To the Editor:
We read with great interest the article by McElvaney and colleagues, “Characterization of the Inflammatory Response to Severe COVID-19 Illness,” published in the Journal (1). Systemic inflammation that characterizes acute respiratory distress syndrome (ARDS) in coronavirus disease (COVID-19) is associated with a high mortality rate (2). The term “cytokine storm” has emerged to explain the immunopathogenesis of most severe forms of COVID-19, because the release of many inflammatory cytokines (e.g., IL-6) was correlated with the disease severity (3). However, immune pathogenesis is still unsettled and comparisons with community-acquired pneumonia (CAP) from other origins are scarce. Interestingly, McElvaney and colleagues showed that COVID-19 cytokinemia is distinct, with circulating levels of IL-6 significantly higher in patients with COVID-19 than in those with non–COVID-19 severe CAP (1). These results are challenged by an editorial by Sinha and colleagues showing that plasma concentrations of IL-6 in patients with severe COVID-19 were lower than those observed in patients with ARDS from other origins (4). However, such comparisons are only possible if the respiratory severity is comparable between both groups.
We performed a prospective study that is part of the LYMPHONIE project (clinicaltrials.gov NCT03505281). We included non–immune-compromised patients with severe pneumonia (at least two criteria of the quick Sequential Organ Failure Assessment score and/or need for mechanical ventilation or vasopressors). Patients with COVID-19 all tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by RT-PCR. Plasma was collected within 48 hours of hospital admission and the concentration of IL-6 quantified using a Luminex assay (R&D Systems). Oral consent was obtained from the patient or their legal representatives. Approval was obtained from the ethics committee (Comité de Protection des Personnes Sud-MEDITERRANEE V) (2017-A03404-49).
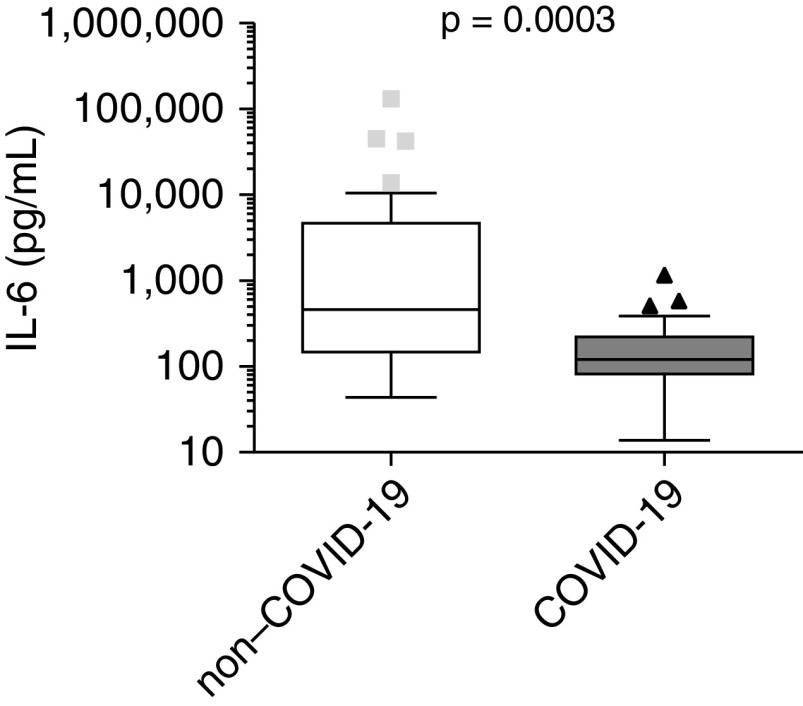
Thirty-six patients without COVID-19 were enrolled between November 2018 and February 2020 (before the COVID-19 pandemic started in Burgundy, France) and 27 patients with COVID-19 in March and April 2020. Median age (interquartile range) was 67.5 (63–76.5) and 64 (57–71) in the non–COVID-19 and COVID-19 groups, respectively (P = 0.0559). ICU admission was needed for 32 (89%) and 27 (100%) patients of the non–COVID-19 and COVID-19 groups, respectively (P = 0.07). PaO2:FiO2 ratios were not different between groups (115.9 [74.6–157.9] vs. 145 [86–175.7] mm Hg, respectively; P = 0.3073). The IL-6 plasma concentrations were higher in the patients without COVID-19 than in those with COVID-19 (460.4 [138.2–4434.7] vs. 121 [75.7–236.6] pg/ml; P = 0.0003) (Figure 1).
Figure 1.
Box plot showing the plasma concentration of IL-6 within 48 hours of hospitalization in 36 patients with non–COVID-19 and 27 patients with COVID-19 severe pneumonia (LYMPHONIE study, 2018–2020). COVID-19 = coronavirus disease.
Here, we showed that despite similar respiratory severity, plasma concentrations of IL-6 were dramatically lower in severe forms of COVID-19 than in CAP from other origins. Thus, although immune modulation is a promising therapeutic avenue that is likely to improve outcomes for COVID-19, the most relevant target and strategy remain to be found.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all investigators of the LYMPHONIE study group, the CIC-EC1432 (Alexandra Lamotte-Felin, Lydie Rossie, and Delphine Pecqueur), the Intensive Care Medicine Research team (Solenne Villot and Mathilde Audry), Serge Monier from the Cytometry core facility, and Shaliha Bechoua from the Centre de Ressource Biologique. They also thank the patients.
LYMPHONIE Study Group: Mathieu Blot, Abderrahmane Bourredjem, Christine Binquet, Lionel Piroth, Jean-Pierre Quenot, Pierre-Emmanuel Charles, François Aptel, Sébastien Prin, Pascal Andreu, Marie Labruyère, Auguste Dargent, Maxime Nguyen, Belaid Bouhemad, Marjolaine Georges, Philippe Bonniaud, Guillaume Beltramo, Marielle Buisson, Bernard Bonnotte, Philip Bielefeld, Jeremy Barben, Alain Putot, Serge Monier, Audrey Large, Suzanne Mouries-Martin, Hervé Devilliers, Pascal Chavanet, and Alexandre Guilhem.
Footnotes
Supported by grants from the AOIc2020 (to M.B.), from the INSERM UMR 1231, ANR-11 LABX-0021-01, Labex Lipstic, the FEDER, the Regional Council of Bourgogne Franche-Comte, and by crowdfunding (https://thellie.org/covid-19).
Author Contributions: Concept and design: M.B., C.B., and L.P. Acquisition, analysis, or interpretation of data: M.B., A.B., C.B., and L.P. Drafting of the manuscript: M.B. Critical revision: C.B. and L.P. Supervision: C.B. and L.P.
Originally Published in Press as DOI: 10.1164/rccm.202007-2924LE on September 21, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the LYMPHONIE Study Group, Mathieu Blot, Abderrahmane Bourredjem, Christine Binquet, Lionel Piroth, Jean-Pierre Quenot, Pierre-Emmanuel Charles, François Aptel, Sébastien Prin, Pascal Andreu, Marie Labruyère, Auguste Dargent, Maxime Nguyen, Belaid Bouhemad, Marjolaine Georges, Philippe Bonniaud, Guillaume Beltramo, Marielle Buisson, Bernard Bonnotte, Philip Bielefeld, Jeremy Barben, Alain Putot, Serge Monier, Audrey Large, Suzanne Mouries-Martin, Hervé Devilliers, Pascal Chavanet, and Alexandre Guilhem
References
- 1.McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Fei D, Li X, Zhao M, Yu K. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med. 2020;46:1475–1476. doi: 10.1007/s00134-020-06065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. doi: 10.1001/jamainternmed.2020.3313. [online ahead of print] 30 Jun 2020; DOI: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.