Abstract
In this study, we examined how emotional arousal interacts with hunger states and the processing of food stimuli. In general, arousal enhances the processing of high-priority information at the expense of lower priority information (Mather & Sutherland, 2011). Because food has been a biologically relevant stimulus in primates throughout evolution, detecting it in the environment and remembering its location has high priority. In our study, inducing arousal enhanced attention to subsequent food stimuli. In addition, we manipulated whether participants were hungry or sated in order to examine how hunger states would influence emotional processing. Previous research reveals that being hungry is associated with increases in norepinephrine, a key neurotransmitter involved in the arousal response. We found that, when sated, participants showed greater pupil dilation to emotional than neutral stimuli. In contrast, when hungry, pupil dilation responses were as strong to neutral as to emotional stimuli. Thus, when hungry, participants were less effective at differentiating the intensity of arousal responses to emotional versus neutral stimuli due to high arousal responses to neutral stimuli. Memory for food stimuli was enhanced compared with memory for nonfood stimuli for all participants, but especially for hungry participants.
Keywords: attention, arousal, goal relevance, hunger, memory
When people are hungry, attention shifts: Food stands out more and seems more attractive (Kringelbach, O’Doherty, Rolls, & Andrews, 2003; Mohanty, Gitelman, Small, & Mesulam, 2008). The attentional effects of being hungry may, however, go beyond focusing on food. In particular, the hormones related to hunger state may increase arousal to maintain a vigilant state (Sakurai, 2014; Soya et al., 2017). For instance, previous studies indicate that orexin released during fasting states activates the locus coerulus-noradrenergic system, which in turn influences arousal level (Soya et al., 2017) and modulates cognition (Markovic, Anderson, & Todd, 2014; Mather, Clewett, Sakaki, &Harley, 2015; Sakurai, 2007; 2014; Soya et al., 2017). In this study, we examined how being hungry influences attentional and memory processes differently depending on the salience of the stimuli.
Many studies indicate that attention and memory are influenced by emotional stimuli (e.g., Knight et al., 2007; Mather, 2007). One challenge in studying the impact of emotion is that emotional stimuli vary on factors that also influence attention and memory, such as stimulus distinctiveness and goal relevance (Brosch, Sander, Pourtois, & Scherer, 2008; Levine & Pizarro, 2006; Montagrin, Brosch, & Sander, 2013; Montagrin & Sander, 2016; Montagrin et al., 2018; Talmi, Luk, McGarry, & Moscovitch, 2007).
To avoid such confounds and show that emotional stimuli modify the nature of attention and memory, one can examine how they influence cognitive processes for other stimuli that are not themselves inherently emotional (for further discussion, see Mather & Sutherland, 2011; Montagrin & Sander, 2016). Doing this reveals an interesting dissociation in which emotional stimuli tend to enhance the processing of subsequent salient stimuli, while impairing the processing of subsequent nonsalient stimuli (Lee, Itti, & Mather, 2012; Lee, Sakaki, Cheng, Velasco, & Mather, 2014; Sutherland & Mather, 2012, 2015). For instance, when participants were briefly shown a circular array of letters that varied in visual salience (i.e., they were printed in either a dark, high salience, or light, low salience, shade of gray against a white background), they were more likely to report the high-contrast (i.e., dark gray) letters than the low-contrast (i.e., light gray) letters. However, when an emotionally arousing sound was presented a few seconds before the visual array of letters, the attentional preference for the high-salience letters was amplified (Sutherland & Mather, 2012; Sutherland & Mather, 2018). Thus, emotional arousal increases the impact of perceptual salience. The arousal biased competition model (ABC model; Mather & Sutherland, 2011) posits that this is one aspect of a broader phenomenon in which arousal increases the processing of high-priority information, while impairing the processing of low-priority information. Priority can be determined by bottom-up salience, as in the experiment with light and dark gray letters, by top-down goals (e.g., Sakaki, Fryer, & Mather, 2014), or by a combination of these factors (Ponzio & Mather, 2014). In addition, priority can be influenced by other factors such as affective salience or relevance (Markovic et al., 2014; Montagrin et al., 2013; Montagrin et al., 2018; Pool, Brosch, Delplanque, & Sander, 2014). For instance, initially neutral stimuli (e.g., the image of a chair) that were goal relevant for a given task were better remembered in a subsequent memory test than were neutral stimuli that had been irrelevant for task success (Montagrin et al., 2013). Along the same lines, an attentional study showed that neutral stimuli associated with chocolate odor were likely to enhance attention in people who like chocolate, but the effect was no longer observed when people were specifically sated for chocolate (Pool et al., 2014). More generally, the relevance of food stimuli increases as hunger increases. In addition to the influence of relevant food stimuli on attention, studies have found that food stimuli are better remembered than nonfood stimuli among hungry people (Morris & Dolan, 2001; Talmi et al., 2013).
Food has been a biologically relevant stimulus in primates throughout evolution. Thus, being able to detect food stimuli and remembering the location in the environment is an adapted behavior, especially when one is hungry. The ABC model hypothesizes that arousal enhances prioritized information at the cost of non-prioritized information, leading to “winner-take-more” and “loser-take-less” effects (Mather & Sutherland, 2011). Thus, experiencing emotional arousal should enhance the processing of relevant food stimuli compared with the processing of lower priority stimuli that are unrelated to food.
The release of noradrenaline induced by an emotionally arousing event is likely to be a key mechanism that enhances high-priority representations and suppresses low-priority representations (Mather et al., 2015). A key factor influencing tonic noradrenaline level is the level of orexin hormones that regulate wakefulness and arousal to seek for food when hungry (Yamanaka et al., 2003). Interestingly, narcolepsy patients who lack orexin hormones show impairment in response to emotional stimuli (Ponz 2010a; 2010b). Thus, given the key role played by norepinephrine in how arousal influences attention and memory selectivity (Mather et al., 2015), hunger level seems likely to modulate the impact of arousing stimuli. The interaction between the noradrenergic system and fasting state on cognitive processing raises interesting questions. We can, for instance, ask whether better memory performance for food stimuli compared with nonfood stimuli in hungry participants is linked to arousal induced by being hungry or/and from the emotional content of food stimuli for hungry participants (Goldstone et al., 2009; Morris & Dolan, 2001; Pool et al., 2014; Talmi et al., 2013).
The effect of hunger on people has been studied for years but little is known about, (a) how being hungry might influence emotional responsivity to neutral and emotional stimuli, (b) how emotional arousal induced by external stimuli can influence attentional and memory processing of relevant stimuli (food), whether this relationship is modulated by hunger level (e.g., additive arousal effect to hungry state), and (c) whether the hunger level when one first encounters an emotional or neutral sound has a lasting influence on how it is assessed, as reflected in ratings 48 hours later. Indeed, in one study (Montagrin et al., 2013), the ratings of initially neutral items associated with emotions at encoding reflected this associated emotional value both immediately and 24 hours later. Thus, the hunger state at the time one first experiences a stimulus might also influence later ratings of those stimuli.
Our study examined these three questions. We compared the effect of emotional arousal among participants in hungry and sated states on the processing of food and household stimuli that compete against one another. Participants performed an adjusted dot-probe task consisting of the detection of a black dot placed on either a food or a household image, which were presented together in pairs. We attempted to manipulate arousal using two different independent variables, (a) induction of hunger, and (b) presentation of emotional (positive and negative) or neutral sounds shortly before the presentation of image pairs. Thus, the external emotional input might shed new light on a potential additive arousal effect to attentional and memory processing when hungry.
To address our first question about how hunger affects emotional and neutral responsivity to stimuli, we measured pupil dilation during the emotional and neutral sounds presented in each trial. Pupil diameter is a reliable psychophysiological measure of emotional arousal (Bradley, Miccoli, Escrig, & Lang, 2008) and is associated with activity in the locus coeruleus, the main source of brain norepinephrine (Joshi, Li, Kalwani, & Gold, 2016; Murphy, O’Connell, O’Sullivan, Robertson, & Balsters, 2014).
To test our second question regarding whether emotionally arousing sounds would enhance attentional and memory processing of high-priority stimuli, that is, food as compared with household stimuli for people in a hungry state, we examined whether attention and memory for inherently high-priority stimuli (i.e., food images) compared with low-priority stimuli (i.e., household images) would be enhanced by emotionally arousing sounds and likely neutral sounds. While being hungry should activate arousal, and increase the relevance for food stimuli (regardless of the external emotional stimuli), being sated should lower arousal, and decrease the current relevance for food stimuli. Because we aimed to investigate whether arousal selectivity is mediated by hunger level specifically for food stimuli, we examined the effect of emotional and non-emotional stimuli on high-priority stimuli which are not biologically relevant in hungry and sated participants. More specifically, we tested whether task-relevant stimuli, compared with task-irrelevant stimuli, would show additional benefits on attention and memory when presented after emotional, but also neutral sounds and whether this effect is mediated by hunger level. To make one picture in the presentation pair more task relevant than the other, we had a target dot appear on one of the pictures. By adding this level to the task, we aimed to disentangle the effect of being hungry and biological relevance (food) on attentional and memory processing. With these manipulations, we could examine whether emotional, but also neutral sounds would selectively enhance memory for stimuli that are relevant for the task (target) in addition to their biological relevance (food), and whether this effect is mediated by hunger level.
Finally, to address our third question, whether hunger level when participants first encounter an emotional or neutral sound has a lasting influence on how it is assessed, as reflected in ratings 48 hours later, we asked participants to rate emotional and neutral sounds when they returned for Session 2.
Method
Participants
Seventy-four University of Southern California undergraduate students and volunteers (53 women, 21 men; M = 20.78 years, SD = 3.05; age calculated for 72 participants) gave their written informed consent to participate in a two-session study and were paid or compensated with course credits (data exclusions are detailed in the results section). We based our sample size on previous independent studies using a similar paradigm with a between-subjects design and similar constraints (i.e., participants asked to refrain from consuming anything other than water in the 7–11 hours preceding the experiment and requested to come in twice to the laboratory; Talmi et al., 2013; Montagrin et al., 2013).
Stimuli Materials
Images.
Stimuli were selected from the Internet to create a homogenous set of images depicting simple household and food items. Photoshop CS4 was used to remove backgrounds in order to have a single item on a gray background.
Post-hoc analyses1 were conducted to compare aesthetic features between food and household stimuli. To test whether food stimuli were more colorful than household stimuli, we extracted the number of green, red and blue pixels for each images (following the procedure outlined in Delplanque, N’Diaye, Scherer, and Grandjean, 2007). T-tests revealed that blue pixels were greater for household stimuli (M = 124.99, SD = 10.11) than for food stimuli (M = 118.80, SD = 6.09), t(142) = −4.45, p < .001,. 95% CI [−8.94, 3.44], d = .74, no significant differences were found for green (household: M =126.30, SD = 9.57; food: M = 123.83, SD = 6.30, t(142) = −1.83, p = .07, d = .30) and red pixels, (household: M =128.50, SD = 9.64; food: M = 130.29, SD = 6.92, t(142) = 1.27, p = .20, d = .21). To examine whether food and household stimuli are processed differently in terms of affective dimensions (see Blechert, Meule, Busch, & Ohla, 2014), we conducted a post-hoc evaluation with 47 independent raters (17 men and 30 women, undergraduate students and volunteers from University of Southern California), aged between 18 and 40 years old (M = 20.00; SD = 3.31). The raters assessed by using continuous scales their hunger level, ranging from 0 (not at all hungry) to 10 (Extremely hungry), and the affective dimensions of arousal (named activation in the survey for more clarity) ranging from 0 (not at all activating) to 100 (extremely activating), valence ranging from −50, (negative) to 100 (positive), attractiveness ranging from 0 (not at all attractive) to 100 (very attractive) for 144 images (72 food and 72 household). Seventy-two food images were rated on two additional dimensions specific to food category, such as palatability from 0 (not at all palatable) to 100 (very palatable), and craving (named desirability in the survey for more clarity) from 0 (you don’t would like at all to eat this item right now if it was in front of you) to 100 (you would like extremely to eat this items right now if it was in front of you) for 72 food images.
Sounds.
Sixty-five sounds were selected from the International Affective Digital Sounds System (IADS; Bradley & Lang, 2007). In addition, because the number of positive sounds from the IADS was not sufficient, we created seven of them with the application SoundFlower. To do so, we obtained different kinds of sounds (music played before American football at the University of Southern California, famous music played by a band, the laugh of a baby, etc.) from the Internet. The 72 sound stimuli (24 positive, 24 negative, and 24 neutral stimuli) were edited with Audacity 2.0.4 to last 4 s and to maintain the meaning of the ecological sound. Decibel levels were adjusted to yield similar levels across stimuli within the same conditions. We also diminished the decibel level for the neutral sounds, as loud noise is in itself arousing (Ilie & Thompson, 2006) and we wanted to maximize the arousal differences between neutral and arousing sound sets. The emotionally arousing sounds were between 33 and 35 decibels louder than the neutral sounds. The sound was adjusted for each participant to be certain that the volume was not too loud or too soft.
Procedure
For the first session, participants were asked not to eat or to consume liquids (including coffee, but excluding water) after 9:00 a.m. until their appointment time (i.e., from 4:00 p.m. to 8:00 p.m.). They were informed that they would receive food upon participation. Depending on the condition, either they ate a regular turkey and cheese sandwich (from Togos, 550 calories) before completing the experimental tasks, or they received a granola bar and almonds after having performed the tasks. The eating restriction was in place only for the first session; before the second session, participants could eat and drink as usual and they did not receive food during the session.
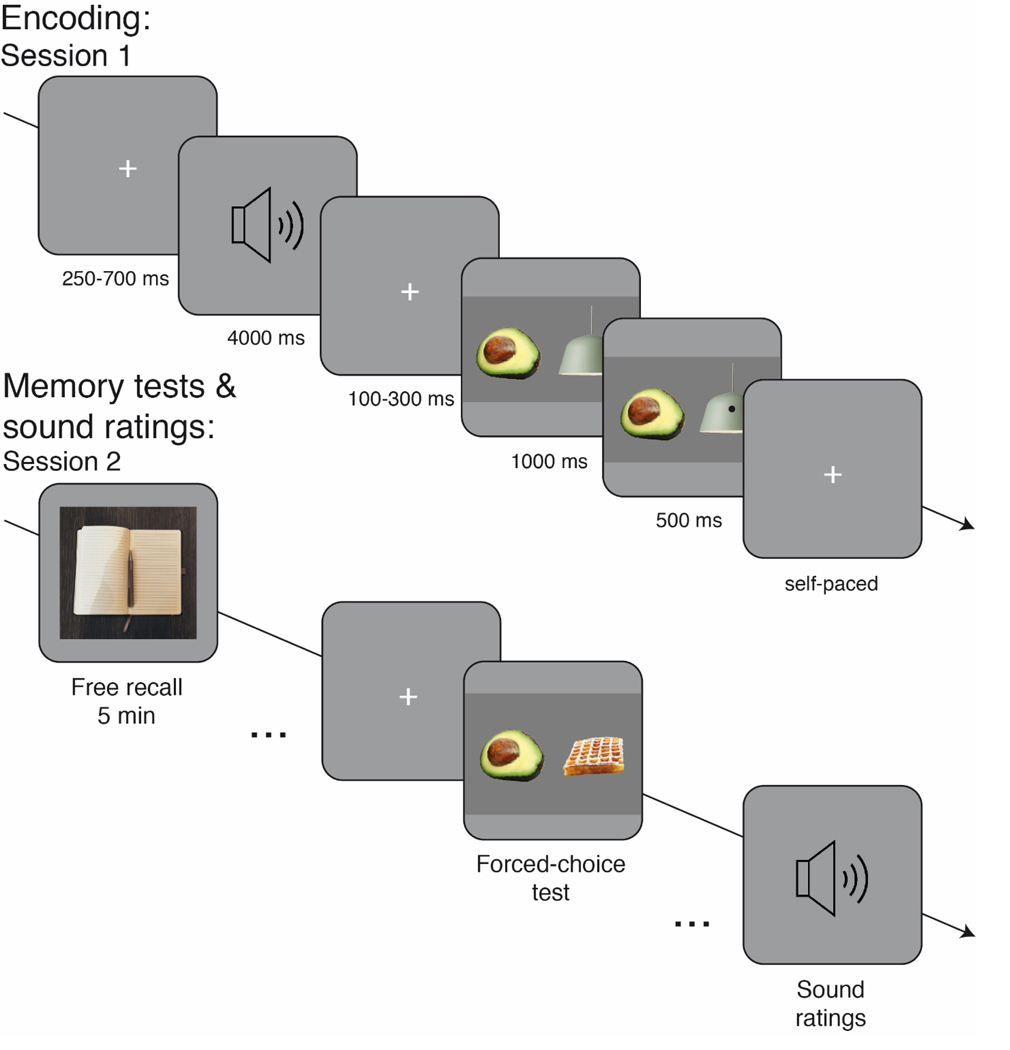
We constructed three lists of 72 trials such that every pair of images appeared with positive, negative, and neutral sounds across participants. Each trial (see details in Figure 1) consisted of the presentation of two images (i.e., food and household items) paired with a sound (i.e., positive, negative, and neutral). Each list comprised 72 food and 72 household items paired with 48 highly arousing sounds (i.e., 24 negative and 24 positive) and 24 neutral sounds from the IADS (Bradley & Lang, 2007).
Figure 1.
Schematic representations of the dot-probe task from Session 1 and memory tests and sound rating trials from Session 2. See the online article for the color version of this figure.
Session 1:
The task in Session 1 was an adjusted dot-probe task (e.g., Mather & Carstensen, 2003). During each trial, a 4-s sound sequence was presented via headphones. This arousing negative, arousing positive, or neutral sound sequence was followed by a brief interstimulus interval (100–300 ms) and then the simultaneous presentation of two images (i.e., one food and one household image; e.g., music box and guacamole, bottle spray and ice cream; keys and spaghetti; towel and peanut butter, etc., see appendix for full list of pair of images presented (Appendix A) and food and household images (Appendix B) for 1,000 ms without the presentation of a dot. Next, the dot appeared on one of the images for 500 ms. The participants’ task was to press the space bar as quickly as possible when the dot appeared. Then there was an intertrial interval (250–700 ms).
Glucose test.
At the end of the 72 trials, the participants were asked to complete a self-administrated blood glucose finger prick with a single-use lancet (Acti-Lance), using a blood glucose monitoring device (OneTouch Ultra 2).
Pupillometry.
Eye tracking was tracked using iView X RED eye-tracking software at a sampling rate of 60 Hz for participants 1 to 20, and 120 Hz for participants 21 to 74 (SensoMotoric Instruments). Fixation and pupil dilation data for each participant were exported with the eye-tracking analysis software program BeGaze 2 (SensoMotoric Instruments). Task events were labeled by using the iView X SDK package for E-prime.
Session 2:
Participants came back to the laboratory 48 hours later without any food consumption restrictions. They were asked to perform an incidental free recall and forced-choice recognition task with the images seen in Session 1. After completing these tasks, the participants were asked to listen to the sound stimuli presented in Session 1 via headphones and to rate them (see Supplemental Material for method and results). Finally, glucose level was measured with a finger prick (using the same procedure as in Session 1).
Free recall test.
The participants were asked to remember the task session from 48 hours earlier in the laboratory (i.e., detecting the dot on the images). We then gave them 5 min to write descriptions of as many images as they could recall from that session. An item was categorized as correctly recalled if the description of the image unequivocally matched the image that was presented. Two judges who were blind to the participant’s condition separately scored all items. Agreement was 67% and mismatches were resolved via discussion.
Forced-choice recognition task.
Two stimuli from the same category (i.e., two food or two household items) were presented on the screen. One of the stimuli was seen 48 hr earlier and the other one was a novel distractor stimulus. The participant’s task was to decide which of the two stimuli was seen during Session 1.
Sound ratings.
After the free recall and the recognition task, participants listened to and evaluated the sound stimuli previously heard on Day 1. We asked the participants about three different affective dimensions: (a) valence (i.e., How positive, negative, or neutral is the sound?) on a continuous scale ranging from 1 (negative) to 8 (positive) with a median neutral position; (b) arousal (i.e., How exciting or not exciting is the sound?) on a continuous scale from 1 (not at all) to 8 (very); and (c) relevance (i.e., How important is the sound for needs or survival?) on a continuous scale from 1 (not at all) to 8 (very much).
Results
Data Exclusions
Seventy-four participants signed up for this two-session study. Sixty-five participants met the primary criteria to be included in our analyses. Nine participants were excluded for the following reasons. Two participants did not return for the memory test, which took place 48 hours after the first session. One participant was excluded for not following task instructions. Based on information from the U.S. National Library of Medicine National Institutes of Health, a normal level of glucose in a fasted state is between 70 and 100 mg/dl (Wisse, Zieve, & Ogilvie, 2014). Thus, three hungry condition participants were excluded for having a level above 100 mg/dl in the first session, as this suggests they ate despite instructions not to do so. Two additional participants from the hungry condition were excluded because their glucose levels were inconsistent with their self-reports. These participants showed a level of between 94 and 98 mg/dl at the first session and between 100 and 104 mg/dl at the second session, although they reported a high appetite level at the first session and a low appetite level at the second session. One other participant was excluded because it was not possible to obtain sufficient blood to activate the glucose sensor.
For the reaction time (RT) analyses, RTs were not recorded for one participant, and for recall analyses, one person did not complete the recall test, yielding 64 participants for each of these analyses.
For the first 20 participants, we used a 60-Hz eye tracker, after which the system was upgraded to 120 Hz. We excluded the first 10 participants (of whom two were among those excluded for the reasons described earlier) from the analysis because we recorded pupil dilation only when they were listening to the sounds and we did not record a baseline. For the pupillary analyses, we used data obtained both from the 60-Hz and 120-Hz systems (the results showed the same pattern of significant results, however, if we included the data only from the 120-Hz system). In total, 57 participants could be included in the pupil dilation analyses (31 participants in the hungry and 26 in the sated condition).
Manipulation Check for Glucose
As expected, an independent t-test revealed that the two groups had different glucose levels during Session 1 after the encoding task. The participants in a hungry state (M = 83.38, SE = 1.57) had a lower glucose level than did those in a sated state (M = 117, SE = 2.82), t(63) = −10.63, p < .001. In Session 1, across sated and hungry participants, hunger levels and glucose levels were negatively correlated, r(65) = −.69, p < .001.
In addition, as expected, in Session 2, participants who previously completed the Session 1 hungry condition did not have significantly different glucose levels (M = 105.67, SE = 4.12) from those who had completed the Session 1 sated condition (M = 101.06, SE = 2.55), t(63) = 0.92, p = .35.
Pupil Dilation
We computed a baseline average pupil dilation for the 200-ms period prior to each trial onset. We calculated the baselined pupil change score for each trial by subtracting the average baseline pupil diameter from each momentary pupil measurement during the trial duration. We then removed any baselined momentary pupil values greater than three standard deviations from the mean for each trial. For each participant, we next took the maximum baselined momentary pupil value for each trial and calculated an average maximum baselined pupil response for each condition type (emotional, neutral), reflecting the average peak pupil increase for that type of trial.
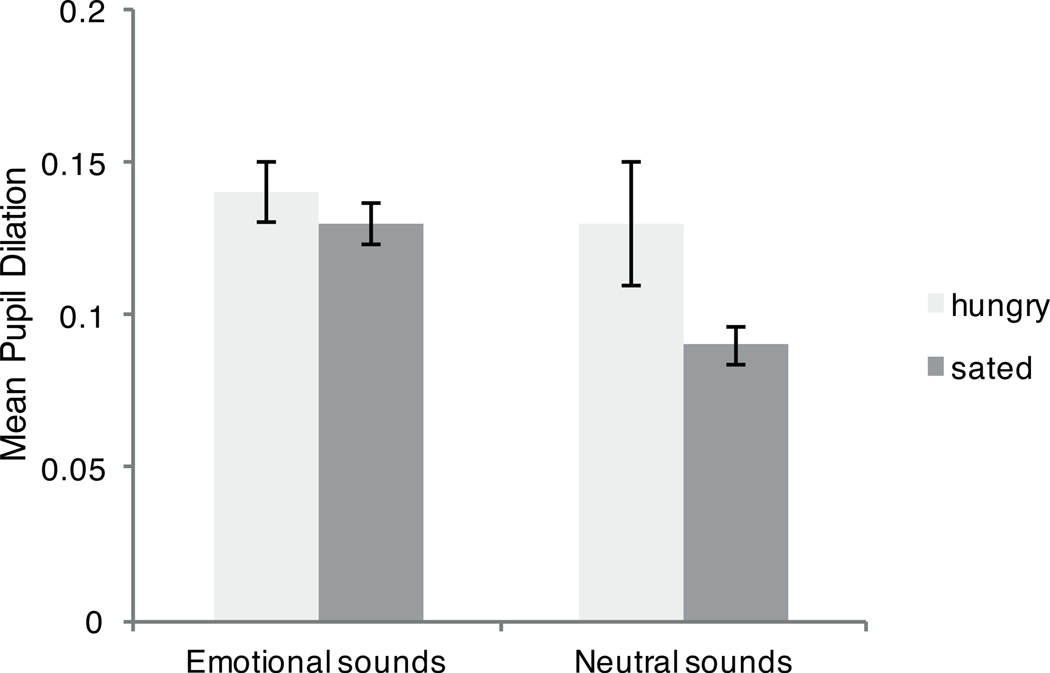
Pupil dilation differed depending on the emotional nature of the sounds, F(1, 55) = 19.89, p < .001, ηp2 = 0.27, (emotional sounds: M = .13, SE = .006; neutral sounds: M = 0.11, SE = .01), and there was a significant interaction between emotion and hunger condition, F(1, 55) = 6.09, p = .02, ηp2 = .10 (Figure 2). Although hungry participants did not show significant differences for pupil dilation during emotional (M = .14, SE = 0.01) compared with neutral sounds (M = .13, SE = .02), t(30) = 1.19, p = .24, mean difference between emotional and neutral pupil dilation M = −.95% CI [−.008, .03], d = 0,12, sated participants showed greater pupil dilation during emotional (M = .13, SE = .007) compared with neutral (M = .09, SE = .006) sounds, t(1, 25) = 7.83, p < .001, mean difference between emotional and neutral pupil dilation M = −.01, 95% CI [−0.03, .01], d = 1.13.
Figure 2.
Mean pupil dilation when participants first hear sounds (error bars represent standard errors of the means).
Reaction Time
We examined whether RT varied across groups and whether they were influenced by the interaction of the hunger condition and the item type. A repeated-measures analysis of variance (ANOVA) was conducted with the between-subjects factor hunger condition (sated/hungry) and the within-subjects factors item type (food/household) and emotion (emotion/neutral), with RTs as the dependent variable. For each individual, RTs from error trials or those with more than three standard deviations and with values below 100 ms were removed.
There was a significant interaction of item type and emotion, F(1, 62) = 8.30, p = .005, ηp2 = .12. For food items, responses varied by emotion condition. Compared with responses to food items on neutral trials (Mneutral = 387.87, SE = 7.4), RTs were faster for food items presented with emotional sounds (Memotional = 367.99, SE = 6.93), t(1, 63) = −2.83, p = .006, mean difference between emotional and neutral sounds for food items M = −19.88 , 95% CI [−33.92, −5.84], d = −0.34. For household items, there were no significant differences by sound emotion type (Mneutral = 372.82, SE = 6.19; Memotional = 381.34, SE = 6.30), t(1, 63) = 1.21, p = 0.23, mean difference between emotional and neutral sounds for household items M = 8.51, 95% CI [−5.51, 22.53], d = .17. Thus, we found that, in general, participants responded faster to food items on emotional trials, as we predicted given that food items are generally highly relevant due to their biological importance.
We also examined whether individual participant’s levels of glucose were associated with average RTs. Within the hungry condition, we found that lower glucose level in Session 1 was associated with faster the RTs for target food items presented with emotional sounds, r(33) = .38, p = .03, and with neutral sounds, r(33) = .51, p < .004. There were no significant correlations between glucose levels and RTs for the other categories (household items for hungry participants, or food or household items for sated participants). Thus, within the hungry condition, having low glucose was associated with faster RTs to food items, regardless of the emotion condition.
Free Recall
A repeated-measures analysis of variance (ANOVA) was conducted with between-subjects factor of condition (sated/hungry) and the within-subjects factors of item type (food/household) and emotion (emotion/neutral), and free recall as the dependent variable. There was a main effect of item type, F(1, 62) = 20.53, p <.001, ηp2 = .25, as food items (M = .84, SE = .07) were better remembered than were household items (M = .50, SE = .05). There was no main effect of emotion or hunger condition for overall recall levels (Fs < 1). However, there was a significant interaction between hunger condition and item type, F(1, 62) = 7.24, p < .01, ηp2 = .10. Planned contrasts indicated that hungry participants remembered food stimuli better than sated participants did (Mhungry = 0.97, SE = .11; Msated = .69, SE = .09), t(1, 62) = −2.01, p < .05, mean difference between sated and hungry participants for food items M = .28, 95% CI [−.56, −.002], d = −.50, and they remembered household stimuli slightly (although not significantly) worse than sated participants did (Mhungry = 0.41, SE = .06; Msated = .55, SE = .07), t(1, 62) = 1.42, p = .16, mean difference between sated and hungry participants for house items M = −.14, .95% CI [−.05, .32], d = .37.
We also examined whether individual glucose levels were related to recall performance. We found that the lower the glucose level was in Session 1, the better the participants recalled food items presented with emotional sounds, r(64) = −.28, p = .03, or neutral sounds, r(64) = −.25, p < .05, suggesting that greater hunger was associated with better memory for food, regardless of the emotion condition. In contrast, there was no significant correlation between glucose level and recall of household items.
Recognition Memory
A repeated-measures ANOVA was conducted with between-subjects factors of condition (sated/hungry), item type (food/household), and emotion (emotion/neutral), with recognition of old items as the dependent variable. Similar to the recall data, there was a main effect of item type, F(1, 63) = 54.62, p <.001, ηp2 =.46. Food items (M = .65, SE = .01) were better remembered than were household items (M = .58, SE = .01), t(1, 64) = 7.38, p < .001, mean difference between food and household items M = .08, 95% CI [.06, .10], d =1.39. There was no main effect of hunger condition for recognition, Fs < 1. However, there was a marginally significant interaction between hunger condition and item type (food items: Mhungry = .66, SE = .02; Msated = .65, SE = .02; household items: Mhungry = .56, SE = .01; Msated = .59, SE = .01), F(1, 63) = 3.57, p = .06, mean difference between sated and hungry participants for house items M = .03, 95% CI [−.01, .06], mean difference between sated and hungry participants for food items M = −.01, 95% CI [−.05, .03], ηp2 =.05 which was consistent with the interaction seen in recall.
Recognition Memory and Dot-Probe Task
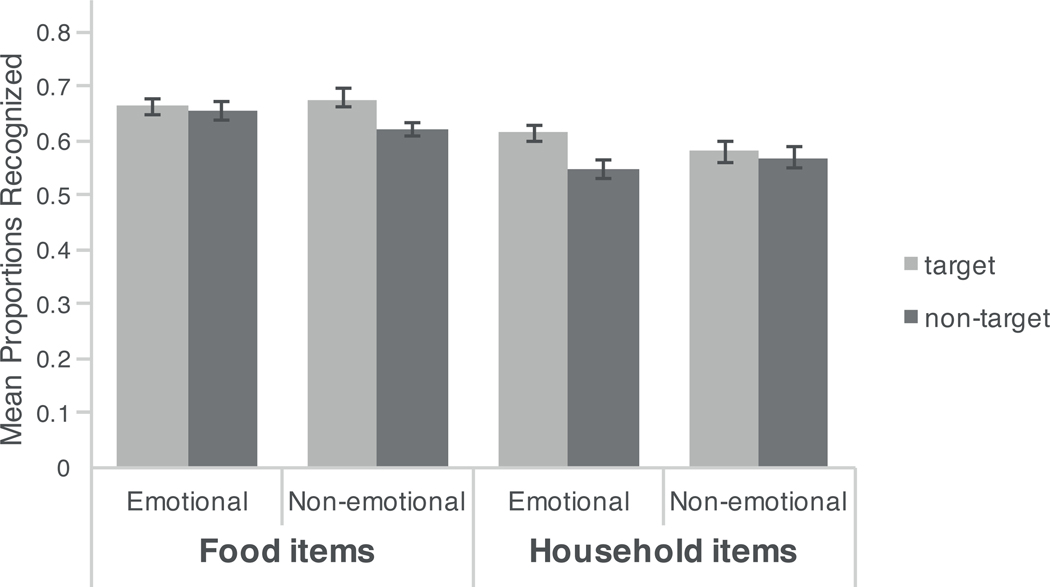
In these analyses, the target type (image shown with target dot/image shown without target dot) was added as a within-subjects factor to the repeated-measures ANOVA of recognition memory. A main effect of target type, F(1, 63) = 16.27, p < .001, mean difference between target and non-target items M = .04, 95% CI [.02, .05], ηp2 =.21 showed that items on which the target dot had appeared (M = .64, SE = .009) were better remembered than were images without a superimposed target (M = .60, SE = .009). There was also a significant three-way interaction between item type, emotion, and target type, F(1, 63) = 6.20, p < .02, ηp2 =.09. To better understand this three-way interaction, we conducted follow-up Emotion × Target Type ANOVAs separately for the food and the household items. For household items, there was a significant effect of target type, F(1, 63) = 7.41, p < .01, ηp2 =.11 and a marginally significant interaction of emotion and target type, F(1, 63) = 3.72, p < .06, ηp2 =.06. As can be seen in Figure 3, and as predicted, emotional sounds enhanced memory for household target items (M = .61, SE = .01) compared with household non-target items (M = .54, SE = .01), t(64) = 4.57, p < .001, mean difference between household target and non-target items for emotional sounds M = .07, 95% CI [.04, .10], d = .74. In contrast, neutral sounds did not significantly enhance target items (M = .58, SE = .01) compared with non-target household items (M = .57, SE = .02), t(64) = .51, p = .61, mean difference between household target and household non-target items for neutral sounds M = .01, 95% CI [−.04, .06], d = 0.07. This interaction is consistent with our predictions that arousal would enhance high-priority items more than it would enhance low-priority items. In contrast, for food items, although they exhibited the overall main effect of target type, F(1, 63) = 6.95, p < .02. , ηp2= .10, we did not find the predicted significant interaction of emotion and target type, F(1, 63) = 2.19, p > .1, ηp2 = .03. In general, food items were enhanced in memory compared with household items regardless of sound type. However, there was a significant effect of emotion for neutral target food items (versus neutral non-target food items), t(64) = −2.37, p < .02, mean difference between target food items and non-target food items for neutral sounds M = .06, 95% CI [.01, .1], d = .43, but no significant effect of emotion on target food items (versus emotional non-target food item), t(64) = −.66, p > .5, mean difference between food target and non-target food items for emotional sounds M = .01, 95% CI [−.02, .04], d = .09. It is not clear why emotion enhanced memory for items that were either inherently salient (i.e., non-target food items) or salient within the task (i.e., target household items), but did not enhance memory for items that were salient for both reasons (i.e., target food items).
Figure 3.
Mean proportions of items correctly recognized on forced-choice test averaged across hungry and sated participants (error bars represent standard errors of the means and .50 is chance level).
Sound Ratings
To examine subjective ratings, we used repeated-measures ANOVAs with the between-subject factor hunger condition at Session 1 (sated/hungry) and the within-subject factor emotion (emotional/neutral) for each rating dimension.2
Arousal Ratings.
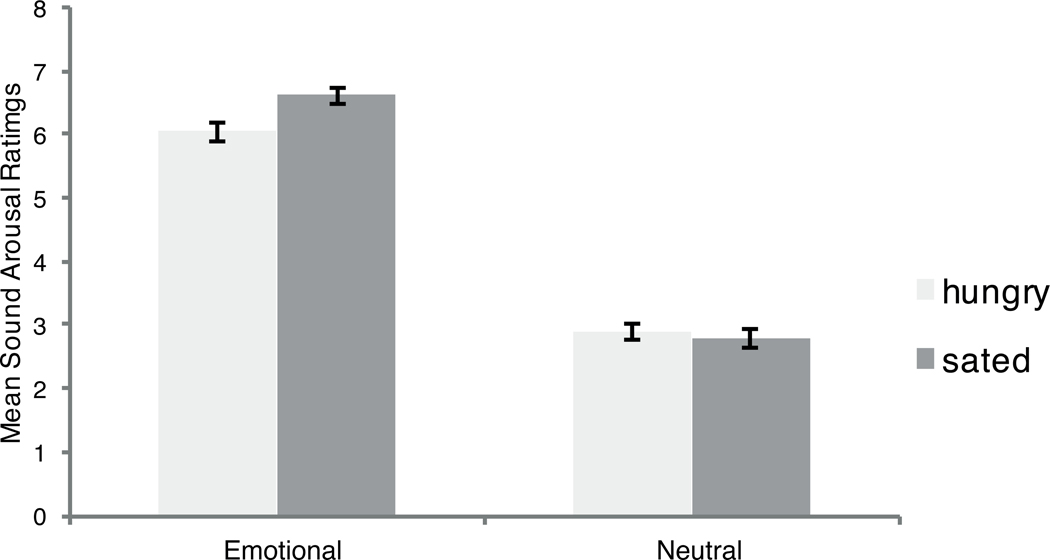
Arousal ratings confirmed the expected main effect of emotion, F(1, 63) = 712.41, p < .001, ηp2=.92, mean difference between arousal and neutral ratings, M = 3.49, 95% CI [3.76, 3.22]. Emotional sounds (M = 6.32, SE = .10) were rated as being more arousing than neutral sounds (M = 2.83, SE = .09). The interaction of Emotion × Hunger Condition was significant, F(1, 63) = 6.21, p = .02, ηp2= .09 (Figure 4). Follow-up pairwise tests showed that differences across groups were significant only for the emotional sounds. For emotional sounds, participants who were previously in a sated state rated them as being more arousing than did the participants who were previously in a hungry state, Msated = 6.61, SE = .11; Mhungry = 6.05, SE = .15; t(1,63) = 2.85, p < .006, mean difference between sated and hungry participants, M = .55, 95% [0.17, 0.94], d = .88. No significant differences were found between previously sated and previously hungry participants for the rating of neutral sounds (Msated = 2.77, SE = .15; Mhungry = 2.88, SE = .12; t(1, 63) = .54, p = .59, mean difference between sated and hungry participants M = .10, 95% CI [−.27, .48], d = −.14. Follow-up t-tests showed that the differences between the emotional and the neutral ratings were greater in sated participants who were previously in a sated state than in sated participants who were previously in a hungry state, Msated = 3.83, SE = .17; Mhungry = 3.18, SE = .19; t(63) = 2.49, p = .02, mean difference between sated and hungry participants, M = −0.65, 95% CI [−1.18, −.13], d = .62. Thus, as also indicated by the pupil dilation results, being sated during initial exposure to the sounds led to more influence of their arousing nature.
Figure 4.
Mean of sound arousal ratings (error bars represent standard errors of the means).
Valence Ratings.
Valence ratings showed an expected main effect of emotion, F(2, 126) = 400.68, p < .001, ηp2 = .86. Follow-up tests revealed that positive sounds were rated as more positive (M = 6.08, SE = .11) than were negative sounds (M = 1.97, SE = .1), t(1, 64) = 24.44, p < .001, mean difference between positive and negative sounds M = 4.11, 95% CI [3.77, 4.44], d = 4.86, or neutral sounds (M = 4.23, SE = .08), t(1, 64) = 15.66, p < .001, mean difference between positive and neutral sounds M = 1.85, 95% [1.61, 2.08], d = 2.39, and negative sounds were rated as more negative than were neutral sounds, t(1, 64) = −15.41, p < .001, mean difference between negative and neutral sounds M = −2.26, 95% CI [−2,56, −1.97], d = 3.12. There were no significant effects of hunger condition.
Post-hoc Analyses Comparing Food and Household Items
See supplemental material for ratings (arousal, attractiveness, valence, craving, palatability) obtained by independent raters and comparisons indicating how food and household items differed on these ratings. As also outlined in the supplemental material, we checked whether the hunger manipulation had differential effects on processing the high- versus low-calorie items (see Appendix C for list of high- and low-calorie items). There were no significant interactions between whether an item was in the low or high calorie category and hunger condition for any of the dependent variables examined (reaction times, recall, recognition and effect of target type on recognition), neither on the ratings (arousal, attractiveness, valence, craving and palatability).
Discussion
The current study revealed two interesting sets of findings. The first set, consistent with what one might expect, showed that food stimuli stood out more than nonfood stimuli for all participants, but especially for the hungry participants. The second (and novel to this study) set of findings was that hungry participants showed similarly high pupil dilation responses to arousing and neutral stimuli, in contrast with sated participants who showed greater pupil dilation responses to arousing stimuli than to neutral stimuli. These results suggest that being hungry increases arousal responses in general, with less discrimination between emotional and non-emotional stimuli.
Half of the participants in the study were in a hungry state, while the other half were in a sated state. During the dot-probe task, in each trial, we presented one food image (high priority) and one household image (low priority) simultaneously with a black dot placed on one of the images. The participant’s task was to detect the dot as quickly as possible. To compare the effect of saliency between hungry and sated participants, we presented an emotional or neutral sound shortly before the two images. To assess the arousal response (physiological state driven by the sympathetic nervous system) to arousing and neutral sounds and whether it is influenced by hunger state, we measured pupil dilation.
Effects of Being Hungry on Attention to and Memory for Food Stimuli
We replicated previous findings that memory in hungry people was enhanced for food items (e.g., Talmi et al., 2013). However, attentional performance did not reveal a similar influence of hunger state. Results indicated that in general, people responded faster to food than to household images. Thus, it seems that food stimuli stood out more than nonfood stimuli for all participants. The ratings from an independent sample also indicate that the food stimuli in our experiment were more subjectively salient than the household stimuli.3
In addition, we found that arousing sounds shortened reaction times to food stimuli similarly in the two hunger conditions. Thus, when a relevant food and a less relevant nonfood stimulus compete with each another, food stimuli continue to enhance attentional priority even when someone is sated. In contrast with the results for RTs, memory performance for food stimuli did not show overall enhancement when encoded after emotional sounds. Instead, food stimuli were remembered better than household stimuli regardless of emotional sounds. These results mirror those found by Talmi et al. (2013), showing that emotional memory was not driven by supplemental attention given to emotional over neutral stimuli (i.e., potentially comparable in our study to food and household items, respectively). Moreover, in our study, glucose levels suggested that greater hunger (not specific to hungry people) was associated with better memory for food, regardless of the emotion condition. It is likely that the biologically relevant information is processed differently. Indeed, the memory findings of the current study showed that food stimuli were remembered better overall (regardless of the nature of the sound). This is in line with the relevance hypothesis, which suggests that goal-relevant information is better remembered than goal-irrelevant information (Montagrin et al., 2013; Montagrin & Sander, 2016; Montagrin et al., 2018). We will discuss in the second section, how these results are also consistent with the idea that under fasting the noradrenergic system is influenced (e.g., via orexin hormones) to increase arousal to enhance food seeking (Yamanaka et al., 2003).
We looked at the effect of emotional arousal on high-priority stimuli, which are not biologically relevant (food or household stimuli presented with the target dot). We tested whether hunger state influenced attention and memory processes, which are not biologically relevant. We found that emotionally arousing sounds increased how much having a target cue (the dot) on a picture enhanced later recognition of that memory in both sated and hungry people. Specifically, for household stimuli, we observed the pattern predicted by the ABC model (Sutherland & Mather, 2012), in which emotional arousal enhanced goal-relevant stimuli (target) more than goal-irrelevant stimuli (non-target). In contrast, for food stimuli, emotion enhanced memory only for goal-irrelevant stimuli (non-target) and did not significantly enhance memory for goal-relevant stimuli (target stimuli, which were already the items most likely to be recognized). Thus, contrary to our predictions based on the ABC model, we did not find an additive effect of arousal on memory due to the goal-relevance task for inherently high-priority stimuli (i.e., food). One possibility is that, as indicated by the RT data, after hearing an arousing sound, participants responded even faster to goal-relevant food stimuli and so likely were focused on that stimulus already when the dot appeared. Thus, the dot location and preceding arousing sound had similar effects on directing attention to food stimuli and so there was no benefit gained from arousal when the food stimulus was a target (goal-relevant) in addition to its biological relevance.
Finally, to better understand how arousal activation related to fasting state influences cognitive processes, we examined whether hungry and sated people differ in emotional responsivity to stimuli.
Effects of Hunger on Arousal Responses to Emotional Stimuli
We used a pupil dilation measure to investigate the differential effect of being sated or hungry on emotional responsivity to emotional and neutral sounds. Sated participants in our study showed more of an arousal response to emotional sounds compared with neutral sounds than did hungry participants. Although sated people showed larger pupil dilation in response to arousing than to neutral sounds, hungry people showed similar pupil dilation to all sounds. Moreover, the pupil dilation responses for emotional and neutral sounds in hungry people was similar to the pupil dilation responses to emotional sounds in sated people. These findings and as indicated above, the fact that memory was better for food stimuli, regardless of the emotional condition, and that greater hunger was associated with this memory performance suggest a generally increased arousal response to stimuli under fasting (Sakurai, 2007; 2014; Soya et al., 2017). In addition, we examined whether the delayed effect of the hunger manipulation on emotional responsivity to emotional and neutral sounds had a lasting effect. Participants made subjective ratings 48 hours later about sounds previously heard on Day 1. Consistent with the pupil dilation responses, subjective ratings of arousal revealed an interaction between hunger state and the emotional nature of the sounds. Participants showed a greater difference in ratings of emotional compared with neutral stimuli first encountered when sated than when hungry. Together, the pupil dilation and arousal rating findings indicate that the emotional system of hungry people might be less effective at differentiating the intensity of arousal in response to emotional versus neutral stimuli. While our findings cannot speak to the exact mechanisms underlying this increase in arousal responses under arousal, one plausible mechanism is that orexin release increases during hunger states, and may stimulate downstream locus coerulus activity (Soya et al., 2017). Increasing sympathetic activity when hungry, despite a lack of energy, might be an adaptive evolutionary behavior to enhance the odds of finding food (Sakurai, 2007; 2011; 2014; Soya et al., 2017). However, other behaviors related to hungry state may be far less adaptive. Indeed, being hungry could promote impulsive behavior, or overacted responses, such as enhanced aggressive behaviors in innocuous situations, to social and moral judgments. For instance, impulsive behaviors were increased by the stimulation of hormones secreted when hungry (i.e., ghrelin) within the ventral tegmental area, brain region involved in reward and aversive system (Anderberg et al., 2016; Matsumoto & Hikosaka, 2009). Thus, it might be interesting to further examine the influence of a fasting state on other behaviors.
Concerning limitations of the present study, the anticipation of the blood test measure by participants may have influenced glucocorticoid levels, as anticipating novel or uncontrollable events can increase glucocorticoids (Lupien, Maheu, Tu, Fiocco, & Schramek, 2007). However, because the blood test procedure was the same for both groups, this should have affected performance similarly across groups.
Conclusion
In this study, we found that being hungry increased arousal responses to stimuli in general, while being sated amplified the sensitivity to arousing versus neutral stimuli. In addition, in line with the ABC model, arousing stimuli increased attention to high-priority information (Sutherland & Mather, 2012). However, the results in our study, extend those reported by Talmi et al. (2013), demonstrating that enhancing arousal prior to presentation of food stimuli did not directly influence memory performance. Emotional sounds improved recognition memory for prioritized neutral stimuli (i.e., household items with a superimposed target dot), but did not interact with target dot placement for inherently prioritized stimuli (i.e., food). Future studies should examine in more detail the influence of being hungry or sated on emotional processing and attention to and memory for stimuli. They should also take into account the fact that being hungry seems to increase arousal responses in a global fashion and might be responsible for a less discriminative activation of the noradrenergic system (via the locus coerulus), which in turn will influence attentional and memory processing (Markovic et al., 2014; Mather et al., 2015; Soya et al., 2017).
Supplementary Material
Acknowledgments
This work was supported by the Swiss National Science Foundation Grant P1GEP1-148618 to AM and by the National Institute on Aging Grant RO1AG025340 to MM. We thank Shawn Nielsen and Rico Velasco for assistance with the eye-tracker setup, Allison Ponzio for assistance with administration procedures and experimental design, and Paul Choi for assistance with participant recruitment and scoring.
Footnotes
Data availability:
Data from the four studies reported in this manuscript are available through the Open Science Framework repository: https://osf.io/k7u4a/
New analyses excluding household items related semantically to food (i.e., cocktail shaker, coffee table, dresser, glass, kettle, tablecloth and toaster) revealed similarly results for memory dependent variables examined (recall and recognition).
The instructions given to the participants apparently did not clearly convey the meaning of the affective dimension of relevance (the pattern of responses for this dimension was similar to the valence ratings, whereas we had expected that both positive and negative sounds would have been more relevant than neutral sounds), and so we do not report these values here.
It is noteworthy that food stimuli used in the experiment were not more colorful than household items and thus colorfulness cannot explain why food items were rated (by independent raters) as more attractive, more positive, and more arousing than household items (see supplemental material). It is likely that food items are more aesthetically pleasing than household items and it could be functional to have food detected faster in the environment.
Contributor Information
Alison Montagrin, Department of Psychology, University of Southern California, 3715 McClintock Avenue, Los Angeles, CA, 90089, Department of Neurosciences and Psychology, University of Geneva, Chemin des Mines 10, CH-1201 Geneva.
Bruna Martins-Klein, University of Massachusetts Amherst, Department of Psychological and Brain Sciences, 135 Hicks Way, Amherst, MA 01003.
David Sander, Department of Psychology, University of Geneva, 40 Boulevard du Pont d’Arve, CH-1205 Geneva.
Mara Mather, Leonard Davis School of Gerontology and Department of Psychology, University of Southern California, 3715 McClintock Avenue, Los Angeles, CA, 90089.
References
- Anderberg RH, Hansson C, Fenander M, Richard JE, Dickson SL, Nissbrandt H, et al. (2016). The Stomach-Derived Hormone Ghrelin Increases Impulsive Behavior. Neuropsychopharmacology, 41, 1199–1209. 10.1038/npp.2015.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Meule A, Busch NA, & Ohla K. (2014). Food-pics: an image database for experimental research on eating and appetite. Frontiers in Psychology, 5, 527 10.3389/fpsyg.2014.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (2007). The International Affective Digitized Sounds (2nd edition; IADS-2): Affective ratings of sounds and instruction manual (Technical Report B-3). Gainesville: University of Florida. [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, & Lang PJ (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45, 602–607. doi: 10.1111/j.1469-8986.2008.00654.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch T, Sander D, Pourtois G, & Scherer KR (2008). Beyond fear: Rapid spatial orienting toward positive emotional stimuli. Psychological Science, 19, 362–370. doi: 10.1111/j.1467-9280.2008.02094.x [DOI] [PubMed] [Google Scholar]
- Delplanque S, N’diaye K, Scherer K, & Grandjean D. (2007). Spatial frequencies or emotional effects? Journal of Neuroscience Methods, 165, 144–150. 10.1016/j.jneumeth.2007.05.030 [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. (2009). Fasting biases brain reward systems towards high-calorie foods. European Journal of Neuroscience, 30, 1625–1635. 10.1111/j.1460-9568.2009.06949.x [DOI] [PubMed] [Google Scholar]
- Ilie G, & Thompson WF (2006). A comparison of acoustic cues in music and speech for three dimensions of affect. Music Perception, 23(4), 319–330. doi: 10.1525/mp.2006.23.4.319?ref=no-x-route:e685973c936c8af114a5fddad440238f [DOI] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, & Gold JI (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron, 89, 221–234. doi: 10.1016/j.neuron.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, & Mather M. (2007). Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion, 7, 705–714. doi: 10.1037/1528-3542.7.4.705 [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, & Andrews C. (2003). Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex, 13, 1064–1071. [DOI] [PubMed] [Google Scholar]
- Lee T-H, Itti L, & Mather M. (2012). Evidence for arousal-biased competition in perceptual learning. Frontiers in Psychology, 3, 241. doi: 10.3389/fpsyg.2012.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-H, Sakaki M, Cheng R, Velasco R, & Mather M. (2014). Emotional arousal amplifies the effects of biased competition in the brain. Social Cognitive and Affective Neuroscience, 9, 2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine LJ, & Pizarro DA (2006). Emotional valence, discrete emotions, and memory In Uttl B, Ohta N, & Siegenthaler AL (Eds.), Memory and emotion: Interdisciplinary perspectives (pp. 37–58). Oxford, UK: Blackwell Publishing. [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, & Schramek TE (2007). The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition, 65, 209–237. doi: 10.1016/j.bandc.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Markovic J, Anderson AK, & Todd RM (2014). Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural Brain Research, 259, 229–241. doi: 10.1016/j.bbr.2013.11.018 [DOI] [PubMed] [Google Scholar]
- Mather M, & Carstensen LL (2003). Aging and attentional biases for emotional faces. Psychological Science, 14, 409–415. doi: 10.1111/1467-9280.01455 [DOI] [PubMed] [Google Scholar]
- Mather M. (2007). Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science, 2, 33–52. doi: 10.1111/j.1745-6916.2007.00028.x.s [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, & Harley CW (2015). Norepinephrine ignites local hot spots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behavioral and Brain Sciences. Advance online publication. doi: 10.1017/S0140525X15000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Sutherland MR (2011). Arousal-biased competition in perception and memory. Perspectives on Psychological Science, 6, 114–133. doi: 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, & Hikosaka O. (2009). Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature, 459, 837–U4. 10.1038/nature08028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, & Mesulam MM (2008). The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cerebral Cortex, 18, 2604–2613. doi: 10.1093/cercor/bhn021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagrin A, & Sander D. (2016). Emotional memory: From affective relevance to arousal. Behavioral and Brain Sciences, 39. doi: 10.1017/S0140525X15001879 [DOI] [PubMed] [Google Scholar]
- Montagrin A, Brosch T, & Sander D. (2013). Goal conduciveness as a key determinant of memory facilitation. Emotion, 13, 622–628. doi: 10.1037/a0033066 [DOI] [PubMed] [Google Scholar]
- Montagrin A, Sterpenich V, Brosch T, Grandjean D, Armony J, Ceravolo L, & Sander D. (2018). Goal-relevant situations facilitate memory of neutral faces. Cognitive, Affective & Behavioral Neuroscience, 18, 1269–1282. 10.3758/s13415-018-0637-x [DOI] [PubMed] [Google Scholar]
- Morris JS, & Dolan RJ (2001). Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. Journal of Neuroscience, 21, 5304–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, & Balsters JH (2014). Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping, 35, 4140–4154. doi: 10.1002/hbm.22466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponz A, Khatami R, Poryazova R, Werth E, Boesiger P, Bassetti CL, & Schwartz S. (2010a). Abnormal activity in reward brain circuits in human narcolepsy with cataplexy. Annals of Neurology, 67, 190–200. 10.1002/ana.21825 [DOI] [PubMed] [Google Scholar]
- Ponz A, Khatami R, Poryazoya R, Werth E, Boesiger P, Schwartz S, & Bassetti CL (2010b). Reduced amygdala activity during aversive conditioning in human narcolepsy. Annals of Neurology, 67, 394–398. 10.1002/ana.21881 [DOI] [PubMed] [Google Scholar]
- Ponzio A, & Mather M. (2014). Hearing something emotional influences memory for what was just seen: How arousal amplifies effects of competition in memory consolidation. Emotion, 14, 1137–1142. doi: 10.1037/a0037983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool E, Brosch T, Delplanque S, & Sander D. (2014). Where is the chocolate? Rapid spatial orienting toward stimuli associated with primary rewards. Cognition, 130, 348–359. doi: 10.1016/j.cognition.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Sakaki M, Fryer K, & Mather M. (2014). Emotion strengthens high-priority memory traces but weakens low-priority memory traces. Psychological Science, 25, 387–395. doi: 10.1177/0956797613504784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. (2007). The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nature Reviews Neuroscience, 8, 171–181. 10.1038/nrn2092 [DOI] [PubMed] [Google Scholar]
- Sakurai T. (2014). The role of orexin in motivated behaviours. Nature Reviews Neuroscience, 15, 719–731. 10.1038/nrn3837 [DOI] [PubMed] [Google Scholar]
- Sakurai T, & Mieda M. (2011). Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends in Pharmacological Sciences, 32, 451–462. 10.1016/j.tips.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Soya S, Takahashi TM, McHugh TJ, Maejima T, Herlitze S, Abe M, et al. (2017). Orexin modulates behavioral fear expression through the locus coeruleus. Nature Communications, 8, 1606 10.1038/s41467-017-01782-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MR, & Mather M. (2012). Negative arousal amplifies the effects of saliency in short-term memory. Emotion, 12, 1367–1372. doi: 10.1037/a0027860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MR, & Mather M. (2015). Negative arousal increases the effects of stimulus salience in older adults. Experimental Aging Research, 41, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MR, & Mather M. (2018). Arousal (but not valence) amplifies the impact of salience. Cognition and Emotion, 32(3), 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Luk BTC, McGarry LM, & Moscovitch M. (2007). The contribution of relatedness and distinctiveness to emotionally-enhanced memory. Journal of Memory and Language, 56, 555–574. [Google Scholar]
- Talmi D, Ziegler M, Hawksworth J, Lalani S, Herman CP, & Moscovitch M. (2013). Emotional stimuli exert parallel effects on attention and memory. Cognition and Emotion, 27, 530–538. doi: 10.1080/02699931.2012.722527 [DOI] [PubMed] [Google Scholar]
- Wisse B, Zieve D, & Ogilvie I. (2014, May 8). Blood sugar test – blood. Retrieved from U.S. National Library of Medicine website: http://www.nlm.nih.gov/medlineplus/ency/article/003482.htm
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. (2003). Hypothalamic Orexin Neurons Regulate Arousal According to Energy Balance in Mice. Neuron, 38, 701–713. 10.1016/S0896-6273(03)00331-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.