To the Editor:
Diagnosing interstitial lung disease (ILD) is frequently challenging. Improvements in the recognition of disease-specific radiological patterns have resulted in many cases of ILD being diagnosed noninvasively. Nonetheless, there remain many circumstances in which further investigations beyond imaging are required to establish a definitive ILD diagnosis. This is reflected in recent diagnostic guidelines for hypersensitivity pneumonitis that suggest bronchoscopy and cellular analysis of BAL in patients with newly identified ILD for whom the differential diagnosis includes fibrotic hypersensitivity pneumonitis (1). The current idiopathic pulmonary fibrosis (IPF) guidelines also provide a conditional recommendation to perform BAL in cases of newly detected ILD of apparently unknown cause, in which the computed tomographic pattern is not one of definite usual interstitial pneumonia (2). However, not all centers perform diagnostic bronchoscopy in patients with ILD; in part, this reflects concerns about safety. A small number of retrospective and anecdotal observations have been used to suggest that bronchoscopy in individuals with IPF could be associated with an increased risk of acute exacerbations or acute respiratory deterioration (3, 4). We therefore aimed to clarify the safety of BAL in patients with IPF using the PROFILE (Prospective Observation of Fibrosis in Lung Clinical Endpoints) study cohort (5).
Incident cases of multidisciplinary diagnosed IPF were recruited prospectively as part of the PROFILE study through the following two coordinating centers in the United Kingdom: Nottingham University Hospitals in Nottingham (NCT01134822) and Royal Brompton Hospital in London (NCT01110694). Patients were assessed at baseline, 1 month, 3 months, and 6 months, and annually for 3 years. Fiberoptic bronchoscopy with BAL was undertaken in a subset of the Brompton cohort at baseline. BAL was performed by instillation of 240 ml of warm saline (in four 60-ml aliquots) into a segment of the right middle lobe with gentle aspiration by hand (6). No other bronchoscopic procedures were performed. Immediate, 30-day, and 90-day adverse events as well as overall survival were evaluated and compared between subjects undergoing BAL and those who did not. Continuous variables are presented as means (SD), and categorical variables are presented as proportions. Differences between subject groups were evaluated with the Mann-Whitney test for continuous variables and the Fisher’s exact test for categorical variables. Time-to-event curves were calculated using the Kaplan-Meier method and compared using the log-rank test. Associations between continuous explanatory variables and overall survival were explored with a Cox proportional hazards model.
Of 614 subjects who were prospectively recruited into the PROFILE study, 223 underwent bronchoscopy (36%). The 391 individuals with IPF who did not undergo BAL were older (71.8 vs. 67.8 yr; P < 0.001) than subjects in the bronchoscopy cohort but otherwise were well matched (Table 1). All subjects in the bronchoscopy cohort tolerated the procedure well, a cell differential was available for all, and there were no immediate (<72 h) complications.
Table 1.
Demographics
| No Bronchoscopy (n = 391) | Bronchoscopy (n = 223) | P Value | |
|---|---|---|---|
| Age, yr, mean (SD) | 71.8 (8.3) | 67.8 (8.1) | <0.001 |
| Sex, M, n (%) | 302 (77.2) | 170 (76.2) | 0.776 |
| Smoking history, ever/current, n (%) | 276 (70.6) | 141 (63.2) | 0.06 |
| FVC, %, mean (SD) | 76.3 (18.1) | 77.8 (18.2) | 0.591 |
| DlCO, %, mean (SD) | 48.5 (17.6) | 47.6 (14.1) | 0.79 |
| FEV1/FVC, mean (SD) | 79.8 (7.8) | 79.6 (7.4) | 0.71 |
In the first 30 days after BAL, six patients (2.7%) reported complications. Two subjects described transient viral-type symptoms after the procedure, one subject described odynophagia (again transient), and three subjects were treated with antibiotics for presumed lower respiratory tract infection, with one case (0.4%) requiring an emergency room attendance but not admission. There was no difference in 30-, 60-, or 90-day all-cause mortality in those undergoing bronchoscopy compared with the no-bronchoscopy cohort. All-cause mortality at 90 days was 1.4% in the bronchoscopy cohort and 3.6% in the nonprocedure cohort.
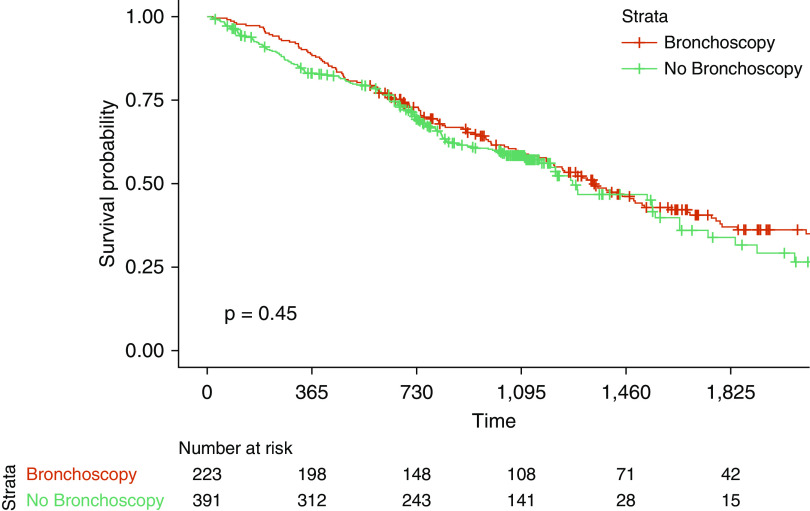
There was no significant difference (P = 0.45) in overall mortality between patients who underwent bronchoscopy and those who did not (Figure 1). The median survival for patients undergoing bronchoscopy was 3.7 years. There remained no difference in survival after adjustment for age, sex, baseline % predicted FVC, baseline % predicted DlCO, smoking status, and recruitment site in a multivariable Cox proportional hazards model (hazard ratio, 0.84; 95% confidence interval, 0.59–1.22; P = 0.364).
Figure 1.
No significant difference in overall mortality in patients with idiopathic pulmonary fibrosis undergoing bronchoscopy. Shown are Kaplan-Meier curves comparing survival between individuals in the PROFILE (Prospective Observation of Fibrosis in Lung Clinical Endpoints) study undergoing bronchoscopy and those not undergoing bronchoscopy. Log-rank P test value is reported.
There are a number of limitations to our work. First, subjects were not randomly assigned to either the procedure or no-procedure arm, as the bronchoscopy component of the study was optional. Second, some of the most severe cases of IPF were not included, as only subjects able to safely undergo bronchoscopy were enrolled; all-cause mortality was used as an endpoint rather than respiratory-related mortality because the necessary level of detail regarding cause of death was not available for the whole cohort. Finally, the no-bronchoscopy cohort was on average older than the intervention cohort, a known risk factor for mortality in IPF. However, there remained no difference in overall survival when incorporating this into a Cox model of survival, implying the age difference between groups had no meaningful impact. Although we demonstrate no negative safety signal, we do not address cost effectiveness; something which may impact local decisions to perform BAL in the diagnostic assessment of ILD.
In summary, this prospectively recruited longitudinal cohort study demonstrates that bronchoscopy is a safe and well-tolerated procedure in individuals with IPF. Although the assessment of BAL in the diagnosis of fibrotic lung disease may have recently been overshadowed by the emergence of cryobiopsy (7) and other novel molecular techniques (8), it remains important in distinguishing specific forms of ILD from IPF (something that is highlighted in recently published diagnostic guidelines for hypersensitivity pneumonitis). Furthermore, BAL has an important role in proof-of-concept clinical trials (9) and as a research tool for understanding disease pathogenesis and discovering novel biomarkers (10, 11).
Supplementary Material
Footnotes
P.L.M. is an Action for Pulmonary Fibrosis Research Fellow. R.G.J. is supported by a National Institute for Health Research Research Professorship (RP-2017-08-ST2-014). T.M.M. is supported by a National Institute for Health Research Clinician Scientist Fellowship (CS-2013-13-017) and a British Lung Foundation Chair in Respiratory Research (C17-3). The PROFILE study was funded by the Medical Research Council (G0901226) and GlaxoSmithKline R&D (CRT114316) and was sponsored by Nottingham University and Royal Brompton and Harefield National Health Service Foundation Trust.
Originally Published in Press as DOI: 10.1164/rccm.202004-1138LE on August 28, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Raghu G, Remy-Jardin M, Ryerson CJ, Myers JL, Kreuter M, Vasakova M, et al. Diagnosis of hypersensitivity pneumonitis in adults: an official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2020;202:e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 3.Hiwatari N, Shimura S, Takishima T, Shirato K. Bronchoalveolar lavage as a possible cause of acute exacerbation in idiopathic pulmonary fibrosis patients. Tohoku J Exp Med. 1994;174:379–386. doi: 10.1620/tjem.174.379. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto K, Taniguchi H, Kondoh Y, Wakai K, Kimura T, Kataoka K, et al. Acute exacerbation of IPF following diagnostic bronchoalveolar lavage procedures. Respir Med. 2012;106:436–442. doi: 10.1016/j.rmed.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Maher TM, Oballa E, Simpson JK, Porte J, Habgood A, Fahy WA, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5:946–955. doi: 10.1016/S2213-2600(17)30430-7. [DOI] [PubMed] [Google Scholar]
- 6.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troy LK, Grainge C, Corte TJ, Williamson JP, Vallely MP, Cooper WA, et al. Cryobiopsy versus Open Lung biopsy in the Diagnosis of Interstitial lung disease alliance (COLDICE) Investigators. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med. 2020;8:171–181. doi: 10.1016/S2213-2600(19)30342-X. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Flaherty KR, Lederer DJ, Lynch DA, Colby TV, Myers JL, et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: a prospective validation study. Lancet Respir Med. 2019;7:487–496. doi: 10.1016/S2213-2600(19)30059-1. [DOI] [PubMed] [Google Scholar]
- 9.Lukey PT, Harrison SA, Yang S, Man Y, Holman BF, Rashidnasab A, et al. A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur Respir J. 2019;53:1801992. doi: 10.1183/13993003.01992-2018. [DOI] [PubMed] [Google Scholar]
- 10.Allden SJ, Ogger PP, Ghai P, McErlean P, Hewitt R, Toshner R, et al. The transferrin receptor CD71 delineates functionally distinct airway macrophage subsets during idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200:209–219. doi: 10.1164/rccm.201809-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molyneaux PL, Willis-Owen SAG, Cox MJ, James P, Cowman S, Loebinger M, et al. Host-microbial interactions in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;195:1640–1650. doi: 10.1164/rccm.201607-1408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.