Abstract
The human brain is an adaptive system that combines the reconfiguration of brain interactions with the evolution of local brain activities. Reoccurring brain activity and connectivity patterns, regarded as “brain states”, are widely observed via different neuroimaging techniques, revealing new insights into the pathophysiology of multiple brain disorders, such as schizophrenia (SZ). However, previous work fails to depict a comprehensive picture of brain dynamics because they only focus on either brain activity or connectivity dynamics, ignoring the temporal co-evolution between them. Here, we propose an analysis pipeline to capture dynamic brain states with covarying activity-connectivity and apply it SZ datasets to probe disease-related brain abnormalities. Results were consistent across different brain parcellation techniques (independent component analysis and atlas-based analysis) and in two independent cohorts who underwent resting-state fMRI scans. We found that the state-based activity patterns and connectivity patterns show high correspondence, where strong and antagonistic connectivity is typically accompanied by strong low-frequency fluctuations across the whole brain while the weak and sparse connectivity co-occurs with weak low-frequency fluctuations, especially in sub-cortical, sensory and cerebellum regions. In addition, a graphical analysis showed that brain activity-connectivity states have different network efficiency and node efficiency is correlated with the low-frequency activities of brain nodes. Compared with healthy controls (HCs), SZ patients spend more time in weakly-connected and -activated brain states but less time in strongly-connected and -activated brain states. Interestingly, the fractional occupancy of one brain state was correlated with attention performance. SZ patients also showed lower node efficiency in thalamic regions in the “strong” brain states. These robust results extend previous findings in dynamic brain analysis and thalamocortical dysfunction in schizophrenia. Capturing covarying brain activity-connectivity dynamics might help to advance our understanding of the co-evolutionary property of brain network and the underlying mechanism in brain disorders which cannot be observed by focusing either of them along.
Keywords: Covarying brain state, reoccurring activity-connectivity states, thalamocortical network dynamics, schizophrenia
Introduction
Despite considerably advancing our knowledge of brain function while studying static brain function, increasing evidence has challenged its oversimplification of the complex modulation and functioning process of the brain (Sakoğlu et al., 2010, Hutchison et al., 2013a). The human brain constantly integrates and coordinates different neural populations across multiple spatiotemporal scales to adapt to the demands of internal and external environments (Buzsáki, 2009, Hutchison et al., 2013a). Such dynamically adaption of brain spans from the condition-dependent variation in brain activity (Lehmann, 1990; Malsburg et al., 2010; Liu and Duyn, 2013) to the time-varying nature of brain coordination (Chang and Glover, 2010; Allen et al., 2014; Zalesky et al., 2014), both of which are associated with cognitive and mental processes (Lehmann et al., 1998; Raichle and Snyder, 2007; Allen et al., 2014, 2018). Numerous brain dynamics have been captured during task conditions (Fornito et al., 2012; Di et al., 2015) and even within the resting-state (Hutchison et al., 2013b; Allen et al., 2014; Zalesky et al., 2014) and it is believed that such fluctuations allow for balancing the information integration among neural populations to facilitate brain function (Bassett et al., 2015).
Although interpreting dynamic patterns from functional magnetic resonance imaging (fMRI) is not necessarily straightforward (Hutchison et al., 2013a), fMRI is a powerful technique to investigate potential functional brain dynamics. One popular hypothesis of the dynamic brain is that brain might switch among discrete states with distinct activity patterns or connectivity patterns, in a conceptual analogy to electroencephalogram (EEG) microstates, a short period during which the clustered patterns remains quasi-stable (Lehmann, 1990), to support different brain function and mental process. This hypothesis has been widely validated in fMRI studies, in which reoccurring brain activity and connectivity are consistently observed and associated with cognitive performance and maturation of brain (Marusak et al., 2017), vigilance (Allen et al., 2018) and brain disorders (Kim et al., 2017; Díez-Cirarda et al., 2018; Du et al., 2018; Fu et al., 2019; Mash et al., 2019; Tu et al., 2019). Like many other biological systems (Hopfield et al., 1983), the human brain might organize as an adaptive network in which the evolution of the topology would entangle with the dynamics of the nodes (Gross and Blasius, 2008). Indeed, previous studies have revealed potential couplings between brain activity and connectivity (Liu and Duyn, 2013; Zhang et al., 2015, 2017; Fu et al., 2017). Both dynamic low-fluctuations (Liao et al., 2019) and connectivity (Allen et al., 2018) in fMRI are shown to associated with EEG power fluctuations, which implies that brain activity and connectivity might covary in time, contributing to the formation of brain states. However, previous work favors only one type of brain dynamics and ignores co-evolutionary relationships across multiple dynamic measures when capturing the brain states, which might fail to accurately depict the dynamic brain. The investigation of such co-varying brain states might shed light on the relationship between local region activity and large-scale functional organization and advance our understanding of how brain network integrates to support fundamental behavior and function.
Schizophrenia (SZ) is a common psychiatric disease with severe cognitive dysfunction which incurs a significant financial burden for both patients and the nation’s economy (Association, 2013). Both brain activity and connectivity abnormalities have been widely reported in SZ, from static to dynamic, revealing consistently disease-related cortical-subcortical dysfunction and abnormal oscillations in subcortical and cortical regions (Hoptman et al., 2010; Salvador et al., 2010; Turner et al., 2013; Damaraju et al., 2014; Sui et al., 2018). However, few studies have linked activity and connectivity abnormalities in SZ, and the underlying relationship between them is still far from understood. Recent work has shown significant temporal associations between brain activity and connectivity and these associations decrease in SZ, indicating uncoupling between brain activity and connectivity in SZ (Fu et al., 2018). Characterizing reoccurring brain states using both activity and connectivity might help to capture the real transient brain conditions that can be potential biomarkers that benefit the understanding of the neural mechanism and the development of new treatments of the disease.
In this study, we characterized both dynamic functional network connectivity (dFNC) and dynamic amplitude of low-frequency fluctuation (dALFF) and used them to capture reoccurring brain activity-connectivity states. We hypothesized that the ALFF and FNC patterns of each dynamic state would be highly coupled and SZ might be associated with the abnormalities in such dynamic states, especially in thalamic networks. We replicated the results on two independent datasets to demonstrate the reliability of our findings. We also performed the same analysis on the time-courses (TCs) extracted from both independent component analysis (ICA) and atlas-based analysis to examine the replicability of the results across different brain parcellation strategies.
Results
Brain Parcellation
The brain was first parcellated into 53 intrinsic connectivity networks (ICNs) using a fully automated spatially constrained group independent component analysis pipeline called Neuromark. The identified ICNs covered the majority of subcortical and cortical gray matter and were arranged into 7 functional domains (Allen et al., 2014; Damaraju et al., 2014): subcortical (SC), auditory (AUD), visual (VS), sensorimotor (SM), cognitive-control (CC), default-mode (DM), and cerebellar domains (CB). To validate the results via another brain parcellation strategy, the automated anatomical labeling (AAL) atlas was also employed to extract TCs for further dynamic activity-connectivity analysis. In total 100 regions of interest (ROIs) from the AAL atlas were selected and arranged into the same 7 functional domains. Details of the two parcellation strategies can be found in the supplementary materials.
Clustering Analysis on Dynamic Activity and Connectivity
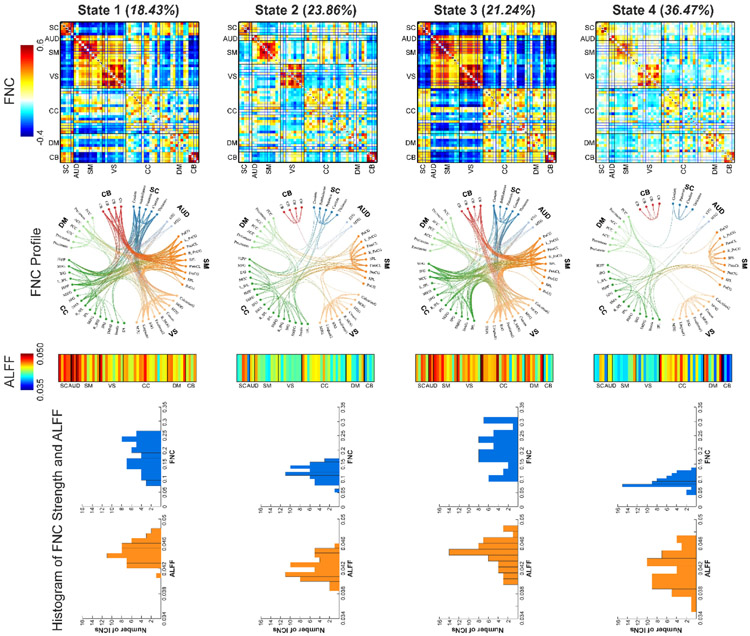
Via a sliding-window approach, dALFF was calculated to measure the time-varying brain activity and dFNC was calculated to measure the time-varying functional connectivity (FC). dALFF and dFNC were concatenated as the input of the clustering analysis for the exploration of reoccurring activity-connectivity states. The number of clusters was determined as k = 4, which was within a reasonable range (4~7) consistent with previous dFNC studies on different brain disorders. Figure 1 displays the clustering results. The first row represents the FNC patterns of each brain state and the third row represents the ALFF patterns of each brain state. The second row displays the functional profile of brain states which retains the strong connectivity (> 0.25). Two sparsely connected states with low ALFF have more frequent occurrences while two strong antagonism connectivity states with high ALFF have less frequent occurrences. State 1 and 3 have strongly positive connectivity within the sensory domains (AUD, SM, and VS) and negative connectivity between the sensory domains and the other domains. There are less negative connectivity patterns in state 1, which shows some homogeneity among sensory and cognitive domains. Similar high ALFF patterns can be observed in these two states, but state 3 has more consistently high ALFF across the whole-brain. The connectivity in state 2 and 4 are relatively weaker and most of the strong connectivity are mainly located within subcortical, sensorimotor and visual domains. State 4 is a dynamic state with highly similar patterns as static FNC. Compared with state 2, state 4 has more sparsely connected patterns and lower ALFF except for some networks in CC domain. The histogram of ICN connectivity strength (average FNC of a given ICN) and ALFF are displayed in the fourth row in Figure 1, showing similar correspondence between activity and connectivity across states.
Figure 1.
Findings of dynamic brain activity-connectivity states. Four brain states are identified. First row: the FNC patterns of brain states. Two states have strong within-domain connectivity and negative between-domain connectivity patterns and two states have weak and sparse connectivity patterns. Second row: top 200 FNC with connectivity strength > 0.25 in each state, representing the strongest functional-relationships between brain regions (absolute value). Each color represents one of the seven domains. Third row: the ALFF patterns of brain state. Two states have high ALFF and two states have low ALFF. Fourth row: the histogram of FNC strength and ALFF of ICNs. FNC strength is calculated as the average FNC between a given ICN and the other ICNs. Functional network connectivity, FNC; the amplitude of low-frequency fluctuations, ALFF; intrinsic connectivity networks (ICNs).
Group Difference in Occurrence of Dynamic Activity-connectivity State
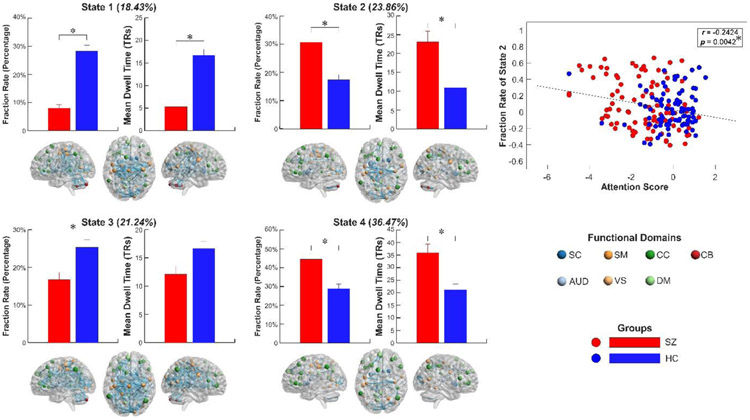
The group differences in occurrence (fraction rate [FRA]: the proportion of time spent in each state; mean dwell time [MDT]: the average length of time in each state) is displayed in Figure 2. Compared with HCs, SZ patients had significantly lower FRA in state 1 and 3 (p < 0.05, false discovery rate [FDR] corrected; age and gender as covariates) but higher FRA in state 2 and 4 (p < 0.05, FDR corrected; age and gender as covariates). Similar differences were observed in the MDT between HCs and SZ. These results show that SZ patients had a significantly lower occurrence in the states with strongly interconnected FNC and high ALFF but higher occurrence in sparsely connected states with low ALFF. In a further analysis of relationships between the abnormal occurrence of brain state and the cognitive decline in SZ patients, we found that the FRA of state 2 is negatively correlated with the attention score (p < 0.05, FDR corrected; age, gender, and diagnosis as covariates), indicating a potential association between the brain dynamic state and cognitive performance. The observed group differences and association were replicated using an independent dataset and details are provided in the following sections and the supplementary materials.
Figure 2.
Group differences in occurrence and its association with cognitive performance. Bars represent the mean of occurrences and error bars represent the standard deviation of mean. SZ patients have significantly decreased FRA and MDT in the “strong” activity-connectivity states (state 1 and state 3) and increased FRA and MDT in the “weak” states (state 2 and state 4). The centroid patterns of states are displayed, with node size representing the ALFF and edge size representing the connectivity value. The FRA of state 2 is negatively correlated with the performance of attention. schizophrenia, SZ; fraction rate, FRA; mean dwell time, MDT; the amplitude of low-frequency fluctuations, ALFF.
Dynamic Brain State with Co-evolved Activity and Connectivity Patterns
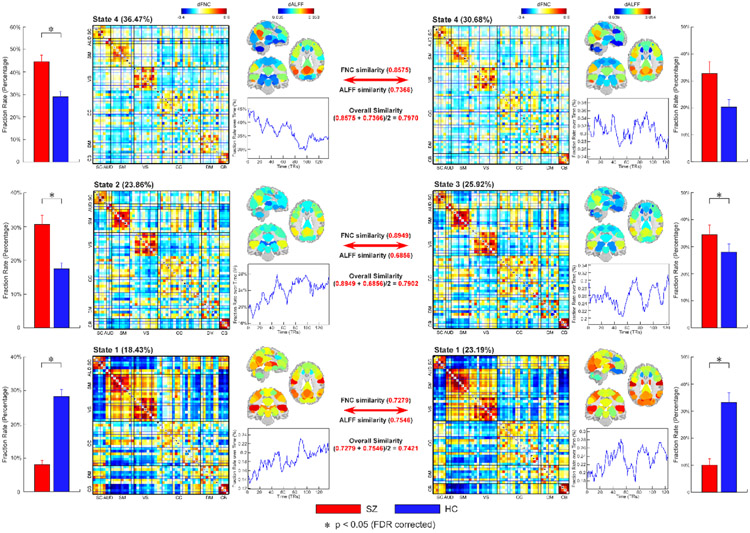
The covarying brain activity-connectivity states were validated using an independent dataset. 53 ICNs and corresponding TCs were estimated via the Neuromark pipeline for the replication dataset (centers of Biomedical Research Excellence [COBRE]). dALFF and dynamic FNC were calculated using the same sliding-window approach and then k-means clustering was applied. Similar to the discovery dataset, two sparsely connected states with low ALFF and two strongly connected states with high ALFF are identified.
We matched the identified states between datasets by evaluating the correlation coefficient between their centroid patterns (FNC and ALFF respectively). An FBIRN state is supposed to be matched to a COBRE state if it has the highest overall similarity (averaged between-FNC similarity and between-ALFF similarity) with this COBRE state than the similarity with the other COBRE states. We only display the pairs of matched states with the overall similarity > 0.7. As seen in Figure 3, three pairs of matched states are identified. Interestingly, for a given matched pair of states, their FNC and ALFF are also matched respectively, with the highest correlation compared with the correlation to the other states. The matched states are arranged in order of overall similarity. State 4 of the COBRE dataset has the highest between-FNC correlation and between-ALFF correlation with the state 4 of the FBIRN dataset (between-FNC correlation = 0.8575; between-ALFF correlation = 0.7366). These states show a similar decreased occurrence in time. State 3 of the COBRE dataset has the highest between-FNC correlation and between-ALFF correlation with the state 2 of the FBIRN dataset (between-FNC correlation = 0.8949; between-ALFF correlation = 0.6856). Slightly increased occurrence across time is observed in these two states. State 1 of the COBRE dataset has the highest between-FNC correlation and between-ALFF correlation with the state 1 of the FBIRN dataset (between-FNC correlation = 0.7279; between-ALFF correlation = 0.7546) and these two states show similar decreasing occurrence in time. The above findings indicate that the state-based ALFF and FNC are highly coupling from one state to another state. We further introduced a permutation test to illustrate that such high correspondence between ALFF and FNC is not due to the clustering effect. The between-ALFF correlations of the matched states are significantly higher than the correlations from the randomly permutated datasets (p < 0.001, 1000 times permutation test).
Figure 3.
Replication of brain activity-connectivity states across datasets. Three pairs of dynamic states are matched as their overall similarity (average similarity between FNC similarity and ALFF similarity) >= 0.7. The similarity is calculated by the correlation coefficient between FNC or ALFF patterns. For each pair of matched states, their FNC and ALFF similarity are always highest compared with the similarity to the other states, indicating the co-existing FNC and ALFF patterns across states. The matched states also have similar temporal trends in FRA and similar FRA abnormalities in SZ. Functional network connectivity, FNC; the amplitude of low-frequency fluctuations, ALFF; fraction rate, FRA.
Similar findings are replicated using a different brain parcellation strategy (see supplementary materials). In the atlas-based analysis, we identified two pairs of matched states between the FBIRN and COBRE datasets. A pair of “strong” dynamic states are matched with strong positive and negative connectivity and high ALFF (between-FNC correlation = 0.8181; between-ALFF correlation = 0.8871) and a pair of “weak” dynamic states are matched with relatively weak and sparsely connected connectivity and low ALFF (between-FNC correlation = 0.7413; between-ALFF correlation = 0.8537).
Differences between HCs and SZ patients were also consistent across datasets (Figure 3). SZ patients had a significantly lower FRA in COBRE state 1 (a state matched the state 1 in FBRIN) and higher FRA in COBRE state 3 (a state matched the state 2 in FBRIN). The group differences are further replicated using the AAL atlas.
Dynamic Network Efficiency
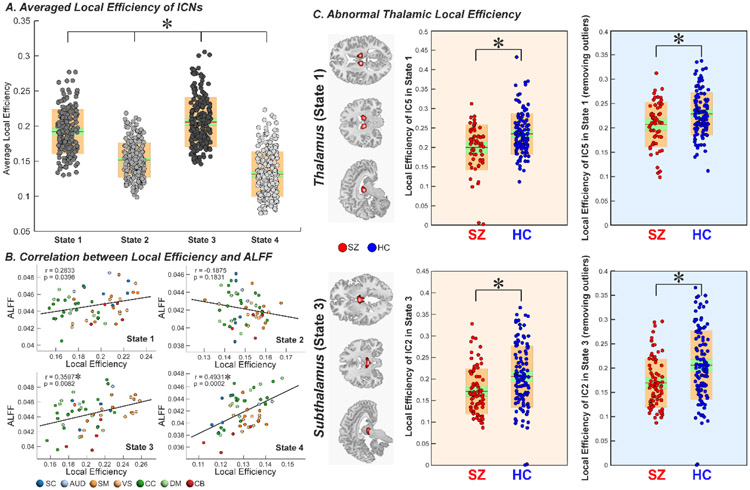
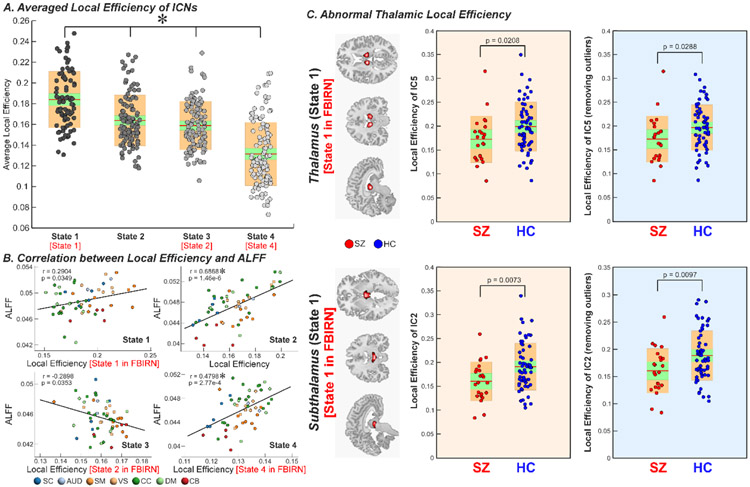
We employed local efficiency, a well-established topological measure of evaluating information transfer in the network node, to investigate the potential relationships between brain activity and its working efficiency. Figures 4 and 5 display the results for the FBIRN and COBRE datasets respectively. The results show that when the brain has high ALFF, the overall local efficiency is high. In contrast, when the brain has low ALFF, the local efficiency is low. Interestingly, for both discovery and replication datasets, we observed significant correlations between ICN efficiency and its ALFF, and such correlated relationships change across states (Figure 4B and Figure 5B). For example, for state 1 and 4 of FBIRN and their matched state 1 and 4 of COBRE, there are positive correlations between ALFF and local efficiency. In contrast, in state 2 of FBIRN and its matched state 3 of COBRE, there is a negative correlation between ALFF and local efficiency. In the analysis of group difference on the local efficiency, we found that SZ patients have lower local efficiency in the thalamus and hypothalamus within those dynamic brain-activity states with high local efficiency (p < 0.05, FDR corrected; age and gender as covariates), and these findings are replicated across datasets (Figure 4C and Figure 5C).
Figure 4.
Graph measure of dynamic activity-connectivity states. A. Average local efficiency across ICNs of each brain activity-connectivity state is displayed using boxplots. Each dot represents an individual’s value and the color represents the efficiency level (Darker → higher efficiency). B. The correlation coefficient between local efficiency and ALFF across ICNs of each state are shown using scatter plots, with different colors representing different functional domains. C. Group comparison in local efficiency of thalamus and subthalamus are displayed using boxplots. SZ patients have decreased efficiency in thalamus and subthalamus in the “high-efficiency” states. Asterisks indicate significance after FDR correction. Intrinsic connectivity network, ICN; the amplitude of low-frequency fluctuation, ALFF; false discovery rate, FDR.
Figure 5.
Replication of graph measure using the COBRE dataset. A. Average local efficiency across ICNs of each brain activity-connectivity state is displayed using boxplots. Brain states show similar efficiency difference (state 1 [state 1 in FBIRN] > state 3 [state 2 in FBIRN] > state 4 [state 4 in FBIRN]). B. The correlation coefficient between local efficiency and ALFF across ICNs of each state are shown using scatter plots, with different colors representing different functional domains. State 1 and 4 which match state 1 and 4 in FBIRN dataset show similar positive efficiency-ALFF correlation while state 3 which matches state 2 in FBIRN shows negative efficiency-ALFF correlation. C. Group comparison in local efficiency of thalamus and subthalamus are displayed using boxplots. SZ patients have decreased efficiency in thalamus and subthalamus in state 1, which is a high-efficiency state. Intrinsic connectivity network, ICN; the amplitude of low-frequency fluctuation, ALFF.
Discussion
Spontaneous fluctuations of brain activity and connectivity have long been appreciated in the scientific literature. Given the dynamic nature of brain activity and connectivity, the investigation of co-varying brain activity and connectivity may unveil neural mechanism as well as the pathophysiology of diseases which cannot be discovered if only examining one of them along. In this study, we investigated dynamic brain activity-connectivity states by evaluating brain activity features and connectivity features simultaneously. Four reoccurring dynamic activity-connectivity states were identified with obviously distinct activity and connectivity patterns. By comparing the results from two independent datasets, we found that the FNC patterns and the ALFF patterns are highly coupling, demonstrating that brain activity and connectivity might co-evolve in time to construct the reoccurring brain states. We also identified significantly different network efficiency between brain states, and moreover network efficiency of connectivity is associated with brain activity. In addition, SZ patients have more occurrences in sparsely connected and less activated brain states. In addition, a higher occurrence of one brain state is associated with a decline in individual attention performance. In contrast, HCs spend more time in strongly connected and more activated states, which have higher information transfer efficiency. Interestingly, within these “strong states”, the thalamus is less efficient in SZ patients.
Reoccurring Dynamic States with Coupling Brain Activity and Connectivity
Given evidence of associations between the low-frequency fluctuation of the fMRI signal and neural activation (Kiviniemi et al., 2000; Yang et al., 2007), ALFF, which measures the low-frequency power of fMRI signals, can be a good representation of regional spontaneous neural activity (Yang et al., 2007; Liao et al., 2019). On the other hand, the FC or FNC from fMRI time series, particularly during the resting-state, has depicted a picture of the functional brain by highlighting various inter-relationships between brain regions (Biswal et al., 1995; Greicius et al., 2003; Allen et al., 2014). Both ALFF and FC have been widely studied, revealing a great deal of knowledge on the spatiotemporal properties of the brain (Greicius et al., 2004; Beckmann et al., 2005; Biswal et al., 2010; Di et al., 2013; Abou Elseoud et al., 2014). Considering the evidence of time-varying brain activity and connectivity captured by high-temporal resolution imaging techniques, such as EEG, electrophysiological recordings of single cells, and LFP, recent fMRI studies assume that the brain representing by fMRI signals are also highly dynamic in a different timescale. While there has been debate on the topic, there is some strong concensus that dynamic patterns in fMRI signals and their interactions likely have a neuronal origin and as such may engender a new understanding of brain organization (McAvoy et al., 2008; Chang and Glover, 2010; Wu et al., 2010; Yaesoubi et al., 2015; Yan et al., 2017). For example, a novel concept of the dynamic brain as measured with fMRI is that the brain can be conceived as a multi-stable process, wherein the brain patterns pass through multiple discrete transient states, in a conceptual analogy to the microstate of EEG (Allen et al., 2014, 2018; Marusak et al., 2017; Fu et al., 2018).
However, previous studies only focus on one type of brain dynamics (activity or connectivity) to search for the presence of reproducible brain states, ignoring the co-evolutionary relationships between them. In this work, by combining features of brain activity (ALFF) and connectivity (FNC), we identified several reoccurring brain states with highly co-existed ALFF and FNC patterns. State 4, which accounts for > 30% of all windows, has weakly connected FNC patterns that resembles the static FNC (Allen et al., 2014; Damaraju et al., 2014; Díez-Cirarda et al., 2018; Tu et al., 2019). This state has overall low ALFF across networks, except for the networks in the posterior DM, such as the precuneus. Previous studies have shown that the precuneus only exhibits affiliation with the DM in only some states, challenging the discussion regarding the inclusion of the precuneus in the default mode network (Buckner et al., 2008; Allen et al., 2014). Our finding suggests that the disparate ALFF in the precuneus and anterior/bilateral DM regions might cause more information transfer among these regions, resulting in stronger connectivity between them. State 2 is another sparsely connected state characterized by strong within-domain FNC but weak between-domain FNC. This state is also the only state showing weak negative connectivity between VS and SM. We speculate that such modular FNC patterns, especially within SM and VS, might be due to the similar moderate low-frequency fluctuations in each domain. State 1 and 3 are two states showing fewer occurrences, both of which show strong coupling between FNC and high ALFF. Our results are in line with previous findings that dynamic states with similar thalamocortical antagonism have increased frequency with time in the eyes open condition (Allen et al., 2018). The thalamocortical antagonism states are suggested to be associated with reduced vigilance since they are accompanied with an increase in delta and theta power and a decrease in alpha power in EEG spectrum, a well-established pattern of reduced vigilance (Makeig and Jung, 1995; Allen et al., 2018). We speculate that the increase of low-frequency fluctuations of fMRI signals in these states might be related to the increase of low-frequency power in the neuroelectric brain activity, indicating the ALFF of BOLD to be a reliable dynamic signature of the brain.
Brain states with co-varying ALFF and FC were further replicated in another independent dataset and via an alternative ROI-based approach, validating our hypothesis of capturing dynamic states using both activity and connectivity features. Three dynamic states in the COBRE dataset are matched to the states from the FBIRN dataset, with high correspondence between their state-based ALFF pattern and FNC pattern. Via a permutation test, we further justify that the correspondence between matched states is significantly higher than the results of the clustering on the permuted data without co-varying activity-connectivity patterns. Results are validated in an ROI-based analysis as well, in which a “strong” state and a “weak” state are replicated between datasets, with simultaneously high correspondence between ALFF and between FC. Along with previous research, our results suggested that the shift in mental and vigilance states during the resting-state might result in co-occurred changes in neuronal activity and synchrony, reflecting by dynamic patterns in low-frequency fluctuations and their interactions from fMRI signals.
Abnormal Dynamic Activity-Connectivity States in SZ
Patients with SZ tended to spend more time in states with weak FNC and low ALFF but less time in states with strongly connected FNC and high ALFF. Schizophrenia is associated with widespread atypical low-frequency fluctuations and FC (Bluhm et al., 2007; Garrity et al., 2007; Manoliu et al., 2014), especially in the cortical-subcortical regions (Hoptman et al., 2010; Lynall et al., 2010; Salvador et al., 2010; Skudlarski et al., 2010; Turner et al., 2013; Damaraju et al., 2014; Klingner et al., 2014; Yu et al., 2014). For example, decreased subcortical low-frequency fluctuations and increased cortical-subcortical connectivity are typical characteristics of schizophrenia (Calhoun et al., 2008; Hoptman et al., 2010; Salvador et al., 2010). However, there is still a lack of focus on the relationships between these two major types of brain abnormalities in schizophrenia. We hypothesize that brain activity and connectivity might be co-affected in schizophrenia because of their co-evolutionary relationships in time. By using two independent datasets and two different approaches (ICA and atlas-based) for time-courses extraction, we identified consistent brain states with high correspondent ALFF and FNC patterns. Results provide statistical support for the linkage between brain activity abnormalities and connectivity abnormalities. High ALFF is typically accompanied with strong connectivity within sensory regions and more antagonism between subcortical and sensory regions, forming a “strong” brain activity-connectivity state. We found that SZ patients spend less time in these strong states, in line with previous dynamic ALFF studies (Fu et al., 2018) and dynamic FNC studies (Damaraju et al., 2014; Rashid et al., 2014) showing that schizophrenia has fewer occurrences in strong ALFF states and FNC states respectively. In contrast, SZ patients spend more time in weak brain states, with sparsely connected FNC and low ALFF. There is a nearly universal acceptance that schizophrenia patients might have an exaggerated focus on self by blurring the boundary between internal and external realities (Lysaker and Lysaker, 2002; Sass and Parnas, 2003). Recent literature has suggested an association between self-focused thought and the brain state with weak and diffused connectivity (Marusak et al., 2017). We argue that exaggerated self-focus in schizophrenia might be relevant to the increased occurrence of weak brain states. Our result of the negative correlation between attention performance and the fraction rate of state 2, a weak state with sparse FNC and low ALFF provides further evidence supporting our hypothesis. The brain of schizophrenia patients has more occurrences of weak brain states due to the hyperreflectivity, a condition with more self-focusing, and in this sense experienced as part of the self, come instead to be taken as objects of focal or objectifying awareness, resulting deficits in external attention (Sass and Parnas, 2003).
Our results also partially explain the inconsistent findings in previous studies based on the static assumption (Hoptman et al., 2010; Huang et al., 2010; Yu et al., 2014). Compared with HCs, SZ patients might not have constantly abnormal brain activity and connectivity through the whole scan. The observed static brain abnormalities in SZ might be because patients spend different time in the same strong and weak brain states. This speculation is further supported by dynamic network efficiency analysis. Complex brain networks show disrupted small-world properties and thus less efficiency in patients with schizophrenia (Bassett et al., 2008; Liu et al., 2008). In this study, we found that brain states show significantly different network efficiency and only high-efficiency states show abnormal efficiency in schizophrenia. Combining the statistic results on state occurrence, these findings indicate that disruption of schizophrenia brain networks might be due to 1) less occurrence in high-efficiency states; 2) decreased efficiency in the high-efficiency states, challenging the previous constant changes based on the static assumption.
More interestingly, the nodes with decreased local efficiency are within the thalamus, a key sensory gate that receives and delivers sensory information among cortical and subcortical regions (Steriade and Llinas, 1988; Sherman and Guillery, 2018). Abnormalities in the thalamus and its connections to the cortical cortex can explain a wide range of symptoms in schizophrenia (Andreasen, 1997). The most consistent abnormalities in the thalamus are ventricular enlargement (Konick and Friedman, 2001) and reductions of brain volume and weight (Steen et al., 2006). Our results might also reflect a hidden relationship between structural abnormalities and functional abnormalities. That is, during the “strong” brain states, when there is a requirement of strong low-frequency fluctuations, the decreased volume of thalamus results in restricted neural activation which might further influence its information transfer to the other brain regions, reflecting by decrease local efficiency in the thalamus. In contrast, during the “weak” brain states, thalamus with pathological changes still has enough volume to exhibit moderate or weak low-frequency fluctuations, so that its local efficiency does not change in schizophrenia.
Method and Materials
Participants and Cognitive Performance Assessment
Two independent cohorts of subjects are used in this study (Table I). Written informed consent is obtained from all participants under protocols approved by the Institutional Review Board (IRB). The discovery cohort is from the Function Biomedical Informatics Research Network (FBIRN). 160 healthy controls (HCs) and 151 patients with schizophrenia (SZs) are selected from this dataset. The replication cohort is from the Centers of Biomedical Research Excellence (COBRE). 89 HCs and 68 SZs from this dataset are used in the analysis. Most of the participants are measured by a neuropsychological battery that includes six neurocognitive domain tests (speed processing, attention/vigilance, working memory, verbal learning, visual learning, and problem solving) called CMINDS (van Erp et al., 2015). Details of the dataset and subject selection criteria are provided in the supplementary materials.
Table 1.
Demographics and cognitive scores of FBIRN and COBRE datasets
| Characteristics | FBIRN |
COBRE |
||
|---|---|---|---|---|
| SZ (n=151) | HCs (n=160) | SZ (n=68) | HCs (n=89) | |
| Age (years) | 38.77 ± 11.63 | 37.04 ± 10.86 | 37.79 ± 14.45 | 38.09 ± 11.67 |
| Gender (female/male) | 45/115 | 36/115 | 11/57 | 25/64 |
| Speed of processing | −1.34±1.08 | 0.06±1.02 | 34.97±11.77 | 53.81±8.62 |
| Attention | −1.36±1.40 | 0.03±0.99 | 37.46±13.20 | 49.55±9.09 |
| Working memory | −1.16±1.08 | 0.05±0.97 | 39.28±12.62 | 48.92±10.67 |
| Verbal learning | −1.38±1.19 | 0.04±1.00 | 38.66±8.78 | 45.53±8.32 |
| Visual learning | −1.09±1.14 | 0.03±0.99 | 35.37±12.70 | 46.02±10.71 |
| Problem solving | −0.85±1.22 | 0.04±0.97 | 44.19±10.80 | 55.59±8.06 |
Image Preprocessing
For the analysis based on the ICA parcellation, the fMRI data were preprocessed through general preprocessing pipelines including slice-timing, realignment, normalization, and smoothing. For the analysis based on the atlas parcellation, besides the above-mentioned procedures, two additional steps including filtering and removing confounding covariates were performed for noise removal. Detailed preprocessing steps can be found in the supplementary materials.
Flowchart of Capturing Dynamic Brain Activity-connectivity States
Flowchart of capturing dynamic brain activity-connectivity states is provided in the supplementary materials Figure S1. The Neuromark pipeline was first used to calculate spatial maps and TCs for FBIRN and COBRE datasets. Via using the Neuromark framework, the targeted ICs of discovery and replication datasets are highly corresponding, leveraging the feasibility of comparing results across independent datasets. The AAL atlas was also used to extract TCs for validating the results on the ROI-based analysis. A sliding window approach is used to estimate dALFF and dFNC. Then k-means clustering is performed on the concatenated dALFF and dFNC features. State occurrences are calculated, and graphic measure analysis is performed in a state-based manner.
Dynamic ALFF and FNC
For each subject, dALFF and dFNC were estimated with a sliding window approach with window size 20 TRs. ALFF of each windowed ICN TC was estimated using the REST toolbox (Zang et al., 2007) and FNC between windowed ICN TCs was estimated using the GIFT toolbox (http://trendscenter.org/software/gift/). K-means clustering was conducted on the concatenated dynamic activity-connectivity features to identify the centroids of the dynamic states for FBIRN and COBRE datasets respectively. We matched the dynamics states from two datasets by evaluating the averaged correlation coefficients between their FNC and ALFF centroid patterns. We introduced a permutation test to further validate the co-existed brain activity-connectivity patterns. For each dataset, the dALFF and dFNC were randomly permuted across windows. Then k-mean clustering was performed on the permuted dALFF and dFNC and the identified states were matched using the same criteria. We calculated the correlation between ALFF for each pair of matched states for the permutated data and examined whether the original correlations between ALFF of matched states are significantly larger than the correlations between ALFF of permutated data.
Statistical comparisons between HCs and SZ were performed on the FRA and MDT of the identified dynamic activity-connectivity states via a general linear model (GLM), controlling the age and gender effects. We further investigated whether the abnormal dynamic features could index cognitive impairments via GLM, controlling age, gender and diagnosis.
Dynamic Graphic Measures
To explore the information transfer efficiency of brain networks and their potential associations with local network activities, we applied a graph theory measure to the dynamic brain activity-connectivity states. Local efficiency of ICNs was estimated based on the sliding-window weighted FNC matrix using the brain connectivity toolbox (BCT, https://sites.google.com/site/bctnet/) and then averaged across windows within each dynamic state. The average local efficiency (across ICNs) of brain states was calculated and compared between states. Then we investigated the potential relationships between the local efficiency and the ALFF for each state. Finally, we examined the local efficiency difference between HCs and SZ within each state using GLM, controlling age and gender.
Supplementary Material
Reference
- Abou Elseoud A, Nissilä J, Liettu A, Remes J, Jokelainen J, Takala T, et al. Altered resting-state activity in seasonal affective disorder. Hum Brain Mapp 2014; 35: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Eichele T, Wu L, Calhoun VD. EEG Signatures of Dynamic Functional Network Connectivity States. Brain Topogr 2018; 31: 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 2014; 24: 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry 1997; 42: 27–33. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®) [Internet]. 2013. [cited 2019 Aug 21] Available from: https://books.google.com/books?hl=zh-CN&lr=&id=-JivBAAAQBAJ&oi=fnd&pg=PT18&dq=DSM-5&ots=ceTQ27LJt7&sig=OQsEb5dPOxvlw5C6FS6SGMxixyM
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and Schizophrenia. J Neurosci 2008; 28: 9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Yang M, Wymbs NF, Grafton ST. Learning-induced autonomy of sensorimotor systems. Nat Neurosci 2015; 18: 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B Biol Sci 2005; 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med 1995; 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 2010; 107: 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RWJ, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: Anomalies in the default network. Schizophr Bull 2007; 33: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Buzsáki G Rhythms of the Brain. 2009 [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp 2008; 29: 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 2010; 50: 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin 2014; 5: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Fu Z, Chan SC, Hung YS, Biswal BB, Zhang Z. Task-related functional connectivity dynamics in a block-designed visual experiment. Front Hum Neurosci 2015; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front Hum Neurosci 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez-Cirarda M, Strafella AP, Kim J, Peña J, Ojeda N, Cabrera-Zubizarreta A, et al. Dynamic functional connectivity in Parkinson’s disease patients with mild cognitive impairment and normal cognition. Neuroimage Clin 2018; 17: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fryer SL, Fu Z, Lin D, Sui J, Chen J, et al. Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. Neuroimage 2018; 180: 632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TGM, Preda A, Turner JA, Callahan S, Calhoun VD, Bustillo JR, et al. Neuropsychological profile in adult schizophrenia measured with the CMINDS. Psychiatry Res 2015; 230: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci U S A 2012; 109: 12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Caprihan A, Chen J, Du Y, Adair JC, Sui J, et al. Altered static and dynamic functional network connectivity in Alzheimer’s disease and subcortical ischemic vascular disease: shared and specific brain connectivity abnormalities. Hum Brain Mapp 2019; 40: 3203–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Tu Y, Di X, Biswal BB, Calhoun VD, Zhang Z. Associations between functional connectivity dynamics and BOLD dynamics are heterogeneous across brain networks. Front Hum Neurosci 2017; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Tu Y, Di X, Du Y, Pearlson GD, Turner JA, et al. Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: An application to schizophrenia. Neuroimage 2018; 180: 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant ‘default mode’ functional connectivity in schizophrenia. Am J Psychiatry 2007; 164: 450–457. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 2003; 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A 2004; 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T, Blasius B. Adaptive coevolutionary networks: A review. J R Soc Interface 2008; 5: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ, Feinstein DI, Palmer RG. ‘Unlearning’ has a stabilizing effect in collective memories. Nature 1983; 304: 158–159. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D’Angelo D, Mauro CJ, et al. Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophr Res 2010; 117: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XQ, Lui S, Deng W, Chan RCK, Wu QZ, Jiang LJ, et al. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage 2010; 49: 2901–2906. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 2013; 80: 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 2013; 34: 2154–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Criaud M, Cho SS, Díez-Cirarda M, Mihaescu A, Coakeley S, et al. Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain 2017; 140: 2955–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Jauhiainen J, Tervonen O, Pääkkö E, Oikarinen J, Vainionpää V, et al. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med 2000; 44: 373–378. [DOI] [PubMed] [Google Scholar]
- Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, et al. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2014; 264: 111–119. [DOI] [PubMed] [Google Scholar]
- Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry 2001; 49: 28–38. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Past, present and future of topographic mapping. Brain Topogr 1990; 3: 191–202. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Strik WK, Henggeler B, Koenig T, Koukkou M. Brain electric microstates and momentary conscious mind states as building blocks of spontaneous thinking: I. Visual imagery and abstract thoughts. Int J Psychophysiol 1998; 29: 1–11. [DOI] [PubMed] [Google Scholar]
- Liao W, Chen H, Li J, Ji GJ, Wu GR, Long Z, et al. Endless Fluctuations: Temporal Dynamics of the Amplitude of Low Frequency Fluctuations. IEEE Trans Med Imaging 2019; 38: 2523–2532. [DOI] [PubMed] [Google Scholar]
- Liu X, Duyn JH. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A 2013; 110: 4392–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain 2008; 131: 945–961. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci 2010; 30: 9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker PH, Lysaker JT. Narrative Structure in Psychosis: Schizophrenia and Disruptions in the Dialogical Self. Theory Psychol 2002; 12: 207–220. [Google Scholar]
- Makeig S, Jung TP. Changes in alertness are a principal component of variance in the EEG spectrum. Neuroreport 1995; 7: 213–216. [PubMed] [Google Scholar]
- von der Malsburg C, Phillips W, Singer W. Dynamic coordination in the brain: from neurons to mind [Internet]. 2010[cited 2019 Aug 20] Available from: https://www.google.com/books?hl=zh-CN&lr=&id=Z95zWxii88kC&oi=fnd&pg=PR5&dq=dynamic+coordination+in+the+brain+&ots=n0mVWplTcW&sig=CnkcKtGCZfPSEJtpvG3ouqPB8sU [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull 2014; 40: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH, et al. Dynamic functional connectivity of neurocognitive networks in children. Hum Brain Mapp 2017; 38: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash LE, Linke AC, Olson LA, Fishman I, Liu TT, Müller RA. Transient states of network connectivity are atypical in autism: A dynamic functional connectivity study. Hum Brain Mapp 2019; 40: 2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy M, Larson-Prior L, Nolan TS, Vaishnavi SN, Raichle ME, D’Avossa G. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J Neurophysiol 2008; 100: 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea [Internet]. 2007. Available from: www.elsevier.com/locate/ynimg [DOI] [PubMed] [Google Scholar]
- Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci 2014; 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoğlu Ü, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: Application to schizophrenia. Magn Reson Mater Physics, Biol Med 2010; 23: 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Sarró S, Gomar JJ, Ortiz-Gil J, Vila F, Capdevila A, et al. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Hum Brain Mapp 2010; 31: 2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass LA, Parnas J. Schizophrenia, Consciousness and the Self. Schizophr Bull 2003; 29: 427–444. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and Its Role in Cortical Function [Internet]. 2018[cited 2019 Sep 4] Available from: https://psycnet.apa.org/record/2006-09311-000 [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, et al. Brain Connectivity Is Not Only Lower but Different in Schizophrenia: A Combined Anatomical and Functional Approach. Biol Psychiatry 2010; 68: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: Systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 2006; 188: 510–518. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 1988; 68: 649–742. [DOI] [PubMed] [Google Scholar]
- Sui J, Qi S, van Erp TGM, Bustillo J, Jiang R, Lin D, et al. Multimodal neuromarkers in schizophrenia via cognition-guided MRI fusion. Nat Commun 2018; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Fu Z, Zeng F, Maleki N, Lan L, Li Z, et al. Abnormal thalamocortical network dynamics in migraine. Neurology 2019; 92: e2706–e2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Damaraju E, Van Erp TGM, Mathalon DH, Ford JM, Voyvodic J, et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Eichele T, Calhoun VD. Reactivity of hemodynamic responses and functional connectivity to different states of alpha synchrony: A concurrent EEG-fMRI study. Neuroimage 2010; 52: 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaesoubi M, Allen EA, Miller RL, Calhoun VD. Dynamic coherence analysis of resting fMRI data to jointly capture state-based phase, frequency, and time-domain information. Neuroimage 2015; 120: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Yang Z, Colcombe SJ, Zuo XN, Milham MP. Concordance among indices of intrinsic brain function: Insights from inter-individual variation and temporal dynamics. Sci Bull 2017; 62: 1572–1584. [DOI] [PubMed] [Google Scholar]
- Yang H, Long X-Y, Yang Y, Yan H, Zhu C-Z, Zhou X-P, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI [Internet]. 2007. Available from: www.elsevier.com/locate/ynimg [DOI] [PubMed] [Google Scholar]
- Yu R, Chien YL, Wang HLS, Liu CM, Liu CC, Hwang TJ, et al. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum Brain Mapp 2014; 35: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Cocchi L, Gollo LL, Breakspear M. Time-resolved resting-state brain networks. Proc Natl Acad Sci U S A 2014; 111: 10341–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y-F, He Y, Zhu C-Z, Cao Q-J, Sui M-Q, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 2007; 29: 83–91. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Jiang XL, Cheng Z, Zhou Y, Xu Q, Zhang ZQ, et al. Decreased Coupling Between Functional Connectivity Density and Amplitude of Low Frequency Fluctuation in Non-Neuropsychiatric Systemic Lupus Erythematosus: a Resting-Stage Functional MRI Study. Mol Neurobiol 2017; 54: 5225–5235. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu Q, Liao W, Wang Z, Li Q, Yang F, et al. Pathological uncoupling between amplitude and connectivity of brain fluctuations in epilepsy. Hum Brain Mapp 2015; 36: 2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.