Abstract
Background
LY-CoV555, a neutralizing monoclonal antibody, has been associated with a decrease in viral load and the frequency of hospitalizations or emergency department visits among outpatients with coronavirus disease 2019 (Covid-19). Data are needed on the effect of this antibody in patients who are hospitalized with Covid-19.
Methods
In this platform trial of therapeutic agents, we randomly assigned hospitalized patients who had Covid-19 without end-organ failure in a 1:1 ratio to receive either LY-CoV555 or matching placebo. In addition, all the patients received high-quality supportive care as background therapy, including the antiviral drug remdesivir and, when indicated, supplemental oxygen and glucocorticoids. LY-CoV555 (at a dose of 7000 mg) or placebo was administered as a single intravenous infusion over a 1-hour period. The primary outcome was a sustained recovery during a 90-day period, as assessed in a time-to-event analysis. An interim futility assessment was performed on the basis of a seven-category ordinal scale for pulmonary function on day 5.
Results
On October 26, 2020, the data and safety monitoring board recommended stopping enrollment for futility after 314 patients (163 in the LY-CoV555 group and 151 in the placebo group) had undergone randomization and infusion. The median interval since the onset of symptoms was 7 days (interquartile range, 5 to 9). At day 5, a total of 81 patients (50%) in the LY-CoV555 group and 81 (54%) in the placebo group were in one of the two most favorable categories of the pulmonary outcome. Across the seven categories, the odds ratio of being in a more favorable category in the LY-CoV555 group than in the placebo group was 0.85 (95% confidence interval [CI], 0.56 to 1.29; P=0.45). The percentage of patients with the primary safety outcome (a composite of death, serious adverse events, or clinical grade 3 or 4 adverse events through day 5) was similar in the LY-CoV555 group and the placebo group (19% and 14%, respectively; odds ratio, 1.56; 95% CI, 0.78 to 3.10; P=0.20). The rate ratio for a sustained recovery was 1.06 (95% CI, 0.77 to 1.47).
Conclusions
Monoclonal antibody LY-CoV555, when coadministered with remdesivir, did not demonstrate efficacy among hospitalized patients who had Covid-19 without end-organ failure. (Funded by Operation Warp Speed and others; TICO ClinicalTrials.gov number, NCT04501978.)
The antiviral drug remdesivir has been shown to decrease the time to recovery in hospitalized patients with coronavirus disease 2019 (Covid-19), and dexamethasone has been shown to decrease mortality.1,2 However, additional effective therapies are urgently needed. The use of passive immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to augment the humoral immune response to infection is a priority for clinical evaluation in patients with Covid-19.
Convalescent plasma, immune globulin, and monoclonal antibodies are all being studied as ways to boost the immune response to SARS-CoV-2. One of these antibodies, LY-CoV555 (also known as LY3819253 or bamlanivimab; AbCellera, Eli Lilly, and the National Institute of Allergy and Infectious Diseases) has been associated with a decrease in viral load and the frequency of hospitalizations or emergency department visits in outpatients with Covid-19.3
To understand the possible role of neutralizing monoclonal antibodies and other antiviral interventions in patients who are hospitalized with Covid-19, the National Institutes of Health established the ACTIV-3/TICO (Therapeutics for Inpatients with Covid-19) platform4 for the efficient conduct of trials. The first trial within the TICO platform was a comparison of LY-CoV5555 and placebo. We report here the preliminary results of this trial.
Methods
Trial Design and Treatments
The TICO platform protocol (available with the full text of this article at NEJM.org) governs the testing of multiple candidate therapies in a multigroup, multistage, double-blind design that allows for the efficient assessment of a range of potential agents with the use of a pooled placebo group when multiple therapies are being tested concurrently.6 In order to respond to pandemic dynamics, this platform protocol incorporates an early futility and safety evaluation after the enrollment of 300 patients (stage 1). Stage 1 is then followed by enrollment to the full sample size (stage 2) for agents that pass the initial futility and safety assessment.
LY-CoV555 was derived from the serum of a Covid-19 survivor. LY-CoV555 binds with high affinity to an epitope within the receptor-binding domain overlapping the binding of the SARS-CoV-2 spike protein by angiotensin-converting enzyme 2. In preclinical models of Covid-19, the use of LY-CoV555 improved clinical outcomes.5 A dose of 7000 mg was chosen on the basis of pharmacokinetic and preliminary safety data.
Hospitalized patients with Covid-19 were randomly assigned in a 1:1 ratio to receive either LY-CoV555 or matching placebo. In addition, all the patients received high-quality supportive care as background therapy, including remdesivir and, when indicated, supplemental oxygen and glucocorticoids. Randomization was stratified according to the trial pharmacy, since each pharmacy could serve more than one trial site. LY-CoV555 or placebo was administered as a single intravenous infusion over a 1-hour period. The infusion was prepared by trial pharmacists. All other personnel, including investigators and research staff, clinical staff, and patients, were unaware of the trial-group assignments.
Other medications were permitted except therapies that may provide exogenous antibodies against SARS-CoV-2. Concurrent enrollment in other randomized trials was not permitted for the first 5 days after randomization.
Patients
We enrolled adult hospitalized patients who had documented SARS-CoV-2 infection and a duration of symptoms attributable to Covid-19 of 12 days or less. Excluded from the trial were patients who had received SARS-CoV-2 intravenous immune globulin, convalescent plasma from a patient who had recovered from Covid-19, or another neutralizing monoclonal antibody against SARS-CoV-2. During stage 1, patients were excluded from the trial if they had end-organ failure (including vasopressor therapy, new renal-replacement therapy, or the receipt of invasive mechanical ventilation, extracorporeal membrane oxygenation, or mechanical circulatory support) or certain extrapulmonary complications. For treatments that passed the early futility assessment, subsequent patients would be enrolled according to expanded eligibility criteria, which permit the presence of end-organ failure and extrapulmonary complications. Additional details regarding the eligibility criteria are provided in the Supplementary Appendix, available at NEJM.org.
The protocol was approved by a central institutional review board or ethics committee at each participating site, and written informed consent was obtained from all the patients or their legally authorized representative.
Primary and Secondary Outcomes
The TICO master protocol stipulates an overall primary outcome (a sustained recovery, as assessed in a time-to-event analysis, through day 90), as well as two ordinal outcomes that are measured at day 5 to direct the early futility assessment. Given the pandemic dynamics, this design allows for a rapid determination with respect to which treatments will be advanced to a complete assessment. The two day 5 outcomes, which are both classified according to seven-level ordinal scales, are termed pulmonary and pulmonary-plus outcomes. The pulmonary outcome is based largely on oxygen requirements, ranging from an ability to perform all normal daily activities to death. The pulmonary-plus outcome captures the range of organ dysfunction that may be associated with progression of Covid-19, such as respiratory dysfunction and coagulation-related complications. Details regarding these outcomes as measured on ordinal scales, which were derived from the scales recommended by the World Health Organization7 and used in the Adaptive Covid-19 Treatment Trial 1 (ACTT-1),1 are provided in the Supplementary Appendix.
Both ordinal outcomes were assessed on days 1 through 7, whereas the pulmonary outcome was also ascertained on days 8 through 14 and on day 28. The choice of day 5 as the primary day for the evaluation was based on an evaluation of data from ACTT-1,1 which showed that remdesivir was associated with a better outcome than placebo on day 5 on an ordinal scale similar to the one used in our pulmonary outcome among patients with characteristics that were similar to those of the patients who were targeted in stage 1 of our trial.
The primary efficacy outcome for treatments that are being studied in TICO is the time to a sustained recovery, which was defined as hospital discharge to home and remaining at home for at least 14 days. A key secondary outcome was death from any cause. Deaths and serious adverse events were assessed during 90 days of follow-up. Data regarding clinical organ failure, serious infections, and clinical adverse events of grade 3 or 4 were collected through day 28.
The primary safety outcome was a composite of death, serious adverse events, or grade 3 or 4 adverse events through day 5.8 Details regarding the collection of adverse-event data are provided in the Supplementary Appendix.
Statistical Analysis
We determined that the enrollment of 300 patients in stage 1 would provide a power of 95% to detect an odds ratio of 1.60 for a more favorable day 5 outcome on the two ordinal scales for LY-CoV555 over placebo, using a one-sided test with a type I error of 0.30. For treatments that advance to stage 2, the planned sample size was 1000 patients (300 enrolled during stage 1 and 700 enrolled during stage 2) with 90 days of follow-up. Additional details regarding the calculations for the stage 1 sample size and total sample size are provided in the Supplementary Appendix. Unless otherwise stated, the analysis cohort for this preliminary report is the modified intention-to-treat population, which includes all the patients who received all or part of the LY-CoV555 or placebo infusion (Fig. S1 in the Supplementary Appendix).
An independent data and safety monitoring board reviewed interim data and used prespecified guidelines to assess futility on the basis of treatment comparisons of the two ordinal outcomes on day 5. These and other guidelines that were provided to the data and safety monitoring board are listed in the protocol and in the Supplementary Appendix.
Follow-up data for all analyses were administratively censored on October 26, 2020, the date on which all trial sites were informed of the recommendations of the data and safety monitoring board to stop the trial for futility. The analysis data set was locked on November 6, 2020, and includes deaths, serious adverse events, organ-failure events, and hospital discharges that occurred up to October 26.
To estimate the treatment effect on the ordinal pulmonary and pulmonary-plus outcomes on day 5, we estimated the summary odds ratio of a better outcome with LY-CoV555 than with placebo using proportional-odds models9 that included the treatment-group indicator. The primary efficacy analysis was adjusted for the ordinal pulmonary category at the time of trial entry and the trial pharmacy; 95% confidence intervals and P values were calculated. P values are two-sided, unless otherwise noted.
We used logistic regression to perform the primary safety analysis, which compared LY-CoV555 with placebo with respect to the percentage of patients who had died or had serious adverse events or new grade 3 or 4 adverse events by day 5 after adjustment for the trial pharmacy. The methods that were used for summarizing time-to-event outcomes, other outcomes, and subgroups are described in the Supplementary Appendix, along with a post hoc analysis of adjusted and unadjusted summary statistics, including adjustments for a chance imbalance in risk factors for severe Covid-19 with the use of a risk score. Statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute) and R software, version 4.0.10
Results
Patients
From August 5 to October 13, 2020, a total of 326 patients were enrolled at 31 trial sites, including 23 in the United States, 7 in Denmark, and 1 in Singapore. The analysis population was restricted to the 314 patients who received an infusion of LY-CoV555 (163 patients) or placebo (151 patients); of the 12 patients who did not receive an infusion, 8 had undergone randomization on the day that the data and safety monitoring board recommended stopping enrollment (Fig. S1). Characteristics of the patients are provided in Table 1. Several baseline characteristics indicated that by chance more patients in the LY-CoV555 group than in the placebo group may have been at greater risk for disease progression.
Table 1. Characteristics of the Patients at Randomization.*.
| Characteristic | LY-CoV555 (N=163) |
Placebo (N=151) |
Total (N=314) |
|---|---|---|---|
| Median age (IQR) — yr | 63 (50–72) | 59 (48–71) | 61 (49–71) |
| Female sex — no. (%) | 66 (40) | 71 (47) | 137 (44) |
| Current pregnancy — no. (%) | 1 (1) | 2 (1) | 3 (1) |
| Race or ethnic group — no. (%)† | |||
| White | 76 (47) | 71 (47) | 147 (47) |
| Hispanic | 41 (25) | 33 (22) | 74 (24) |
| Black | 33 (20) | 34 (23) | 67 (21) |
| Other | 13 (8) | 13 (9) | 26 (8) |
| Body-mass index — no. (%)‡ | |||
| ≥30 | 81 (50) | 83 (55) | 164 (52) |
| ≥40 | 20 (12) | 22 (15) | 42 (13) |
| Coexisting illness — no. (%) | |||
| Any | 117 (72) | 98 (65) | 215 (68) |
| Hypertension requiring medication | 82 (50) | 72 (48) | 154 (49) |
| Diabetes requiring medication | 54 (33) | 36 (24) | 90 (29) |
| Renal impairment | 24 (15) | 9 (6) | 33 (11) |
| Asthma | 14 (9) | 14 (9) | 28 (9) |
| Heart failure | 12 (7) | 1 (1) | 13 (4) |
| Median no. of days since symptom onset (IQR) | 7 (5–9) | 8 (5–9) | 7 (5–9) |
| Medication — no. (%) | |||
| Remdesivir | 60 (37) | 66 (44) | 126 (40) |
| Antibacterial agent | 54 (33) | 36 (24) | 90 (29) |
| Glucocorticoid | 80 (49) | 74 (49) | 154 (49) |
| Antiplatelet or anticoagulant agent§ | 106 (65) | 95 (63) | 201 (64) |
| ACE inhibitor or ARB | 41 (25) | 31 (21) | 72 (23) |
| NSAID | 17 (10) | 16 (11) | 33 (11) |
| Oxygen requirement — no. (%) | |||
| Supplementary oxygen | |||
| None | 44 (27) | 42 (28) | 86 (27) |
| <4 liters/min | 60 (37) | 57 (38) | 117 (37) |
| ≥4 liters/min | 29 (18) | 34 (23) | 63 (20) |
| Noninvasive ventilation or high-flow device | 30 (18) | 18 (12) | 48 (15) |
| Invasive ventilation or ECMO | 0 | 0 | 0 |
| Laboratory measures | |||
| Median C-reactive protein (IQR) — mg/liter | 94 (47–156) | 90 (45–139) | 92 (47–151) |
| Median B-lymphocyte count (IQR) — cells/mm3 | 784 (560–1056) | 810 (550–1310) | 799 (552–1116) |
Percentages may not total 100 because of rounding. ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, ECMO extracorporeal membrane oxygenation, IQR interquartile range, and NSAID nonsteroidal antiinflammatory drug.
Race or ethnic group was reported by the patients.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Details regarding the types of antiplatelet and anticoagulation medications that were used are provided in Table S1 in the Supplementary Appendix.
A total of 298 patients (95%) had begun to receive remdesivir before or on the day of randomization; 40% had already started receiving it at the time of randomization. In addition, 49% were receiving glucocorticoids, and 51% were receiving heparinoids at baseline (Table 1 and Table S1). Concomitant treatments that had been prescribed on day 5 are summarized in Table S2.
Data were available with respect to the day 5 outcomes in all but 3 patients (99%). The median duration of follow-up was 31 days. At the time of this report, 279 patients (89%) had been discharged from the hospital.
Efficacy Outcomes
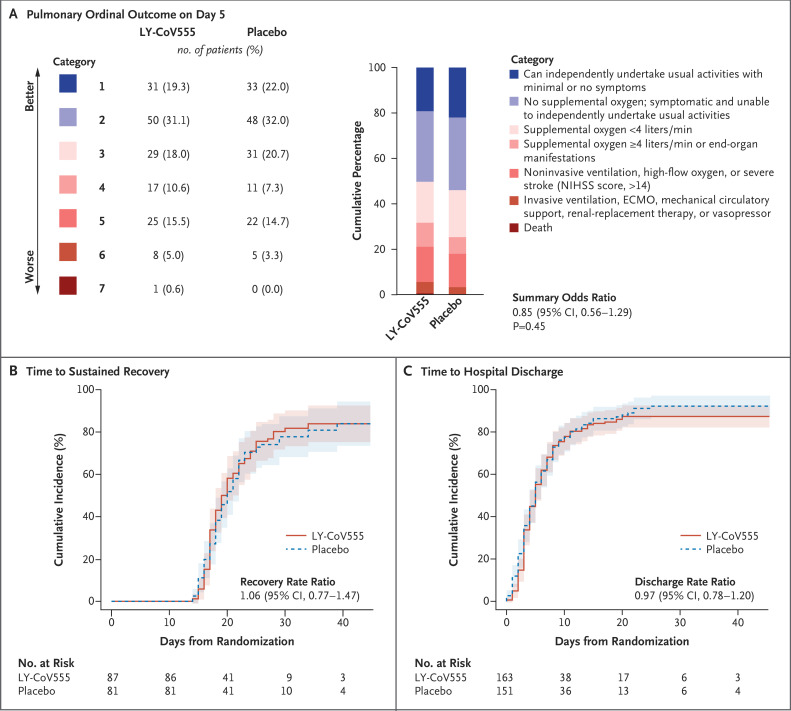
The distribution of patients across the seven categories of the pulmonary ordinal outcome on day 5 were similar in the LY-CoV555 group and the placebo group (Figure 1A). A total of 81 patients (50%) in the LY-CoV55 group were in one of the two most favorable categories, as compared with 81 patients (54%) in the placebo group; 90 patients (55%) and 85 patients (56%), respectively, had been discharged from the hospital by day 5. The odds ratio of being in a more favorable category in the LY-CoV555 group than in the placebo group was 0.85 (95% confidence interval [95% CI], 0.56 to 1.29; P=0.45) (Figure 1A and Table 2). The pulmonary and pulmonary-plus outcomes were almost identical; only 2 patients (both in the placebo group) were in a worse category of the pulmonary-plus outcome than their pulmonary category (1 owing to vasopressor use and 1 owing to stroke). The between-group comparison showed similar results for the pulmonary-plus outcome (odds ratio, 0.87; 95% CI, 0.57 to 1.31; P=0.50) (Table 2). Results for both outcomes met the prespecified criteria for futility.
Figure 1. Pulmonary Ordinal Outcome at Day 5 and Time until Sustained Recovery and Hospital Discharge.
Panel A shows the pulmonary ordinal outcome at day 5 in the LY-CoV555 group and the placebo group. The summary odds ratio was estimated with the use of a proportional-odds model after adjustment for the baseline pulmonary category and trial pharmacy. In Panels B and C, the cumulative time until a sustained recovery and hospital discharge, respectively, are Aalen–Johansen estimates; rate ratios were calculated with the use of Fine–Gray models, stratified according to trial pharmacy. The rate ratios estimate the subdistribution hazard ratios after accounting for the competing risk of death. ECMO denotes extracorporeal membrane oxygenation, and NIHSS National Institutes of Health Stroke Scale.
Table 2. Summary of Major Outcomes.*.
| Outcome | LY-CoV555 (N=163) |
Placebo (N=151) |
Comparison (95% CI)† |
P Value |
|---|---|---|---|---|
| Efficacy outcomes ‡ | ||||
| Pulmonary ordinal outcome at day 5 — no./total no. (%) | 0.85 (0.56–1.29)§ | 0.45 | ||
| Category 1: best | 31/161 (19) | 33/150 (22) | ||
| Category 2 | 50/161 (31) | 48/150 (32) | ||
| Category 3 | 29/161 (18) | 31/150 (21) | ||
| Category 4 | 17/161 (11) | 11/150 (7) | ||
| Category 5 | 25/161 (16) | 22/150 (15) | ||
| Category 6 | 8/161 (5) | 5/150 (3) | ||
| Category 7: worst | 1/161 (1) | 0 | ||
| Pulmonary-plus ordinal outcome at day 5 — no./total no. (%) | 0.87 (0.57–1.31)§ | 0.50 | ||
| Category 1: best | 31/161 (19) | 33/150 (22) | ||
| Category 2 | 50/161 (31) | 47/150 (31) | ||
| Category 3 | 29/161 (18) | 31/150 (21) | ||
| Category 4 | 17/161 (11) | 12/150 (8) | ||
| Category 5 | 25/161 (16) | 21/150 (14) | ||
| Category 6 | 8/161 (5) | 6/150 (4) | ||
| Category 7: worst | 1/161 (1) | 0 | ||
| Sustained recovery through Oct. 26 — no./total no. (%) | 71/87 (82) | 64/81 (79) | 1.06 (0.77–1.47)¶ | NA |
| Discharge from hospital through Oct. 26 — no. (%) | 143 (88) | 136 (90) | 0.97 (0.78–1.20)¶ | NA |
| Safety outcomes ‖ | ||||
| Infusion reaction — no. (%)** | 23 (14) | 14 (9) | 1.64 (0.79–3.44)†† | 0.19 |
| Composite safety outcome — no. (%)‡‡ | ||||
| Through day 5 | 31 (19) | 21 (14) | 1.56 (0.78–3.10)†† | 0.20 |
| Through day 28 | 38 (23) | 30 (20) | 1.22 (0.75–1.98)§§ | 0.42 |
| Composite safety outcome, organ dysfunction, or serious coinfection through day 28 — no. (%) | 49 (30) | 37 (25) | 1.25 (0.81–1.93)§§ | 0.31 |
| Death through Oct. 26 — no. (%) | 9 (6) | 5 (3) | 2.00 (0.67–5.99)§§ | 0.22 |
NA denotes not applicable.
The difference between the LY-CoV555 group and the placebo group was calculated as an odds ratio, rate ratio, or hazard ratio, as indicated in the table.
The pulmonary and pulmonary-plus outcomes were calculated with the use of a seven-level ordinal scale, ranging from an ability to do usual activities with minimal or no symptoms (category 1) to death (category 7). The pulmonary outcome is based largely on oxygen requirements, and the pulmonary-plus outcome also captures the range of organ dysfunction that may be associated with progression of Covid-19, such as respiratory dysfunction and coagulation-related complications. Estimates for efficacy outcomes that are greater than 1.0 favor LY-CoV555 over placebo.
This odds ratio was estimated from a proportional-odds model that was adjusted for baseline ordinal category and trial pharmacy.
This rate ratio was estimated from a Fine–Gray model to account for the competing risk of death, stratified according to trial pharmacy.
Estimates for safety outcomes that are greater than 1.0 favor placebo over LY-CoV555.
Types of infusion reactions are provided in Table S7 in the Supplementary Appendix.
This odds ratio was estimated from a logistic-regression model that was adjusted for the trial pharmacy.
The composite safety outcome was defined as death, a serious adverse event, or an adverse event of grade 3 or 4. Additional details are provided in Tables S8 and S9.
This hazard ratio was estimated from a proportional-hazards regression model stratified according to trial pharmacy.
The proportional-odds assumption was met for both models. The primary findings were unaffected by further adjustment with the use of a risk score that considered potential baseline risk factors for the pulmonary outcome on day 5 (Table S3). The odds ratios for both ordinal outcomes were less than 1.0 for all assessed time points, including days 1 through 7, day 14, and day 28 (Table S4).
Among 167 patients who were followed for at least 28 days or who died within this time frame, 71 of 87 patients (82%) in the LY-CoV555 group and 64 of 81 patients (79%) in the placebo group had a sustained recovery (rate ratio, 1.06; 95% CI, 0.77 to 1.47). In the overall cohort of 314 patients, hospital discharge occurred in 143 of 163 patients (88%) in the LY-CoV555 group and in 136 of 151 patients (90%) in the placebo group (rate ratio, 0.97; 95% CI, 0.78 to 1.20) (Table 2, Figure 1B and 1C, and Table S3).
Tables S5A and S5B summarize the association of the day 5 pulmonary ordinal outcome with the time until a sustained recovery. The median time until a sustained recovery was longer for worse categories of the pulmonary outcome on day 5. As compared with the best category of the pulmonary outcome (category 1: ability to perform usual activities with minimal or no symptoms), the rate ratio for a sustained recovery was significantly below 1 for patients in each of the other categories, with a clear trend toward a longer sustained recovery time in patients with more severe illness.
Organ Dysfunction and Serious Infection
The percentages of patients in whom organ dysfunction or serious infection developed during follow-up were similar in the LY-CoV555 group and the placebo group (16% and 14%, respectively) (Table S6). Most of the organ-dysfunction events were due to respiratory dysfunction (in 10% and 11%, respectively), whereas other rarer events (i.e., seen in <4%) were thromboembolic events, acute delirium, and hypotension leading to vasopressor treatment. Intercurrent serious coinfection was seen in only 3% of the cohort.
Safety Outcomes
Overall, 99% of the patients completed the infusion of LY-CoV555; the infusion was paused temporarily in 3%. Most of the signs or symptoms that were associated with the infusion were of grade 1 or 2 in severity (Table 2 and Table S7).
Through day 5, the primary safety outcome (a composite of death, serious adverse events, or incident grade 3 or 4 adverse events) occurred in 31 of 163 patients (19%) in the LY-CoV555 group and in 21 of 151 patients (14%) in the placebo group (odds ratio, 1.56; 95% CI, 0.78 to 3.10) (Table 2 and Table S8). Through day 28, with the inclusion of organ dysfunction and serious infection along with the composite safety outcome, the hazard ratio was 1.25 (95% CI, 0.81 to 1.93). Most events in the composite safety outcome were grade 3 or 4 adverse events (Table 2 and Fig. S2C).
During 28 days of follow-up, a primary safety event occurred in 38 of 163 patients (23%) in the LY-CoV555 group and in 30 of 151 patients (20%) in the placebo group (Table 2, Table S9, and Fig S2A). Most of the events were classified as respiratory, thoracic, mediastinal, general, or psychiatric, according to the criteria of the Medical Dictionary for Regulatory Activities, version 23.1 (Table S10).
A total of 14 participants died, 9 in the LY-CoV555 group and 5 in the placebo group (hazard ratio, 2.00; 95% CI, 0.67 to 5.99). Of the 14 deaths, 12 were attributed to worsening of Covid-19 and 2 to cardiopulmonary arrest (Table 2 and Fig. S2B).
Except for a slight decrease in the mean serum creatinine level from baseline to day 5 in the LY-CoV555 group as compared with no change in the placebo group, no significant between-group differences were seen with respect to intrapatient changes in key laboratory values (Table S11).
Subgroup Analyses
Patients who entered the trial in a worse category on the pulmonary ordinal scale tended to be in a worse category on day 5 (Fig. S3 and Tables S12 and S13). No evidence for a differential treatment effect across baseline categories was seen (P=0.78 for interaction). The distributions across the seven categories of the pulmonary outcome on day 5 were similar in the two groups within each baseline category. LY-CoV555 showed no evidence of benefit in any of the baseline categories. No significant interaction was observed for the effect size of either ordinal outcome at day 5 with respect to several other prespecified subgroups that were defined according to baseline status, including categories of symptom duration (≤5 days, 6 to 8 days, and ≥9 days) (Table S13).
Discussion
In this preliminary report of the results of the first TICO trial, we found that hospitalized patients with Covid-19 who received a single infusion of the neutralizing monoclonal antibody LY-CoV555 (at a dose of 7000 mg) did not have better clinical outcomes at day 5 than those who received placebo. Most of the patients (95%) were also receiving remdesivir. Thus, LY-CoV555 met the prespecified criteria for futility and further enrollment was stopped. The day 5 outcomes that were used for an early assessment of futility were closely associated with the primary outcome of the time until a sustained recovery, which was no better in the LY-CoV555 group than in the placebo group. Taken together, these results indicate a low likelihood that LY-CoV555 improves outcomes among hospitalized patients with Covid-19.
The sample size of more than 300 patients for the early futility assessment provided high statistical power for determining whether recruitment should continue to the full sample size of 1000 patients. The selection of the day 5 timing for determining early efficacy and futility was based on results from other trials, including the ACTT-1 evaluation of remdesivir as compared with placebo.1 As in ACTT-1, similar results in the two groups were observed at all time points evaluated in our trial.
We observe that, in general, patients underwent randomization at a point in the disease course before the development of organ failure. The enrollment of such patients before the early futility assessment was by design, under the assumption that the greatest effect of an antiviral agent would be observed in patients with less severe illness. Reasons for the lack of benefit for LY-CoV555 in this trial are unknown and may include slow or ineffective penetration of the antibody into infected tissue, minimal intrinsic potency, rapid selection of escape mutants no longer neutralized by the agent,11,12 and harmful effects of the antibody. It has been hypothesized that such harmful effects (which have been described as “antibody-dependent enhancement”) could theoretically be associated with increased viral replication or exaggerated inflammation.13,14 Additional research will be required to clarify whether antibody-dependent enhancement will be observed in patients with Covid-19.
Although the trial was not adequately powered for robust subgroup analyses, we identified no evidence that the effect of LY-CoV555 on the ordinal outcomes at day 5 differed according to any subgroup, including the baseline pulmonary ordinal category and the duration of symptoms before enrollment. Assessments of serologic status and viral load in the patients at baseline are ongoing. Despite chance imbalances in illness severity at baseline, adjusted analyses did not suggest benefit for LY-CoV555 in this patient population.
In addition, we found no between-group differences with respect to the primary outcome of sustained recovery or the related outcome of hospital discharge. An analysis of the association between the day 5 ordinal category and the time until a sustained recovery suggested a strong relationship, which supports the use of the day 5 ordinal category for early futility assessment. The evaluations of clinical status at later time points (e.g., days 7 and 28) were also consistent with the status on day 5. These results support our approach of early evaluation of futility using data at day 5 to decide on whether a treatment should proceed to full enrollment. This initial trial thus confirms the approach taken in the TICO platform.
On the basis of these preliminary results with a median of only 31 days of follow-up, the safety of LY-CoV555 as compared with placebo remains uncertain. None of the between-group differences that were observed in the prespecified safety outcomes met the criteria for statistical significance. A limitation of the trial is our inability to make definitive statements about the safety of LY-CoV555 as compared with placebo. Since the sample size was smaller and the duration of follow-up was shorter than planned, the confidence intervals around major safety outcomes are wide.
Our results should be interpreted in the context of a preliminary study assessing three doses of LY-CoV555 (700, 2800, and 7000 mg) in outpatients with Covid-19.3 Although no dose-response effect was noted, patients who received LY-CoV555 may have had slightly increased viral clearance from the nasopharynx and may have had a lower risk of hospitalization. In contrast, the current trial enrolled inpatients, the majority of whom had hypoxemia, and tested the effect of LY-CoV555 on a background of remdesivir and substantial glucocorticoid therapy.
Other forms of passive immunity have been only minimally investigated to date, although randomized trials are ongoing. We note that in prior experience with neutralizing monoclonal antibodies for acute infections (e.g., in Ebola virus disease15), various antibodies differed dramatically in efficacy, and certain antibodies had efficacy even in advanced disease. Rapid and rigorous assessment of potential antiviral therapies for Covid-19, including the use of additional monoclonal antibodies, remains a high priority.
The TICO platform will proceed with the evaluation of additional Covid-19 treatments, including the use of new neutralizing monoclonal antibodies. The clinical benefit from other antibody agents, given either as individual or combination therapies, may differ from that of LY-CoV555 owing to differences in epitope target, binding-site specificity, affinity, tissue levels, effector functions, and pharmacokinetic profile.
Acknowledgments
We thank the members of the TICO data and safety monitoring board — Merlin L. Robb, M.D. (chair), David Glidden, Ph.D., Graeme A. Meintjes, M.B., Ch.B., Ph.D., Barbara E. Murray, M.D., Stuart Campbell Ray, M.D., Valeria Cavalcanti Rolla, M.D., Ph.D., Haroon Saloojee, M.B., B.Ch., Anastasios A. Tsiatis, Ph.D., Paul A. Volberding, M.D., Jonathan Kimmelman, Ph.D., and Sally Hunsberger, Ph.D. (executive secretary) — for their review of the protocol and their guidance based on interim reviews of the data.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
The members of the writing committee are as follows: Prof. Jens D. Lundgren, M.D., D.M.Sc., Birgit Grund, Ph.D., Christina E. Barkauskas, M.D., Thomas L. Holland, M.D., Robert L. Gottlieb, M.D., Ph.D., Uriel Sandkovsky, M.D., Samuel M. Brown, M.D., Kirk U. Knowlton, M.D., Wesley H. Self, M.D., M.P.H., D. Clark Files, M.D., Mamta K. Jain, M.D., M.P.H., Thomas Benfield, M.D., D.M.Sc., Michael E. Bowdish, M.D., Bradley G. Leshnower, M.D., Jason V. Baker, M.D., Jens-Ulrik Jensen, M.D., Ph.D., Edward M. Gardner, M.D., Adit A. Ginde, M.D., M.P.H., Estelle S. Harris, M.D., Isik S. Johansen, M.D., D.M.Sc., Norman Markowitz, M.D., Michael A. Matthay, M.D., Lars Østergaard, M.D., Ph.D., D.M.Sc., Christina C. Chang, M.D., Ph.D., Victoria J. Davey, Ph.D., M.P.H., Anna Goodman, F.R.C.P., D.Phil., Elizabeth S. Higgs, M.D., Daniel D. Murray, Ph.D., Thomas A. Murray, Ph.D., Roger Paredes, M.D., Ph.D., Mahesh K.B. Parmar, Ph.D., Andrew N. Phillips, Ph.D., Cavan Reilly, Ph.D., Shweta Sharma, M.S., Robin L. Dewar, Ph.D., Marc Teitelbaum, M.D., Deborah Wentworth, M.P.H., Huyen Cao, M.D., Paul Klekotka, M.D., Ph.D., Abdel G. Babiker, Ph.D., Annetine C. Gelijns, Ph.D., Virginia L. Kan, M.D., Mark N. Polizzotto, M.D., Ph.D., B. Taylor Thompson, M.D., H. Clifford Lane, M.D., and James D. Neaton, Ph.D.
This article was published on December 22, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the U.S. Operation Warp Speed program; the National Institute of Allergy and Infectious Diseases and Leidos Biomedical Researchfor the INSIGHT Network; the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the PETAL (Prevention and Early Treatment of Acute Lung Injury) Network and the Cardiothoracic Surgical Trials Network; and the U.S. Department of Veterans Affairs and grants from the governments of Denmark (no. 126 from the National Research Foundation), Australia (from the National Health and Medical Research Council), and the United Kingdom (MRC_UU_12023/23 from the Medical Research Council). Trial medications were donated by Gilead Sciences and Eli Lilly.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020;383:1813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. DOI: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. DOI: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). 2020. (https://www.nih.gov/research-training/medical-research-initiatives/activ).
- 5.Jones BE, Brown-Augsburger PL, Corbett KS, et al. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. October 9, 2020. (https://www.biorxiv.org/content/10.1101/2020.09.30.318972v3). preprint.
- 6.Murray DD, Babiker AG, Baker JV, et al. Design and implementation of the multi-arm, multi-stage Therapeutics for Inpatients with COVID-19 (TICO) platform master protocol: an Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative. November 13, 2020. (https://www.medrxiv.org/content/10.1101/2020.11.08.20227876v2). preprint.
- 7.World Health Organization. Novel coronavirus: COVID-19 therapeutic trial synopsis. 2020. (https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf).
- 8.Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events: corrected version 2.1, July 2017 (https://rsc.niaid.nih.gov/clinical-research-sites/grading-severity-adult-pediatric-adverse-events-corrected-version-two-one).
- 9.McCullagh P. Regression models for ordinal data. J R Stat Soc B 1980;42:109-127. [Google Scholar]
- 10.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2020. [Google Scholar]
- 11.Baum A, Fulton BO, Wloga E, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020;369:1014-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. July 22, 2020. (https://www.biorxiv.org/content/10.1101/2020.07.21.214759v1). preprint. [DOI] [PMC free article] [PubMed]
- 13.Arvin AM, Fink K, Schmid MA, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020;584:353-363. [DOI] [PubMed] [Google Scholar]
- 14.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 2020;5:1185-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019;381:2293-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.