Abstract
End stage kidney disease (ESKD) is associated with low fertility with rates of conception estimated to occur in women on dialysis at 1/100th of the general population. However, live birth rates are increasing over time in women on hemodialysis, while they remain lower and static in women on peritoneal dialysis. Intensification of hemodialysis, targeting a serum BUN <35 mg/dL or 36 hours of dialysis per week in women with no residual kidney function, is associated with improved live birth rates and longer gestational age. Even in intensively dialyzed cohorts, rates of prematurity and need for neonatal intensive care are high, upwards of 50%. While women on peritoneal dialysis in pregnancy do not appear to be at increased risk of delivering preterm compared to those on hemodialysis, their infants are more likely to be small for gestational age. As such, hemodialysis has emerged as the preferred dialysis modality in pregnancy. Provision of specialized nephrology, obstetric, and neonatal care is necessary to manage these complex pregnancies and family planning counseling should be offered to all women with ESKD.
Keywords: pregnancy, dialysis, ESKD, obstetrics, fetal
Introduction
Dialysis revolutionized health care for patients with end stage kidney disease (ESKD), but does not fully restore many normal physiologic processes that are aberrant in severe chronic kidney disease (CKD). One such process important to women is pregnancy and childbirth.1 Despite many advances in the last five decades, pregnancy in a woman on dialysis remains a rare and relatively high-risk event. However, a growing body of literature now describes the epidemiology, outcomes, and clinical management of pregnancy in women with ESKD on dialysis, which are addressed in this review.
Preconception Counseling
Family planning is an important, but often overlooked aspect of care for women of childbearing age on dialysis,2 the goals of which are to support informed and values-based decision making. Contraception is recommended for all pre-menopausal women who are sexually active and not intending pregnancy. Estrogen-containing contraceptives should be avoided in the dialysis population due to their association with increased cardiovascular disease risk whereas progesterone-based pills and intrauterine devices are considered safe. Depot medroxyprogesterone should be avoided due to its association with decreased bone density.3 For those women who do desire motherhood, a full discussion regarding pregnancy, timing, and transplant is warranted, along with alternative options including adoption and surrogacy. Fertility and pregnancy outcomes in kidney transplant recipients are generally accepted to be better than for women on dialysis. The fertility rate of kidney transplant recipients and women on dialysis is estimated at 1/10th and 1/100th, respectively, of the general population.4,5 A recent large meta-analysis of over 6000 pregnancies reported a live birth rate of 72.9% (95% CI 70.0–75.6%) in kidney transplant recipients.6 Preterm delivery and maternal morbidity are also less likely in transplant recipients when compared to women on dialysis.7 However, avoidance of pregnancy for one year following transplant is recommended8 and post-transplant kidney function is not guaranteed. This may be particularly relevant to counseling if a woman is approaching the end of her reproductive life span. One must also consider sensitization that occurs with pregnancy, particularly for women with high panel reactive antibodies, as a pregnancy while on dialysis may hinder future opportunities for transplant. All women of childbearing age with ESKD should be informed of their family planning options accounting for individual nuances. As such, 90% of women with CKD found a multi-disciplinary approach to be informative and helpful in deciding about pregnancy.9
Fertility and ESKD
ESKD is associated with low estradiol levels and decreased clearance of prolactin, which can inhibit pulsatility of gonadotropin releasing hormone and the midcycle surge of luteinizing hormone that leads to ovulation.10,11 Related to the changes in the hypothalamic-pituitary-gonadal axis, endometrial atrophy is common in women on hemodialysis12 and may impair implantation even if ovulation does occur. Approximately 70% of women on hemodialysis are oligo- or amenorrheic.13 In addition, up to 84% of women with ESKD on hemodialysis report low sexual function or sexual activity,14 which compounds the difficulties these women face trying to conceive.
Restoration of normal menstrual cycles and fertility may be improved in women of childbearing age who switch from conventional thrice-weekly hemodialysis to intensified regimens, such as nocturnal home hemodialysis.15,16 Fertility evaluations for women with ESKD should be performed in conjunction with a specialist in reproductive endocrinology and offered early. This may include a careful assessment of menstrual and reproductive history, along with measurements of estradiol and follicular stimulating hormone in cycle days 2–5, mid-luteal phase progesterone, and a radiographic evaluation for structural abnormalities. Anti-mullerian hormone, a 140 kDa glycoprotein that is synthesized in developing follicles, has received attention as a marker of ovarian reserve which may be useful in fertility evaluations. Anti-mullerian hormone decreases across the reproductive lifespan, and is used to predict timing of last menses17 and response to ovarian stimulating fertility treatments.18 However, a large prospective study of healthy women showed that women with a low anti-mullerian hormone had no difference in probability of conceiving within 6 to 12 months compared to women with a normal anti-mullerian hormone.19 Studies are conflicting as to whether women on hemodialysis have differences in serum anti-mullerian compared to normal healthy controls, with some reporting lower anti-mullerian levels in women on hemodialysis20 and others reporting similar levels to healthy women.21,22 Among women on hemodialysis, anti-mullerian hormone levels were significantly lower in women with regular menstrual cycles than those with menstrual disorders, and anti-mullerian hormone levels were observed to decline for 6 months after kidney transplant20 – a period which is typically associated with resumption of regular menses and improvement in fertility.23,24 Together, this suggests that anti-mullerian hormone alone is unlikely to independently correlate with fertility potential in women on hemodialysis.
Women with ESKD on dialysis may be anuric with irregular menstrual cycles, limiting the utility of home urine pregnancy testing. Thus quantitative serum human chorionic gonadotropin (HCG) levels are used for the diagnosis of pregnancy in this population. Low levels of HCG positivity (<25 mIU/mL) have been observed in 14.5% of non-pregnant women of childbearing age on dialysis.25 To confirm a pregnancy, serial testing should show a rapid rise in HCG level and a viability ultrasound is recommended to identify a gestational sac early in first trimester.
Incidence of pregnancy and delivery in women on dialysis:
The first case reports in the literature of pregnancy in women on hemodialysis arose in the 1970s.26 Thereafter, systematic attempts to report pregnancy outcomes in women on dialysis in the 1980s and 1990s largely relied on surveys of dialysis centers and voluntary registries. The first of its kind, in 1980, the European Renal Association-European Dialysis Transplant Association (ERA-EDTA) reported 16 births to women on dialysis from more than 1300 women of childbearing age in 19 countries.27 Subsequent population-based surveys reported 2–7% of women on dialysis became pregnant during follow up (2.4% in United States,28 3.4% in Japan,29 7% in Saudi Arabia30), however response rate varied considerably from 33–68% in these studies. With 100% response rate from dialysis centers surveyed in Belgium, the pregnancy rate was 0.3 per 100 patient-years amongst women 18–44 on dialysis.31
More recently, compulsory registry and administrative data have provided estimates of pregnancy and delivery rates in women with ESKD. The Australian and New Zealand Dialysis and Transplant Registry (ANZDATA) observed an overall pregnancy rate of 2.07 per 1000 patient-years (PTPY) with a live birth rate of 1.26 PTPY between 1966–2008.32 Notably, pregnancy rates were lower amongst women treated with peritoneal dialysis. The United Kingdom’s Obstetric Surveillance System similarly found 1.4 pregnancies PTPY amongst women on dialysis between 2012 and 2014.11 Utilizing the US Renal Data System (USRDS), a compulsory registry of individuals with ESKD with additional administrative claims data for those insured by Medicare, Shah and colleagues found an unadjusted rate of 17.7 pregnancies PTPY (95% CI, 17.0–18.5) in women aged 15–44 on hemodialysis or peritoneal dialysis from 2005–2013.33 The pregnancy rate was higher in women on hemodialysis at 19.3 pregnancies PTPY compared to 9.3 PTPY amongst women on peritoneal dialysis. While pregnancy rates did not appear to be changing over the study period in Shah’s analysis, in a similar analysis using USRDS, delivery rates did appear to be increasing over time among women 18–45 with ESKD.34 The observed delivery rate rose from 2.1 to 3.6 deliveries PTPY from 2005 to 2016 amongst women on hemodialysis, and was lower and relatively static at approximately 1 delivery PTPY in women on peritoneal dialysis. Both studies found that white women, diabetics, and those with longer time on dialysis were less likely to have a pregnancy or delivery.33,34
Hemodialysis prescription and birth outcomes in women with ESKD
Studies using national administrative data from different countries have reported a wide range of live birth rates. ANZDATA reported a 79% live birth rate amongst women with ESKD between 1966 and 2008.32 Using USRDS, Shah and colleagues reported a higher pregnancy rate than ANZDATA, as well as a significantly lower live birth rate of 27.1%, with 2.6% of pregnancies ending in stillbirth, 29.4% spontaneous abortion, 7.6% therapeutic abortion, and 2.7% ectopic or trophoblastic pregnancy. 31% of identified pregnancies had an unknown outcome.33 Changes in the validated algorithm to identify pregnancies and their outcomes35 may have altered the accuracy in ascertaining these outcomes in USRDS, however overall the observed live birth rate to women with ESKD in the US is likely much lower than that observed in ANZDATA.
Neither ANZDATA nor USRDS data were able to control for residual kidney function or dialysis prescription. However, early observations showed improved outcomes in women with more weekly time on dialysis or those who started dialysis after conceiving,28,30,31,36 thus it was hypothesized that clearance, whether delivered or native, may have a meaningful impact on pregnancy outcomes in women on hemodialysis. The association of hours of dialysis and biochemical parameters, such as pre-dialysis blood urea nitrogen, has been interrogated in a number of observational studies.
Comparison of 22 pregnancies from the Toronto Pregnancy and Kidney Disease (“PreKid”) clinic and 70 pregnancies from the American Registry of Pregnancy in Dialysis revealed an association of increased dialytic time with a significant improvement in live birth rate, gestational age, and gestational weight.37 Specifically, the live birth rate for women in the Canadian cohort with established ESKD was 83.3%, whereas it was just 52.6% in the American cohort (P=0.02). The Canadian women dialyzed 43 +/− 6 hours per week on average, compared to 17 +/− 5 hours per week in the American cohort (P<0.001). Importantly, a dose-response correlation of dialysis intensity and pregnancy outcomes was observed when pooling data from both cohorts: with increasing hours of hemodialysis live birth rate improved from 48% in those who received <=20 hours to 85% in those who received >=37 hours per week (p=0.02), while gestational age significantly increased (38 weeks vs. 28 weeks, p=0.002). This data was further supported by a meta-regression analysis that showed a continuous correlation effect with reduction in frequency of preterm delivery and delivering an infant small for gestational age (most commonly defined as weight below the 10th percentile for gestational age) as weekly hours of dialysis increase (R2=0.22; P=0.044).38
Additional analyses have evaluated the association of mid-week pre-dialysis blood urea nitrogen (BUN) on pregnancy outcomes. Asamiya and colleagues compared successful and unsuccessful pregnancies in women with ESKD. The average blood urea nitrogen (BUN) level was significantly lower in the successful group than in the unsuccessful group (45.3±8.3 versus 66.9±10.9 mg/100 ml respectively; P<0.001), and using regression modeling, a BUN of <48 mg/dL correlated with a successful outcome defined as a birthweight greater than 1500 grams and gestational age greater than 32 weeks.39 In 2018, Luders and colleagues reported that an adaptive hemodialysis prescription to target a mid-week BUN <35 mg/dL was associated with improvement in a composite fetal outcome of perinatal death or extremely premature (<30 weeks gestational age) birth in 93 pregnancies.40
Compiling this data, the Kidney Disease Outcomes Quality Initiative gave an ungraded recommendation for long frequent hemodialysis in the setting of pregnancy in 2015,41 and in 2019 clinical practice guidelines from the United Kingdom supported provision of dialysis to meet a mid-week pre-dialysis BUN <35 mg/dl (12.5 mmol/L).42 It is unknown whether dialysis membrane type (for example, high versus low flux) is instrumental in meeting this goal though high flux membranes are commonly used and theoretically provide better middle molecule clearance. Hemodiafiltration has also been shown to be safe and well-tolerated with 100% live birth rate in a single center study of 5 patients, though it is not widely available.43 Kt/V is not validated in pregnancy and thus is not routinely used as a marker of dialysis adequacy in pregnancy.
Peritoneal dialysis prescription and birth outcomes in women with ESKD
Patients on peritoneal dialysis often maintain residual kidney function, thus one might expect greater likelihood of pregnancy and delivery; however, several studies suggest that pregnancy and subsequent delivery is less likely in women on peritoneal dialysis.32–34 It has been hypothesized that hypertonic dialysate may impair normal ovulation. Metaplasia and band-like fibrosis of the ovaries and fallopian tube have been observed in women receiving peritoneal dialysis, suggesting that peritoneal dialysis may induce mechanical barriers to normal ovulation leading to low fertility.44 A compilation of case reports comprising 14 pregnancies amongst women on peritoneal dialysis observed a median gestational age of 34 weeks (range, 29–39 weeks) with a mean birth weight of 1780 g (range, 900–2700 gm),38 with similar results reported in other case series.45 Use of peritoneal dialysis in pregnancy was not associated with a higher likelihood of preterm delivery, however was associated with a greater prevalence of delivering a small for gestational age infant when compared to women on hemodialysis in pregnancy (67% on peritoneal dialysis vs. 31% on hemodialysis; P = 0.015), and 29% of infants born to mothers on peritoneal dialysis required neonatal intensive care.38 Weighing the observational evidence in support of intensified hemodialysis and the relative lack of evidence in peritoneal dialysis, as well as the lower observed incidence of pregnancy and delivery in women on peritoneal dialysis,32–34 switching to hemodialysis prior to pregnancy or in the first trimester is recommended.42 Given potential time constraints and burdens of intensive hemodialysis, alternative strategies have been reported with success such as addition of intermittent hemodialysis to peritoneal dialysis46 and switching from peritoneal dialysis to hemodialysis in the second trimester,47 however should be undertaken with caution and careful counseling.
Maternal pregnancy complications in women with ESKD on dialysis
The frequency of maternal complications for pregnant women with ESKD on dialysis is difficult to assess owing to the heterogeneity of reported data and treatment regimens. Forthcoming prospectively collected data from pregnancies amongst women with advanced CKD and ESKD in the Australasian Maternity Outcomes Surveillance System may help elucidate this.48 In general, women with ESKD on dialysis are at significantly increased odds of preterm delivery, caesarean delivery, blood transfusion at delivery, and severe maternal morbidity and mortality compared to the delivery hospitalizations for women without CKD or ESKD.7 Caesarean delivery occurred in 39% of deliveries in 664 women on hemodialysis and in 41% of deliveries in 47 women on peritoneal dialysis in the US between 2002 and 2015,34 though rates of caesarean delivery are reported up to 74.2%.40
Preeclampsia is reported to occur between 5–20% of pregnancies in women on dialysis.32,37,38,40 Pre-eclampsia should be suspected after 20 weeks gestation in any dialysis patient with worsening hypertension, symptoms of headache, blurry vision, epigastric or right upper quadrant pain, and signs of hemolysis, transaminitis, or thrombocytopenia. The constellation of intrauterine growth restriction combined with an impedance of uterine artery flow by Doppler pulsatility may also help detect women at increased risk of developing preeclampsia.49,50 Angiogenic serum markers of preeclampsia (soluble fms-like tyrosine kinase-1 “sFlt-1” and placental growth factor “PlGF”) are promising, specifically using a sFlt-1 to PlGF ratio of less than 38 to rule out preeclampsia in low-risk populations.51 However, further study of their utility in high risk populations such as women with ESKD is needed.
Frequency of polyhydramnios is variably reported, occurring in between 5–53% of pregnancies of women on hemodialysis.32,38,40 In Luders and colleagues’ Brazilian cohort, persistent polyhydramnios was an indication to augment hemodialysis time.40 Similarly, this complication occurred only in 1 pregnancy of the intensively dialyzed Toronto PreKid cohort, and was improved with increased ultrafiltration.37 Exclusive of twin gestations, in the Toronto PreKid cohort, shortened cervix was also observed in 14% of pregnancies.37
Fetal complications in women with ESKD on dialysis
Preterm birth occurs frequently to women with ESKD on dialysis. Preterm birth was identified in 41% of delivering women on hemodialysis and peritoneal dialysis in the US from 2002–2015;34 this estimate may be low due to reliance on administrative codes for the diagnosis of preterm delivery. Intensified hemodialysis is associated with increased gestational age at delivery, and preterm delivery occurred in 50% of live births in the Toronto PreKid cohort.37 In a large meta-analysis, median gestational age for all live births was 33 weeks (range, 26–39 weeks) and 32% (49/154) of infants were born small for gestational age.38 Even in more intensively dialyzed cohorts with high live birth rates, up to 70% of infants needed neonatal intensive care.40 Fetal malformations have not been observed more frequently in the ESKD population.38
There are few studies of outcomes of the children born to women on dialysis outside of the perinatal period. In a single center study of 10 children born to 7 women on dialysis, none of the children had obvious developmental delay in infancy or childhood (median follow up time 4.5 years, range 0.8–25.2 years).52 Amongst 17 children ages 2–13 born to women on dialysis in Italy, 2 children had clinically apparent pervasive developmental problems including delays in socialization and communication.53 This outcome may be mediated by the increased risk of preterm delivery and low birthweight in women with ESKD, as these are also associated with increased likelihood of reporting autism spectrum disorder traits in adulthood.54 Larger studies are needed to be able to better counsel women on long-term risks.
Supportive alterations to hemodialysis care in pregnancy
Dialysate composition should be altered in pregnancy, both in response to augmented dialytic clearance and to support appropriate growth of the developing fetus. With increased hemodialysis, most nutritional restrictions can be liberalized. A dietician should follow closely to ensure pregnant women have adequate protein intake of 1.5–1.8 mg/kg/day. Given the increased frequency of dialysis, dialysate potassium composition can typically be maintained at 3 meq/L. To support normal bone development of the fetus, maternal serum calcium and phosphorous levels should target the normal range, and adjustment of calcium dialysate as well as phosphorous supplementation may be necessary. Calcium-based binders and vitamin D analogues are considered safe in pregnancy as long as maternal calcium levels are maintained in the normal range, however, sevelamer is associated with reduced or irregular ossification of fetal bones in animal models55 and thus is not recommended.
The physiologic anemia of pregnancy compounds that of ESKD and escalating doses of erythropoietin stimulating agents two to three times the pre-pregnancy dose may be required to target a goal hemoglobin of 10–11 mg/dL. Recombinant erythropoietin is a large molecule which does not appear to cross the placenta.56 Increases in iron sucrose supplementation may also be required. Medications commonly used in patients on dialysis, and the recommended alterations in pregnancy, are shown in Table 1.
Table 1.
Commonly used medications in dialysis patients and safety in pregnancy and lactation
| Pregnancy Safety | Considerations for pregnant women with ESKD | Lactation Considerations | |
|---|---|---|---|
| Anti-hypertensives | |||
| ACE-Inhibitors | Fetotoxic in second and third trimesters | No apparent increased risk in first trimester. | Enalapril and captopril safely used in lactation |
| However, given potential for delayed diagnosis of pregnancy in women with ESKD, stop prior to attempting to conceive | |||
| Angiotensin Receptor Blockers | Fetotoxic in second and third trimesters with limited data in first trimester | Stop prior to pregnancy | No sufficient data; avoid use |
| Beta-blockers | Labetalol considered a preferred agent and licensed for use in pregnancy | Can cause fetal bradycardia Atenolol is contraindicated due to increased risk of intrauterine growth restriction | Considered safe in lactation |
| Calcium Channel Blockers | Nifedipine (long-acting) considered a preferred agent in pregnancy | Nifedipine has also been evaluated as a short term tocolytic | Considered safe in lactation |
| Methyldopa | Considered a preferred agent in pregnancy | Side effects include: fatigue, nausea, vomiting | May worsen post-partum depression; monitor closely |
| Loop diuretics | Can be used safely in pregnancy | In pregnant women on dialysis, favor increasing dialysis time and ultrafiltration if volume control is suboptimal | Use judiciously, may hinder breastmilk production |
| Hydralazine | Considered safe in pregnancy | Increased risk of neonatal thrombocytopenia, lupus-like syndrome, fetal tachycardia | Considered safe in lactation |
| Erythropoeitin stimulating agents | Considered safe in pregnancy, large molecule which is unlikely to cross the placenta | May need twice the typical dose to meet goal Hgb 10–11 mg/dL | Considered safe in lactation |
| Iron sucrose | Considered safe in pregnancy | Considered safe in lactation | |
| Heparin | Considered safe in pregnancy | Stop heparin use with dialysis prior to delivery to allow for placement of epidural | Preservative-free preferred post-partum to minimize potential infant exposure and toxicity to benzyl alcohol |
| Bone mineral metabolism | |||
| Calcium based binders | Considered safe in pregnancy | Adjust to maintain maternal calcium and phosphorous levels in the normal range; Not often needed in women undergoing intensive hemodialysis | Considered safe in lactation |
| Sevelamer | Associated with fetal bone ossification abnormalities in animal studies; avoid | Use calcium-based binders if needed | Limited data though not expected to be present in large quantities in breastmilk; avoid in lactation |
| Calcitriol | Considered safe in pregnancy | Adjust to maintain target parathyroid hormone levels | Considered safe in lactation |
| Cinacalcet | Limited though successful case reports; avoid in pregnancy | Limited data; avoid in lactation |
Ultrafiltration should be re-evaluated frequently, and should account for anticipated weight gain as well as obstetric assessments of blood pressure and amniotic fluid. While recommended gestational weight gain varies based on pre-pregnancy weight, 11.5–16 kg (25–35 lbs) in total is recommended for women with a normal pre-pregnancy body mass index.57 Weight gain is typically minimal in the first trimester, followed by 0.3–0.5 kg/week in the second and third trimesters. There are no rigorous studies of blood pressure targets in pregnant women with CKD or ESKD. In women with pre-existing hypertension or gestational hypertension, controlling diastolic blood pressure to target 85 mmHg versus 100 mmHg reduced the frequency of severe maternal hypertension >=160/110 mmHg in pregnancy and did not increase the likelihood of pregnancy loss or need for high level neonatal care after birth.58 There is a paucity of clinical trial data to guide blood pressure treatment goals in ESKD in general41 and no studies to guide blood pressure goals in pregnant women with ESKD. Obstetrical guidelines differ on treatment goals for healthy pregnant women,59–61 though some suggest a goal blood pressure of <140/90 mmHg or diastolic of 85 mmHg in women with chronic hypertension.60,61 For women with ESKD, this can be achieved through ultrafiltration and anti-hypertensive agents. Intra-dialytic hypotension < 120/70 mmHg should be avoided due to potential negative impact on placental perfusion.
Obstetrical care for women with ESKD on dialysis
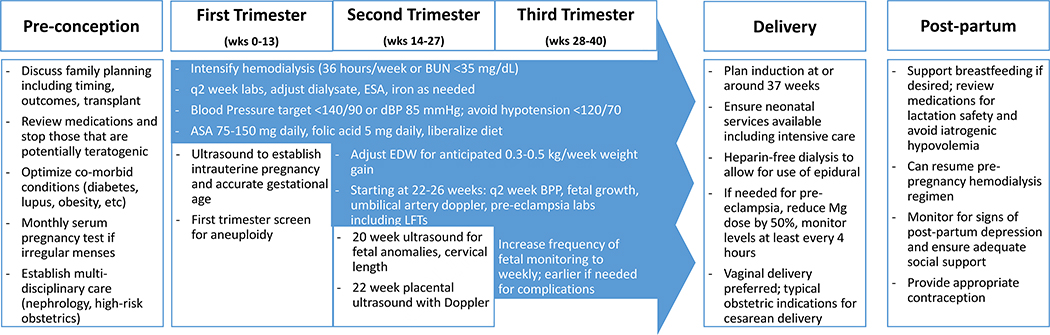
The optimal timing of prenatal visits, additional testing, and supportive care for pregnant women with ESKD has not been rigorously tested, however recommendations can be extrapolated from published protocols with a high rate of live births,37,40 systematic reviews,38 and expert guidelines.42,62 These recommendations along with alterations in usual hemodialysis provisions are summarized in Figure 1.
Figure 1. Adaptations to routine prenatal and peripartum care for women with ESKD on dialysis.
BUN: blood urea nitrogen; ESA: erythropoietin stimulating agent; ASA: aspirin; EDW: estimated dry weight; BPP: biophysical profile; LFTs: liver function tests; Mg: magnesium
First trimester:
After establishing presence of a viable and desired pregnancy, multi-disciplinary care with specialists in high-risk obstetrics is recommended. Low dose aspirin (81–150 mg) should be prescribed for preeclampsia prevention prior to 12 weeks’ gestation. High dose folic acid supplementation (5 mg) is recommended due to increased losses in intensified hemodialysis. Trisomy screening in the first trimester may be falsely abnormal, as B-HCG and PAPP-A can be falsely elevated in kidney disease;63 and further testing may include nuchal translucency ultrasound or amniocentesis.
Second trimester and onward:
An anatomic survey to assess for fetal anomalies and cervical length is typically performed at 20 weeks. After 20 weeks, monitoring for signs and symptoms of preeclampsia should occur at least every two weeks, including assessments for headache, visual changes, right upper quadrant and epigastric pain as well as thrombocytopenia, transaminitis, and hemolysis. Fetal growth and wellbeing should be assessed frequently, at minimum every 4 weeks in a stable ambulatory patient40 and commonly every 2 weeks,37 with more frequent assessments when complications are encountered. This includes measurement of interval fetal growth, biophysical profile (fetal heart rate, muscle tone, breathing, body movement, and quantity of amniotic fluid), and umbilical artery pulsatility by Doppler starting at 20–24 weeks.
Third trimester and delivery:
Induction of labor may be arranged for logistical reasons to coordinate dialysis care, and typically is recommended around 37 weeks in women without complications. Vaginal delivery is preferred, if possible. ESKD itself is not a specific indication for caesarean delivery, and the usual obstetric indications for caesarean delivery apply to women with ESKD. If magnesium is required for treatment of pre-eclampsia/eclampsia, loading and continuous doses should be decreased by 50% and levels followed closely.
Post-partum care:
Medical, social, and emotional support is important for women with ESKD following delivery. Resumption of the pre-pregnancy hemodialysis schedule can occur immediately following delivery. Women who undergo a caesarean delivery should wait to resume peritoneal dialysis until the incision is healed, typically 4–6 weeks.47,64 For those who desire to breastfeed, medications should be carefully reviewed for incompatibility with breastfeeding. Care should be taken to avoid excessive ultrafiltration, which may hinder breastmilk production. Psychosocial awareness and support is critical, as women with chronic medical conditions are at higher odds of developing postpartum depression.65
Initiating dialysis for patients who progress to ESKD in pregnancy
Recommendations for initiating dialysis in pregnancy for progressive CKD reflect expert opinion and practice as data is lacking. Initiation of dialysis should be considered when the usual indications including uremic symptoms, volume overload, and electrolyte abnormalities arise in a woman with chronically deteriorating kidney function during pregnancy. Often prior to these usual indications, a careful analysis should also take into account maternal BUN, fetal growth and wellbeing, and relative risk-benefit balance of early delivery versus dialysis initiation. A vegetarian, low protein, amino acid supplemented diet has been one strategy proposed to help avoid the need for dialysis in pregnancy and appears to be safe.66,67 Clinically, discussion of the risks and benefits is warranted as maternal BUN rises >40 mg/dL, and dialysis initiation strongly considered when BUN rises >45–50 mg/dL prior to 34 weeks gestation.42
Summary
Pregnancy in women on dialysis remains a challenging and high-risk clinical scenario, benefiting from multi-disciplinary and interprofessional expertise. Shared, informed decision making should be the goal for family planning for all women of childbearing age with ESKD. Nephrologists, high-risk obstetricians, neonatologists, dieticians, nurses, social workers all must come together to deliver this highly specialized care, which has been noted in contemporary observational studies to increase live birth rates. Long-term studies of outcomes of mothers on dialysis and their babies are lacking, but would add to the growing body of literature with which to counsel our patients.
Clinical Summary:
Fertility is depressed in women with ESKD on dialysis. However, pregnancy remains possible and live birth rates are increasing over time in women on hemodialysis
Intensified hemodialysis, targeting an average mid-week BUN<35 mg/L or 36 hours/week if no residual kidney function, is the recommended treatment for pregnant women with ESKD on dialysis
Intensified hemodialysis is associated with improved outcomes including increased gestational age and birthweight
Informed family planning is necessary for all women of ESKD of childbearing age
Acknowledgements:
ALO is supported by KL2 TR002241/UL1 TR 002240.
Footnotes
Financial Disclosures:
Andrea L. Oliverio: None.
Michelle A. Hladunewich: Medical Lead for Glomerulonephritis and Specialty Clinics, Ontario Renal Network
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrea L. Oliverio, Division of Nephrology, University of Michigan Medical School, Ann Arbor, MI.
Michelle A. Hladunewich, Divisions of Nephrology and Obstetrics, Sunnybrook Health Sciences Center, University of Toronto, Toronto, CA.
References
- 1.Tong A, Jesudason S, Craig JC, Winkelmayer WC. Perspectives on pregnancy in women with chronic kidney disease: systematic review of qualitative studies. Nephrol Dial Transplant 2015;30:652–61. [DOI] [PubMed] [Google Scholar]
- 2.Ramesh S, James MT, Holroyd-Leduc JM, et al. Sex Hormone Status in Women With Chronic Kidney Disease: Survey of Nephrologists’ and Renal Allied Health Care Providers’ Perceptions. Can J Kidney Health Dis 2017;4:2054358117734534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modesto W, Bahamondes MV, Bahamondes L. Prevalence of Low Bone Mass and Osteoporosis in Long-Term Users of the Injectable Contraceptive Depot Medroxyprogesterone Acetate. J Womens Health (Larchmt) 2015;24:636–40. [DOI] [PubMed] [Google Scholar]
- 4.Piccoli GB, Cabiddu G, Daidone G, et al. The children of dialysis: live-born babies from on-dialysis mothers in Italy--an epidemiological perspective comparing dialysis, kidney transplantation and the overall population. Nephrol Dial Transplant 2014;29:1578–86. [DOI] [PubMed] [Google Scholar]
- 5.Levidiotis V, Chang S, McDonald S. Pregnancy and maternal outcomes among kidney transplant recipients. J Am Soc Nephrol 2009;20:2433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S, Venkatesan RL, Gupta A, et al. Pregnancy outcomes in women with kidney transplant: Metaanalysis and systematic review. BMC Nephrol 2019;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliverio AL, Admon LK, Mariani LH, Winkelman TNA, Dalton VK. Health Outcomes and Health Care Utilization Among Obstetric Deliveries With Concurrent CKD in the United States. Am J Kidney Dis 2020;75:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay DB, Josephson MA, Armenti VT, et al. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005;5:1592–9. [DOI] [PubMed] [Google Scholar]
- 9.Wiles KS, Bramham K, Vais A, et al. Pre-pregnancy counselling for women with chronic kidney disease: a retrospective analysis of nine years’ experience. BMC Nephrol 2015;16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim VS, Henriquez C, Sievertsen G, Frohman LA. Ovarian function in chronic renal failure: evidence suggesting hypothalamic anovulation. Ann Intern Med 1980;93:21–7. [DOI] [PubMed] [Google Scholar]
- 11.Wiles KS, Nelson-Piercy C, Bramham K. Reproductive health and pregnancy in women with chronic kidney disease. Nat Rev Nephrol 2018;14:165–84. [DOI] [PubMed] [Google Scholar]
- 12.Matuszkiewicz-Rowinska J, Skorzewska K, Radowicki S, et al. Endometrial morphology and pituitary-gonadal axis dysfunction in women of reproductive age undergoing chronic haemodialysis--a multicentre study. Nephrol Dial Transplant 2004;19:2074–7. [DOI] [PubMed] [Google Scholar]
- 13.Chakhtoura Z, Meunier M, Caby J, et al. Gynecologic follow up of 129 women on dialysis and after kidney transplantation: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol 2015;187:1–5. [DOI] [PubMed] [Google Scholar]
- 14.Strippoli GF, Collaborative D, Sexual Dysfunction in Hemodialysis Working G, et al. Sexual dysfunction in women with ESRD requiring hemodialysis. Clin J Am Soc Nephrol 2012;7:974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Eps C, Hawley C, Jeffries J, et al. Changes in serum prolactin, sex hormones and thyroid function with alternate nightly nocturnal home haemodialysis. Nephrology (Carlton) 2012;17:42–7. [DOI] [PubMed] [Google Scholar]
- 16.Barua M, Hladunewich M, Keunen J, et al. Successful pregnancies on nocturnal home hemodialysis. Clin J Am Soc Nephrol 2008;3:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman EW, Sammel MD, Lin H, Boorman DW, Gracia CR. Contribution of the rate of change of antimullerian hormone in estimating time to menopause for late reproductive-age women. Fertil Steril 2012;98:1254–9 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CH, Chen YC, Wu HH, Yang JG, Chang YJ, Tsai HD. Serum anti-Mullerian hormone predicts ovarian response and cycle outcome in IVF patients. J Assist Reprod Genet 2009;26:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA 2017;318:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayed A, Soliman A, Naguib M, Soliman M, Salaheldin M. Ovarian reserve in an Egyptian cohort with end-stage kidney disease on hemodialysis and after successful kidney transplantation: a prospective study. Int Urol Nephrol 2019;51:737–43. [DOI] [PubMed] [Google Scholar]
- 21.Stoumpos S, Lees J, Welsh P, et al. The utility of anti-Mullerian hormone in women with chronic kidney disease, on haemodialysis and after kidney transplantation. Reprod Biomed Online 2018;36:219–26. [DOI] [PubMed] [Google Scholar]
- 22.Sikora-Grabka E, Adamczak M, Kuczera P, Szotowska M, Madej P, Wiecek A. Serum Anti-Mullerian Hormone Concentration in Young Women with Chronic Kidney Disease on Hemodialysis, and After Successful Kidney Transplantation. Kidney Blood Press Res 2016;41:552–60. [DOI] [PubMed] [Google Scholar]
- 23.Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron 2002;92:735–7. [DOI] [PubMed] [Google Scholar]
- 24.Watnick S, Rueda J. Reproduction and contraception after kidney transplantation. Curr Opin Obstet Gynecol 2008;20:308–12. [DOI] [PubMed] [Google Scholar]
- 25.Haninger-Vacariu N, Herkner H, Lorenz M, et al. Exclusion of pregnancy in dialysis patients: diagnostic performance of human chorionic gonadotropin. BMC Nephrol 2020;21:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Confortini PGG, Ancona G, et al. Full term pregnancy and successful delivery in a patient on chronic hemodialysis. Proc Eur Dial Transplant Assoc 1971;8:74–80. [Google Scholar]
- 27.Successful pregnancies in women treated by dialysis and kidney transplantation. Report from the Registration Committee of the European Dialysis and Transplant Association. Br J Obstet Gynaecol 1980;87:839–45. [DOI] [PubMed] [Google Scholar]
- 28.Okundaye I, Abrinko P, Hou S. Registry of pregnancy in dialysis patients. Am J Kidney Dis 1998;31:766–73. [DOI] [PubMed] [Google Scholar]
- 29.Toma H, Tanabe K, Tokumoto T, Kobayashi C, Yagisawa T. Pregnancy in women receiving renal dialysis or transplantation in Japan: a nationwide survey. Nephrol Dial Transplant 1999;14:1511–6. [DOI] [PubMed] [Google Scholar]
- 30.Souqiyyeh MZ, Huraib SO, Saleh AG, Aswad S. Pregnancy in chronic hemodialysis patients in the Kingdom of Saudi Arabia. Am J Kidney Dis 1992;19:235–8. [DOI] [PubMed] [Google Scholar]
- 31.Bagon JA, Vernaeve H, De Muylder X, Lafontaine JJ, Martens J, Van Roost G. Pregnancy and dialysis. Am J Kidney Dis 1998;31:756–65. [DOI] [PubMed] [Google Scholar]
- 32.Shahir AK, Briggs N, Katsoulis J, Levidiotis V. An observational outcomes study from 1966–2008, examining pregnancy and neonatal outcomes from dialysed women using data from the ANZDATA Registry. Nephrology (Carlton) 2013;18:276–84. [DOI] [PubMed] [Google Scholar]
- 33.Shah S, Christianson AL, Meganathan K, Leonard AC, Schauer DP, Thakar CV. Racial Differences and Factors Associated with Pregnancy in ESKD Patients on Dialysis in the United States. J Am Soc Nephrol 2019;30:2437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliverio AL, Bragg-Gresham JL, Admon LK, Wright Nunes JA, Saran R, Heung M. Obstetric Deliveries in US Women With ESKD: 2002–2015. Am J Kidney Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornbrook MC, Whitlock EP, Berg CJ, et al. Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res 2007;42:908–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jesudason S, Grace BS, McDonald SP. Pregnancy outcomes according to dialysis commencing before or after conception in women with ESRD. Clin J Am Soc Nephrol 2014;9:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hladunewich MA, Hou S, Odutayo A, et al. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J Am Soc Nephrol 2014;25:1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccoli GB, Minelli F, Versino E, et al. Pregnancy in dialysis patients in the new millennium: a systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol Dial Transplant 2016;31:1915–34. [DOI] [PubMed] [Google Scholar]
- 39.Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int 2009;75:1217–22. [DOI] [PubMed] [Google Scholar]
- 40.Luders C, Titan SM, Kahhale S, Francisco RP, Zugaib M. Risk Factors for Adverse Fetal Outcome in Hemodialysis Pregnant Women. Kidney Int Rep 2018;3:1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Kidney F. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis 2015;66:884–930. [DOI] [PubMed] [Google Scholar]
- 42.Wiles K, Chappell L, Clark K, et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol 2019;20:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haase M, Morgera S, Bamberg C, et al. A systematic approach to managing pregnant dialysis patients--the importance of an intensified haemodiafiltration protocol. Nephrol Dial Transplant 2005;20:2537–42. [DOI] [PubMed] [Google Scholar]
- 44.Hosfield EM, Rabban JT, Chen LM, Zaloudek CJ. Squamous metaplasia of the ovarian surface epithelium and subsurface fibrosis: distinctive pathologic findings in the ovaries and fallopian tubes of patients on peritoneal dialysis. Int J Gynecol Pathol 2008;27:465–74. [DOI] [PubMed] [Google Scholar]
- 45.Chou CY, Ting IW, Lin TH, Lee CN. Pregnancy in patients on chronic dialysis: a single center experience and combined analysis of reported results. Eur J Obstet Gynecol Reprod Biol 2008;136:165–70. [DOI] [PubMed] [Google Scholar]
- 46.Ross LE, Swift PA, Newbold SM, Bramham K, Hurley A, Gallagher H. An Alternative Approach to Delivering Intensive Dialysis in Pregnancy. Perit Dial Int 2016;36:575–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw J, Katopodis C, Hladunewich MA, Ryz K. Changing Dialysis Modality during Pregnancy: A Case Report. Perit Dial Int 2018;38:456–8. [DOI] [PubMed] [Google Scholar]
- 48.Safi N, Sullivan E, Li Z, et al. Serious kidney disease in pregnancy: an Australian national cohort study protocol. BMC Nephrol 2019;20:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrington K, Carpenter RG, Goldfrad C, Campbell S. Transvaginal Doppler ultrasound of the uteroplacental circulation in the early prediction of pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol 1997;104:674–81. [DOI] [PubMed] [Google Scholar]
- 50.Coleman MA, McCowan LM, North RA. Mid-trimester uterine artery Doppler screening as a predictor of adverse pregnancy outcome in high-risk women. Ultrasound Obstet Gynecol 2000;15:7–12. [DOI] [PubMed] [Google Scholar]
- 51.Zeisler H, Llurba E, Chantraine F, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med 2016;374:13–22. [DOI] [PubMed] [Google Scholar]
- 52.Abou-Jaoude P, Dubourg L, Bessenay L, et al. What about the renal function during childhood of children born from dialysed mothers? Nephrol Dial Transplant 2012;27:2365–9. [DOI] [PubMed] [Google Scholar]
- 53.Piccoli GB, Postorino V, Cabiddu G, et al. Children of a lesser god or miracles? An emotional and behavioural profile of children born to mothers on dialysis in Italy: a multicentre nationwide study 2000–12. Nephrol Dial Transplant 2015;30:1193–202. [DOI] [PubMed] [Google Scholar]
- 54.Pyhala R, Hovi P, Lahti M, et al. Very low birth weight, infant growth, and autism-spectrum traits in adulthood. Pediatrics 2014;134:1075–83. [DOI] [PubMed] [Google Scholar]
- 55.(Accessed April 15, 2020, at https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022127s011lbl.pdf.)
- 56.Sienas L, Wong T, Collins R, Smith J. Contemporary uses of erythropoietin in pregnancy: a literature review. Obstet Gynecol Surv 2013;68:594–602. [DOI] [PubMed] [Google Scholar]
- 57.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol 2009;21:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med 2015;372:407–17. [DOI] [PubMed] [Google Scholar]
- 59.ACOG Practice Bulletin No. 203: Chronic Hypertension in Pregnancy. Obstet Gynecol 2019;133:e26–e50. [DOI] [PubMed] [Google Scholar]
- 60.Butalia S, Audibert F, Cote AM, et al. Hypertension Canada’s 2018 Guidelines for the Management of Hypertension in Pregnancy. Can J Cardiol 2018;34:526–31. [DOI] [PubMed] [Google Scholar]
- 61.Webster K, Fishburn S, Maresh M, Findlay SC, Chappell LC, Guideline C. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ 2019;366:l5119. [DOI] [PubMed] [Google Scholar]
- 62.Cabiddu G, Castellino S, Gernone G, et al. A best practice position statement on pregnancy in chronic kidney disease: the Italian Study Group on Kidney and Pregnancy. J Nephrol 2016;29:277–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coskun A, Bicik Z, Duran S, et al. Pregnancy-associated plasma protein A in dialysis patients. Clin Chem Lab Med 2007;45:63–6. [DOI] [PubMed] [Google Scholar]
- 64.Lim TS, Shanmuganathan M, Wong I, Goh BL. Successful multigravid pregnancy in a 42- year-old patient on continuous ambulatory peritoneal dialysis and a review of the literature. BMC Nephrol 2017;18:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown HK, Qazilbash A, Rahim N, Dennis CL, Vigod SN. Chronic Medical Conditions and Peripartum Mental Illness: A Systematic Review and Meta-Analysis. Am J Epidemiol 2018;187:2060–8. [DOI] [PubMed] [Google Scholar]
- 66.Attini R, Leone F, Parisi S, et al. Vegan-vegetarian low-protein supplemented diets in pregnant CKD patients: fifteen years of experience. BMC Nephrol 2016;17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cabiddu G, Castellino S, Gernone G, et al. Best practices on pregnancy on dialysis: the Italian Study Group on Kidney and Pregnancy. J Nephrol 2015;28:279–88. [DOI] [PubMed] [Google Scholar]