | <500 | 401 |
To convert the values for urea nitrogen to millimoles per liter, multiply by 0.357. To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for lactic acid to milligrams per deciliter, divide by 0.1110.
Reference values are affected by many variables, including the patient population and the laboratory methods used. The ranges used at Massachusetts General Hospital are for adults who are not pregnant and do not have medical conditions that could affect the results. They may therefore not be appropriate for all patients.
The next day, SARS-CoV-2 RNA was detected in the nasopharyngeal swab, and the patient had a telemedicine visit through the urgent care clinic. He reported headache, nausea, anorexia, and weakness. Four days later, the headache worsened, and he had presyncope; he sought evaluation at the emergency department of this hospital.
On arrival at the emergency department, multiple episodes of vomiting occurred. The patient described headache and pain localized behind the eyes, as well as persistent shortness of breath, pleuritic pain, myalgias, nausea, vomiting, and anorexia. There was no fever, cough, abdominal pain, or diarrhea. The patient had undergone an appendectomy during childhood; he reported no other known medical conditions, was taking no medications, and had no known allergies to medications.
The patient was born in Central America and was a university student there when he immigrated to the United States 3 months before admission. He worked in landscaping. He had had heterosexual contacts before immigration and reported that he had used condoms. He did not smoke tobacco or electronic cigarettes, drink alcohol, or use illicit drugs. He lived in an apartment in an urban area of Massachusetts with nine other people, including his brother and sister and their families. The patient did not know his family medical history.
On examination, the temperature was 36.1°C, the blood pressure 134/90 mm Hg, the heart rate 62 beats per minute, the respiratory rate 16 breaths per minute, and the oxygen saturation 99% while the patient was breathing ambient air. The body-mass index (the weight in kilograms divided by the square of the height in meters) was 21.3. The patient appeared fatigued, diaphoretic, and lethargic. He was intermittently agitated and increasingly somnolent, falling asleep multiple times during a conversation. He frequently squeezed his eyes closed and used his hands to hit his head, stating that it hurt. The pupils were equal and reactive to light; there was no nuchal rigidity. He was alert and oriented and followed commands. Cranial nerve examination was normal, but rotation of the head or lifting of the leg caused pain in the head, eyes, and neck. The lungs were clear. The remainder of the examination was normal.
Results of urinalysis and urine and blood toxicologic screening were normal. Other laboratory test results are shown in Table 1. Chest radiography revealed no opacities. Acetaminophen and intravenous fluids were administered.
Ninety minutes later, the patient was found on the ground next to the stretcher, on his hands and knees, incontinent of urine. Intravenous vancomycin, ceftriaxone, and acyclovir were administered empirically.
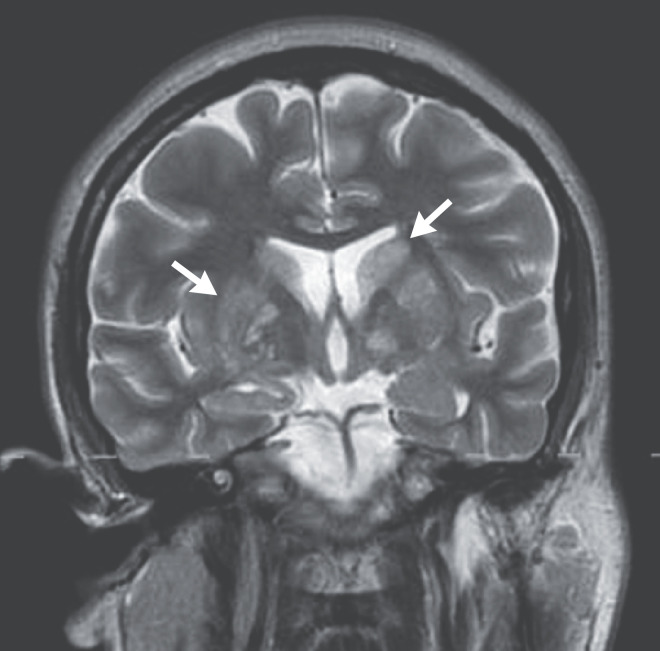
Dr. R. Gilberto Gonzalez: Computed tomography (CT) of the head, performed without the administration of intravenous contrast material, revealed no intracranial hemorrhage, mass, or large infarct. There was subtle hypodensity of the basal ganglia. There was posterior flattening of both globes and a partially empty sella turcica. Magnetic resonance imaging (MRI) of the head, performed before and after the administration of intravenous contrast material, revealed posterior flattening of both globes and tapering of the transverse sinuses. T2-weighted images and T2-weighted, fluid-attenuated inversion recovery images showed multiple small hyperintense foci in the caudate nuclei and putamina (Figure 1); several foci appeared to be cystic. In addition, there were scattered hyperintense foci in the subcortical and periventricular white matter of the frontal lobes and the anterior corona radiata.
Figure 1. MRI of the Head.
A coronal, T2-weighted image shows abnormal hyperintense foci in the basal ganglia, including the caudate nuclei and putamina (arrows). Several of the more inferiorly located abnormalities have signal characteristics suggestive of small cysts.
Dr. Shatzman: Lumbar puncture was performed. The opening pressure was higher than 55 cm of water, and the cerebrospinal fluid (CSF) was colorless. On CSF analysis, the total protein level was 47 mg per deciliter (reference range, 5 to 55), and the glucose level 42 mg per deciliter (2.3 mmol per liter; reference range, 50 to 75 mg per deciliter [2.8 to 4.2 mmol per liter]). There were 108 white cells per microliter (reference range, 0 to 5), of which 81% were lymphocytes and 18% were monocytes.
The patient was admitted to the neurology intensive care unit (ICU) 17 hours after presentation. A diagnostic test was performed.
Differential Diagnosis
Dr. Howard M. Heller: This 24-year-old man presented with a 3-week history of indolent progression of headache and respiratory and gastrointestinal symptoms. Four days before admission, he had received a diagnosis of Covid-19. He did not have a fever, and the results of physical examination were consistent with signs of meningeal inflammation. He had very slight absolute lymphopenia and mild anemia. Lumbar puncture was notable for an elevated opening pressure, and CSF analysis showed lymphocytic pleocytosis, a slightly low glucose level, and a normal protein level.
There are numerous epidemiologic, clinical, and laboratory clues in this case. We need to sort out which of these might be “red herrings,” or distractions unrelated to the diagnosis, and to avoid anchoring and being misled by other clues.
Covid-19
Could this patient’s illness be attributed to Covid-19? During the Covid-19 pandemic, this diagnosis has certainly been on the minds of clinicians and patients. This patient’s oxygen saturation was normal while he was breathing ambient air, and a chest radiograph showed no opacities. If he had a decreased oxygen saturation with activity and diffuse ground-glass opacities on chest radiography, then CT of the chest would be appropriate, since it is a sensitive method for the diagnosis of Covid-19 pneumonia.
Covid-19 has been associated with a hypercoagulable state that can lead to pulmonary emboli, but this patient had a normal d-dimer level, a finding that makes pulmonary emboli unlikely. In addition, Covid-19 has been associated with encephalitis, but Covid-19 encephalitis usually occurs in the presence of severe pulmonary disease and is typically associated with frontotemporal hypoperfusion, leptomeningeal enhancement, or evidence of strokes on MRI.1,2 Venous sinus thrombosis can occur in patients with Covid-19, but there is no evidence of venous sinus thrombosis on MRI in this patient. I think Covid-19 is a coincidental diagnosis in this case and is not the most likely cause of the neurologic illness.
Tickborne Diseases
Whenever we hear the words “landscaper” or “hiking in New England,” we tend to anchor on tickborne diseases, especially in the spring. As a landscaper, the patient was not able to work from home during the shutdown for the Covid-19 pandemic. When headache is the predominant symptom, we need to be concerned about cerebral vasculitis and Rocky Mountain spotted fever. However, in the absence of fever and rash 3 weeks into the illness, this diagnosis is unlikely.
The patient did not have leukopenia, thrombocytopenia, or elevated aminotransferase levels, so anaplasmosis is not a major diagnostic consideration. He had mild anemia but normal aspartate aminotransferase and lactate dehydrogenase levels; these findings point us away from an infection that causes hemolysis, such as babesiosis. Furthermore, neither anaplasmosis nor babesiosis would cause the central nervous system (CNS) findings seen in this patient.
Borrelia miyamotoi can cause severe, sometimes relapsing, febrile illness and lymphocytic meningitis. Powassan virus can cause encephalitis and meningitis, but these manifestations usually involve the temporal lobes rather than the basal ganglia. No cases of infection with Powassan virus or any arbovirus were reported in Massachusetts during the first 6 months of 2020, when this patient’s illness occurred.
Early disseminated Lyme borreliosis can cause lymphocytic meningitis, and increased intracranial pressure with pseudotumor cerebri has been described, but these manifestations are more common in children than adults.3 Lyme encephalitis can lead to a variety of MRI findings but not the abnormalities described in this case.4,5 Another occupational hazard for landscapers is sporotrichosis, which can cause lymphocytic meningitis, but this patient did not have the skin lesions typically associated with this infection.6
Sexually Transmitted Infections
Although this patient’s sexual history is not particularly suggestive of sexually transmitted infections, we need to consider this possibility, since some patients are initially reluctant to share details of their sexual history. The sexually transmitted infections that can cause lymphocytic meningitis include acute human immunodeficiency virus (HIV) infection, syphilis, and herpes simplex virus type 2 infection. The patient did not have any relevant findings on examination, such as oral or genital sores or an erythematous rash.
Other Infections
Given that this patient had recently immigrated to the United States, we need to consider possible diagnoses linked to Central America. Tuberculosis can cause meningitis with mononuclear pleocytosis, but with this infection, the CSF protein level is typically much higher than the level seen in this patient. In addition, he had no calcified granulomata on chest imaging; on brain imaging, we would be likely to see signs of meningitis or tuberculomas but not cystic-appearing lesions located in the basal ganglia. Cysticercosis is typically associated with either multiple, scattered enhancing cysts surrounded by edema in patients with active disease or calcifications of old cysts. Toxoplasmosis often involves the basal ganglia but typically causes ring-enhancing lesions with edema in immunocompromised patients. Chagas’ disease can cause meningoencephalitis and focal lesions during reactivation of infection in immunocompromised patients. Paracoccidioidomycosis is endemic in Central America, but neurologic involvement is uncommon and ring-enhancing lesions are usually seen. Coccidioidomycosis commonly causes meningitis, even in immunocompetent people, and although it is not endemic in Central America, we are not told how the patient traveled from Central America to Massachusetts; many immigrants undergo an arduous journey through the Sonoran Desert in northwestern Mexico. Both histoplasmosis and cryptococcosis can cause lymphocytic meningitis and are possible diagnoses in this case.7 Finally, because the patient did not have a fever and his inflammatory markers were not markedly abnormal, we need to consider noninfectious causes, specifically CNS lymphoma.
Cryptococcosis
The condition that is most commonly associated with a cystic, grapelike appearance in the brain, especially in the basal ganglia, and typically causes a very high intracranial pressure is cryptococcosis.8 Cryptococcal meningitis can occur in seemingly healthy people, but it usually occurs in people who are much older than this patient; it most commonly occurs in immunosuppressed patients, especially in the presence of advanced HIV infection. This patient had no identifiable risks for HIV infection or relevant findings on examination, such as thrush or lymphadenopathy. Hypergammaglobulinemia is a hallmark of the humoral dysregulation associated with HIV infection, especially at the late stage, but this patient’s globulin level and albumin:globulin ratio were normal.9 In addition, his history was not suggestive of hypogammaglobulinemia or another underlying immunodeficiency. Given that this patient’s presentation is most consistent with cryptococcal meningitis, I suspect that he also has a new diagnosis of advanced HIV infection. To establish these diagnoses, I would perform a CSF test for cryptococcal antigen and a fungal wet preparation. If cryptococcal disease is identified, the patient will need to undergo evaluation for an underlying immunodeficiency, including an HIV test. If the HIV test is negative, characterization of T-cell subsets by flow cytometry should be performed to rule out idiopathic CD4+ lymphocytopenia.
Clinical Impression
Dr. Brian L. Edlow: The patient’s history, MRI findings, and lumbar puncture results suggested meningoencephalitis due to a viral or fungal infection. We considered cryptococcosis to be a likely diagnosis, given the elevated intracranial pressure. Although few cases of intracerebral lesions associated with Covid-19 had been reported, Covid-19 infectious and parainfectious encephalitis were also on our differential diagnosis.
Clinical Diagnosis
Meningoencephalitis, most likely due to cryptococcus.
Dr. Howard M. Heller’s Diagnosis
Cryptococcal meningitis and immunosuppressed state, most likely in the context of advanced human immunodeficiency virus type 1 infection.
Pathological Discussion
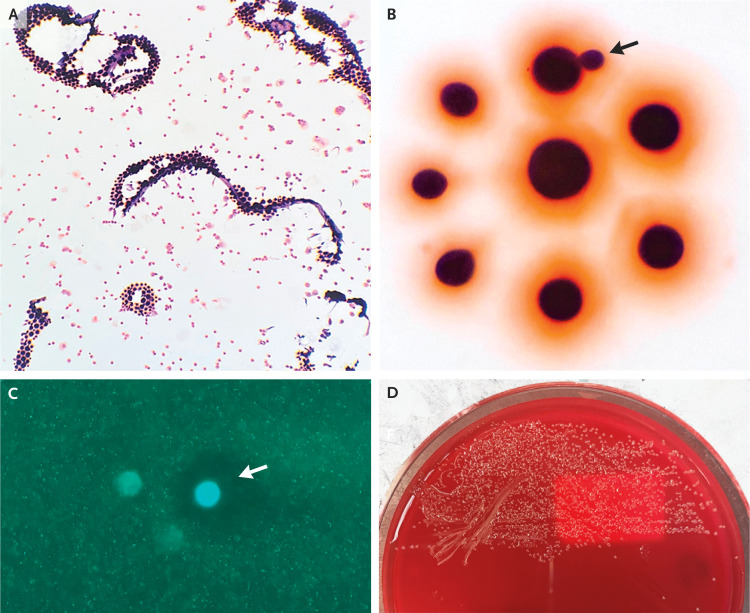
Dr. Tasos Gogakos: The first diagnostic test in this case was Gram’s staining of the CSF specimen after centrifugation, which revealed the presence of small, round gram-positive organisms (Figure 2A). At higher magnification, these organisms were of various sizes, well circumscribed, and surrounded by a salmon hue, with some of them budding on a narrow base (Figure 2B). The forms were not abutting one another; this finding is indicative of an encapsulated organism. A fungal wet preparation revealed fungal forms with circumferential clearance (Figure 2C). A latex agglutination test for cryptococcal antigen was positive, with a titer of 1:8192. Culture of the CSF specimen on a blood agar plate showed growth of small, translucent, glistening mucoid colonies after 2 days of incubation (Figure 2D). The isolate was identified as Cryptococcus neoformans by means of matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) mass spectrometry.
Figure 2. Cerebrospinal Fluid Specimen.
Gram’s staining of the cerebrospinal fluid (Panel A) shows small, round gram-positive organisms. At higher magnification (Panel B), these organisms are of various sizes, well circumscribed, surrounded by a salmon hue, and well separated, with occasional budding on a narrow base (arrow). Calcofluor white staining of a fungal wet preparation (Panel C) shows intense central staining with circumferential clearing (arrow) due to dye exclusion by the polysaccharide capsule. Culture on a blood agar plate (Panel D) shows small, glistening colonies with a mucoid appearance.
Cryptococcal infection usually occurs in the presence of severe immunosuppression characteristic of advanced HIV type 1 (HIV-1) infection (CD4+ T-cell count, <50) and is often associated with an elevated opening pressure on lumbar puncture.10 To assess the immunocompetence of this patient, HIV testing was performed. A combined antigen and HIV-1 and HIV-2 antibody screen was positive, and the diagnosis of HIV-1 infection was confirmed with the use of an HIV differentiation assay. The HIV-1 RNA viral load was 138,000 copies per milliliter. The CD4+ T-cell count was 16 per microliter, establishing the diagnosis of acquired immunodeficiency syndrome (AIDS).
HIV-positive immunocompromised patients are at an increased risk for opportunistic CNS infections, such as cryptococcal meningitis, cerebral toxoplasmosis, and tuberculous meningitis.11 Antitoxoplasma IgG antibodies were detected in this patient, but in light of the radiologic findings, cerebral toxoplasmosis was considered to be unlikely. Mycobacterial cultures of blood and CSF specimens were negative, as was an enzyme-linked immunospot assay for interferon-γ.
Pathological Diagnosis
Advanced human immunodeficiency virus infection and cryptococcal infection of the central nervous system.
Discussion of Management
Dr. Edlow: In a patient with meningoencephalitis in the absence of obstructive hydrocephalus on head CT, the mechanism that is most likely to cause intracranial hypertension is impaired CSF absorption within the arachnoid granulations. This type of intracranial hypertension is treated with CSF diversion by means of serial lumbar puncture,12 which we performed on days 1 and 2 of the patient’s admission to the ICU. CSF diversion is an essential function-sparing and lifesaving intervention in patients with cryptococcal meningoencephalitis, given that elevated intracranial pressure is a well-established predictor of mortality.13,14
During each lumbar puncture, we measured the opening and closing pressure with the patient in the lateral decubitus position, because this information informs decision making with regard to the frequency of subsequent lumbar punctures. Infectious Diseases Society of America guidelines recommend that CSF be drained to bring the pressure below 20 cm of water or reduce it by 50%.12 We removed a large volume of CSF during each lumbar puncture, but the closing pressure remained above 20 cm of water, and by the next day, the opening pressure was again higher than 55 cm of water. These recurrent elevations in intracranial pressure were associated with worsening retro-orbital headache and intermittent alterations of arousal (i.e., somnolence).
Concurrent with CSF diversion, we administered pharmacologic therapies to reduce the intracranial pressure. Cerebral edema can develop in patients with cryptococcal meningoencephalitis if cryptococcal polysaccharides block the flow of interstitial fluid into the subarachnoid space.15 Thus, we administered mannitol, a hyperosmolar therapy that reduces intracerebral water content. We also administered acetazolamide, a carbonic anhydrase inhibitor that inhibits the production of new CSF. Data supporting the efficacy of mannitol and acetazolamide in patients with cryptococcal meningoencephalitis are lacking,12,16 but we thought that the potential benefits of these therapies outweighed the minimal risks. We did not administer glucocorticoids, which are contraindicated in HIV-infected patients with cryptococcosis.12 Because there had been a question of seizure when the patient was in the emergency department, we initially administered levetiracetam. However, no seizures were observed in the ICU, and continuous electroencephalography on ICU days 2 to 3 showed no evidence of epileptiform activity. Thus, we discontinued levetiracetam.
We performed repeat head CT on ICU days 1 and 2 to confirm that no mass lesion or obstructive hydrocephalus had developed, because fatal brain-stem herniation has been reported in HIV-infected patients who have undergone repeat lumbar puncture for cryptococcal meningitis.17 On ICU day 3, we transitioned from daily lumbar puncture to placement of a lumbar drain, a decision motivated by the need to optimize patient comfort, reduce the risk of agitation, and minimize provider exposure to a patient with Covid-19. We initiated CSF diversion through the lumbar drain at 15 to 20 ml per hour, the rate at which new CSF is made by the choroid plexus. We clamped the drain frequently to avoid overly rapid drainage, which can cause intracranial hypotension and subdural hemorrhage.
The patient continued to have headaches, altered arousal, and elevated intracranial pressure after 2 weeks of CSF diversion through the lumbar drain. Therefore, our neurosurgical team placed a ventriculoperitoneal shunt on ICU day 20 for long-term CSF diversion. A ventriculoperitoneal shunt is required in approximately 30% of HIV-infected patients with cryptococcal meningitis who have elevated intracranial pressure on the initial lumbar puncture,18 as was seen in this patient.
Dr. Kevin L. Ard: We considered what role, if any, Covid-19 played in this patient’s illness. Although the shortness of breath he had reported on presentation could have been due to Covid-19, it resolved quickly, and the chest radiograph was clear. In contrast, his primary symptoms of headache, fatigue, and vomiting that occurred over a period of weeks were all classic symptoms of cryptococcal meningoencephalitis. Covid-19 is associated with lymphopenia, and there is one case report of an opportunistic infection, pneumocystis, in a patient with Covid-19–associated lymphopenia and no other known cause of immunocompromise.19 We wondered whether the decline in his CD4+ T-cell count due to Covid-19 might have accelerated the presentation of cryptococcal meningitis, but we ultimately thought that this was unlikely, given his markedly abnormal CD4:CD8 T-cell ratio. A normal CD4:CD8 T-cell ratio is approximately 2, and early data indicate that the ratio tends to be preserved in patients with Covid-19–associated lymphopenia.20 In patients with AIDS, the CD4+ T-cell population becomes depleted and the CD8+ T-cell population usually expands, and the ratio falls; this patient’s CD4:CD8 T-cell ratio was quite low, at 0.02, a finding compatible with advanced HIV infection. Our conclusion was that Covid-19 was not a major contributor to his clinical presentation.
Our central focus was management of AIDS-associated cryptococcal meningoencephalitis. This infection should prompt four key steps. The first step is administration of antifungal therapy, which consists of amphotericin B and flucytosine, for at least 2 weeks, followed by consolidation and maintenance therapy with fluconazole.21 The second step, and often one of the most challenging aspects, is management of the elevated intracranial pressure. The third step is evaluation for complications of AIDS and other opportunistic infections, such as tuberculosis, Mycobacterium avium complex, and cytomegalovirus. During the Covid-19 pandemic, there has been a desire to limit opportunities for transmission and conserve personal protective equipment, and many specialty consultations have occurred by video or telephone. However, such an approach would not have been appropriate for this patient; in addition to laboratory investigations for opportunistic infections, his illness warranted a detailed physical examination, with palpation of lymph nodes and inspection of his skin and oropharynx. Ultimately, no additional opportunistic infections or AIDS complications were identified. The fourth and final step in the management of AIDS-associated cryptococcal meningoencephalitis is determination of when to start antiretroviral therapy (ART).
We usually begin ART soon after a diagnosis of HIV is established, often on the same day the diagnosis is made, because of the personal and public health benefits of treatment. However, in patients with cryptococcal meningoencephalitis, we make a rare exception, because such patients have increased mortality associated with early ART initiation that is attributed to immune reconstitution inflammatory syndrome.22,23 We know to delay ART initiation in such patients but do not know the optimal time for initiation. Department of Health and Human Services guidance specifies that ART should be initiated within 2 to 10 weeks after the start of antifungal treatment,21 which is a wide time frame. In tertiary care settings such as ours, where patients can be monitored closely and we have the ability to provide advanced and immediate management of elevated intracranial pressure, we often begin ART closer to 2 weeks after diagnosis.
Follow-up
Dr. Ard: Approximately 2.5 weeks after the initiation of antifungal therapy, after sterilization of CSF cultures and placement of a ventriculoperitoneal shunt, this patient started ART with coformulated emtricitabine–tenofovir alafenamide–bictegravir, a standard first-line regimen in the United States. Unfortunately, he did not pick up his ART prescription after discharge from a rehabilitation facility, but this was detected promptly by his outpatient clinician and he resumed the medication within 1 week. At a follow-up clinic visit 2.5 months after diagnosis, the patient was doing well and had started working again. He reported adherence to both emtricitabine–tenofovir alafenamide–bictegravir and fluconazole, and he had no evidence of lingering neurologic deficits.
Final Diagnosis
Cryptococcal meningoencephalitis and advanced human immunodeficiency virus infection.
Disclosure Forms
Footnotes
This case was presented at the Medicine Case Conference.
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology 2020;95(13):e1868-e1882. [DOI] [PubMed] [Google Scholar]
- 2.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 2020;143:3104-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nord JA, Karter D. Lyme disease complicated with pseudotumor cerebri. Clin Infect Dis 2003;37(2):e25-e26. [DOI] [PubMed] [Google Scholar]
- 4.Hildenbrand P, Craven DE, Jones R, Nemeskal P. Lyme neuroborreliosis: manifestations of a rapidly emerging zoonosis. AJNR Am J Neuroradiol 2009;30:1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindland ES, Solheim AM, Andreassen S, et al. Imaging in Lyme neuroborreliosis. Insights Imaging 2018;9:833-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mialski R, Nobrega de Almeida J Jr, Honorato da Silva L, et al. Chronic meningitis and hydrocephalus due to Sporothrix brasiliensis in immunocompetent adults: a challenging entity. Open Forum Infect Dis 2018;5(5):ofy081-ofy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina N, Samayoa B, Lau-Bonilla D, et al. Burden of serious fungal infections in Guatemala. Eur J Clin Microbiol Infect Dis 2017;36:965-969. [DOI] [PubMed] [Google Scholar]
- 8.Xia S, Li X, Li H. Imaging characterization of cryptococcal meningoencephalitis. Radiol Infect Dis 2016;3:187-191. [Google Scholar]
- 9.Serpa J, Haque D, Valayam J, Breaux K, Rodriguez-Barradas MC. Effect of combination antiretroviral treatment on total protein and calculated globulin levels among HIV-infected patients. Int J Infect Dis 2010;14:Suppl 3:e41-e44. [DOI] [PubMed] [Google Scholar]
- 10.Tan IL, Smith BR, von Geldern G, Mateen FJ, McArthur JC. HIV-associated opportunistic infections of the CNS. Lancet Neurol 2012;11:605-617. [DOI] [PubMed] [Google Scholar]
- 11.Bowen LN, Smith B, Reich D, Quezado M, Nath A. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol 2016;12:662-674. [DOI] [PubMed] [Google Scholar]
- 12.Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Clin Infect Dis 2000;30:710-718. [DOI] [PubMed] [Google Scholar]
- 13.Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. Clin Infect Dis 2000;30:47-54. [DOI] [PubMed] [Google Scholar]
- 14.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med 1997;337:15-21. [DOI] [PubMed] [Google Scholar]
- 15.Lee SC, Casadevall A. Polysaccharide antigen in brain tissue of AIDS patients with cryptococcal meningitis. Clin Infect Dis 1996;23:194-195. [DOI] [PubMed] [Google Scholar]
- 16.Newton PN, Thai LH, Tip NQ, et al. A randomized, double-blind, placebo-controlled trial of acetazolamide for the treatment of elevated intracranial pressure in cryptococcal meningitis. Clin Infect Dis 2002;35:769-772. [DOI] [PubMed] [Google Scholar]
- 17.Antinori S, Ridolfo AL, Gianelli E, Piazza M, Gervasoni C, Monforte AA. The role of lumbar puncture in the management of elevated intracranial pressure in patients with AIDS-associated cryptococcal meningitis. Clin Infect Dis 2000;31:1309-1311. [DOI] [PubMed] [Google Scholar]
- 18.Cherian J, Atmar RL, Gopinath SP. Shunting in cryptococcal meningitis. J Neurosurg 2016;125:177-186. [DOI] [PubMed] [Google Scholar]
- 19.Menon AA, Berg DD, Brea EJ, et al. A case of COVID-19 and Pneumocystis jirovecii coinfection. Am J Respir Crit Care Med 2020;202:136-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol 2020;95:834-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: cryptococcosis. ClinicalInfo. 2016 (https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/cryptococcosis).
- 22.Makadzange AT, Ndhlovu CE, Takarinda K, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-Saharan Africa. Clin Infect Dis 2010;50:1532-1538. [DOI] [PubMed] [Google Scholar]
- 23.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014;370:2487-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.