Abstract
The therapeutic application of heat is very effective in cancer treatment. Both hyperthermia, i.e. heating to 39–45°C to induce sensitization to radiotherapy and chemotherapy, and thermal ablation, where temperatures beyond 50°C destroy tumor cells directly are frequently applied in the clinic. Achievement of an effective treatment requires high quality heating equipment, precise thermal dosimetry, and adequate quality assurance. Several types of devices, antennas and heating or power delivery systems have been proposed and developed in recent decades. These vary considerably in technique, heating depth, ability to focus, and in the size of the heating focus. Clinically used heating techniques involve electromagnetic and ultrasonic heating, hyperthermic perfusion and conductive heating. Depending on clinical objectives and available technology, thermal therapies can be subdivided into three broad categories: local, locoregional, or whole body heating. Clinically used local heating techniques include interstitial hyperthermia and ablation, high intensity focused ultrasound (HIFU), scanned focused ultrasound (SFUS), electroporation, nanoparticle heating, intraluminal heating and superficial heating. Locoregional heating techniques include phased array systems, capacitive systems and isolated perfusion. Whole body techniques focus on prevention of heat loss supplemented with energy deposition in the body, e.g. by infrared radiation. This review presents an overview of clinical hyperthermia and ablation devices used for local, locoregional, and whole body therapy. Proven and experimental clinical applications of thermal ablation and hyperthermia are listed. Methods for temperature measurement and the role of treatment planning to control treatments are discussed briefly, as well as future perspectives for heating technology for the treatment of tumors.
Keywords: Hyperthermia, thermal therapy, heating equipment, ablation
Graphical Abstract:
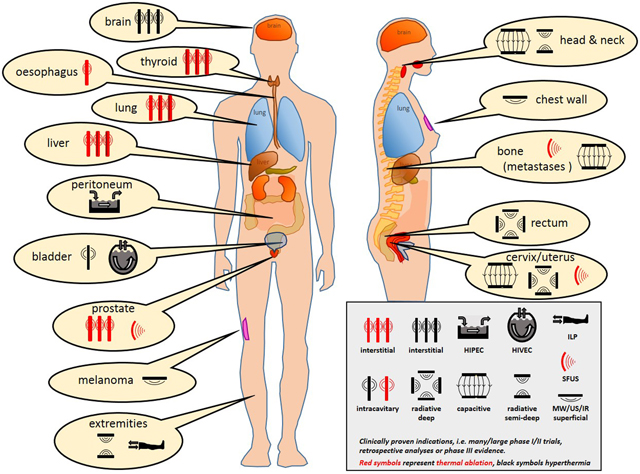
Schematic overview of clinically proven heating techniques used for various indications and tumor locations.
1. Introduction
Thermal medicine involves the manipulation of body or tissue temperatures for the treatment of diseases. The application of heat has a long history in the treatment of malignant tumors and was already used by the ancient Greeks. In the late 19th century spontaneous regression of malignant tumors was reported in patients suffering from high fevers due to bacterial infections[1]. Since then, heating techniques have been developed to apply more controlled heating to maximize the therapeutic effect while minimizing unwanted side effects. Therapeutic heating of tumor tissue can be roughly categorized as hyperthermia or thermal ablation.
Hyperthermia treatments aim to induce relatively mild tumor heating up to a maximum temperature of 45°C. The therapeutic effect depends on the temperature and the duration of heating (thermal dose)[2–4]. This is often expressed as the cumulative equivalent minutes at 43°C (CEM43)[5, 6], since the widely accepted target temperature is 43°C for up to 1h[6]. The use of CEM43 is based on the Arrhenius relationship, describing the time-temperature dependent cytotoxic effect of hyperthermia[7]. For short exposure times at temperatures below 43°C, the cell survival versus exposure time curve shows a shoulder, which is indicative of accumulation of sub-lethal thermal damage. For increased exposure time, this shoulder turns into a constant rate of cell death, followed after several hours by a lower rate of cell death due to the development of thermotolerance. For heat exposure at temperatures above 43°C, cell death occurs at a constant rate. Although the CEM43 thermal iso-effect dose concept has some limitations, it is commonly used as a practical normalization to convert a given time–temperature exposure to an equivalent exposure time at 43°C[5]. Hyperthermia is typically applied once or twice a week in combination with radiotherapy and/or chemotherapy schedules and has been shown to enhance the therapeutic effect of these adjuvant treatment modalities. Known mechanisms for this enhancement include inhibition of DNA damage repair, increased blood flow and reoxygenation[8–12]. Furthermore, hyperthermia stimulates the immune response[13]. Optimal dose differs for these mechanisms. Increased blood flow and reoxygenation become active at relatively low temperatures of ~39⁰C, peak around 41–42⁰C, and reverse when temperatures exceed 44⁰C[14]. Effective inhibition of DNA damage repair inhibition requires higher temperatures (exceeding ~41⁰C) and strongly increases with increasing thermal dose[15]. The combination of all mechanisms above effectively display a dose-effect relationship within the hyperthermia temperature range. The applied timing of delivery is either simultaneous (often for chemotherapy) or sequential with a relatively short time interval between hyperthermia and radiotherapy/chemotherapy.
Thermal ablation aims at generation of temperatures over 50°C for a few minutes in a single session to destroy tumor cells by heat alone. These excessive temperatures cause very rapid cell death by coagulation and protein denaturation, leading to both necrosis and apoptosis, depending on the dose. As explained in the previous paragraph, Arrhenius relationships can also be applied to thermal ablation to predict thermal damage and physical changes associated with thermal exposure in several tissue types[16]. Depending on the mode of energy delivery, thermal ablation can be used as a minimally invasive alternative to surgical resection. However, in the tumor periphery beyond the border of immediate tissue coagulation, non-lethal, but hyperthermic temperatures are achieved and therefore the combination of thermal ablation with chemotherapy, radiotherapy, or immunotherapy, is also attracting growing clinical interest for effectively treating both the tumor and its periphery[17–19]. Figure 1 represents the characteristics of hyperthermia and ablation in terms of temperature levels. Panel B shows a schematic representation of the clear division between the different realms of hyperthermia versus thermal ablation based on data presented in Dewhirst et al[20]. The dividing line shows that the exposure time needed to achieve cell death decreases with increasing temperatures.
Figure 1:
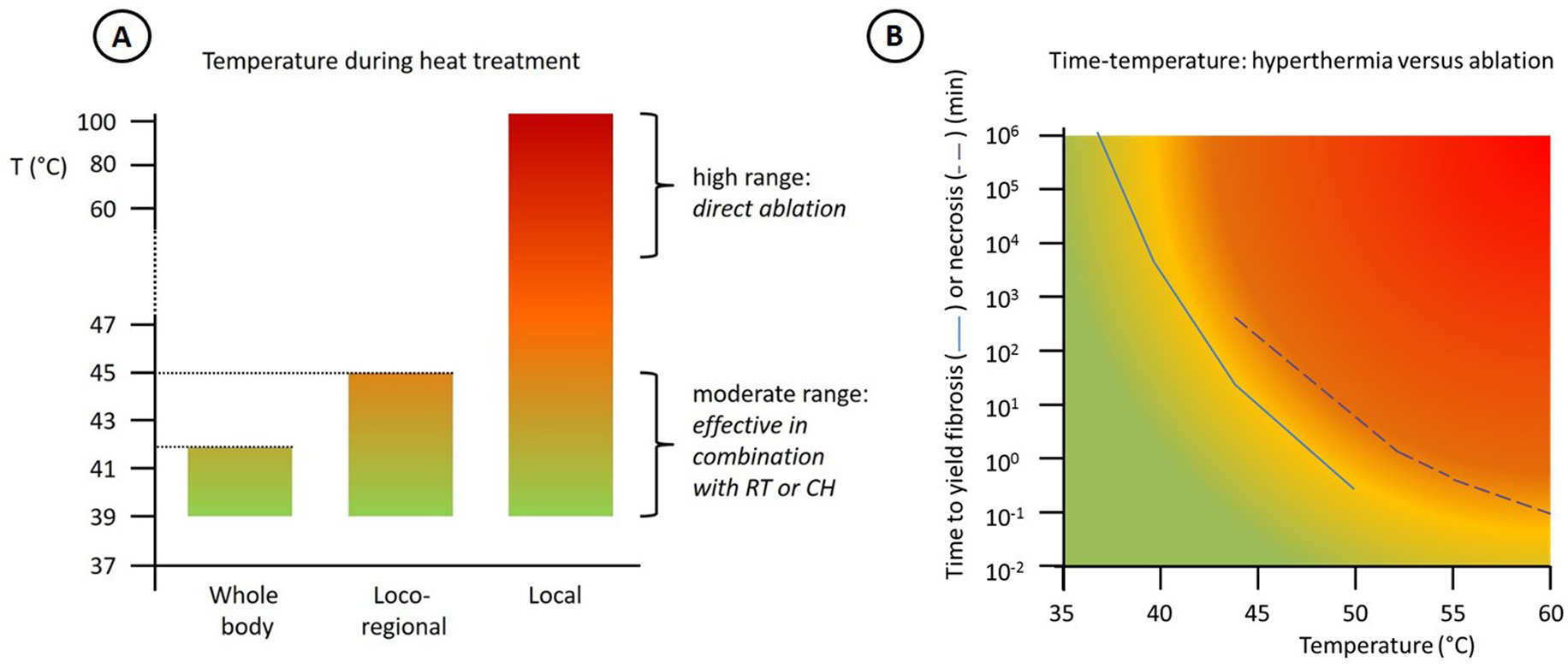
A: Characteristics of the three main treatment approaches in terms of temperature level. B: Time to yield muscle fibrosis (continuous curve) or necrosis (dotted curve) at specific temperatures for hyperthermia and ablation (based on Dewhirst et al [20]).
Over the past several decades increasing knowledge gained about the mechanisms of action of both hyperthermia and thermal ablation have had positive effects on treatment outcome in several randomized clinical trials for both hyperthermia and thermal ablation[21]. Based on these positive clinical trials, hyperthermia and thermal ablation are recognized in clinical oncology for a number of tumor indications. Hyperthermia is routinely used for locally advanced cervical cancer patients unfit for chemotherapy, for recurrent breast cancer, non-muscle invasive bladder cancer and soft tissue sarcoma. Thermal ablation is currently in routine clinical use for a large number of indications, including hepatocellular carcinoma, renal cell carcinoma (the most common type of kidney cancer), primary and secondary lung tumors, prostate cancer, non-surgical liver metastases and adrenal metastases.
The realization of good clinical results requires high quality heating equipment, and thus a significant amount of research has been undertaken toward the development of robust and reliable heating devices. The tumor location is often decisive in selecting whether invasive or external heating is preferred. Several types of instruments, antennas and systems have been proposed. These vary widely in physical mechanism of energy transduction, techniques for energy delivery, heating depth and directional control of the delivered energy, as well as in the size of the volume that can be heated[22]. These developments have resulted in dedicated heating equipment for specific tumor sites and anatomical locations, driving significant progress in both the control and technology of heating.
In this review we present an overview of equipment used clinically for hyperthermia and thermal ablation. Different techniques of heating are first explained, followed by an overview of equipment for local, locoregional and whole body heating with a description of tumor sites and clinical applications. Since monitoring is important to ensure treatment quality and thermal dose delivery, control methods involving thermometry and treatment planning are also briefly summarized. Finally, future perspectives are discussed.
2. Methods of heating
Hyperthermia and thermal ablation can be produced using a variety of techniques. Clinically used heating techniques are electromagnetic heating, ultrasound, hyperthermic perfusion and conductive heating. The principles of these techniques are discussed below and a schematic representation of the heating mechanisms is shown in Figure 2.
Figure 2:
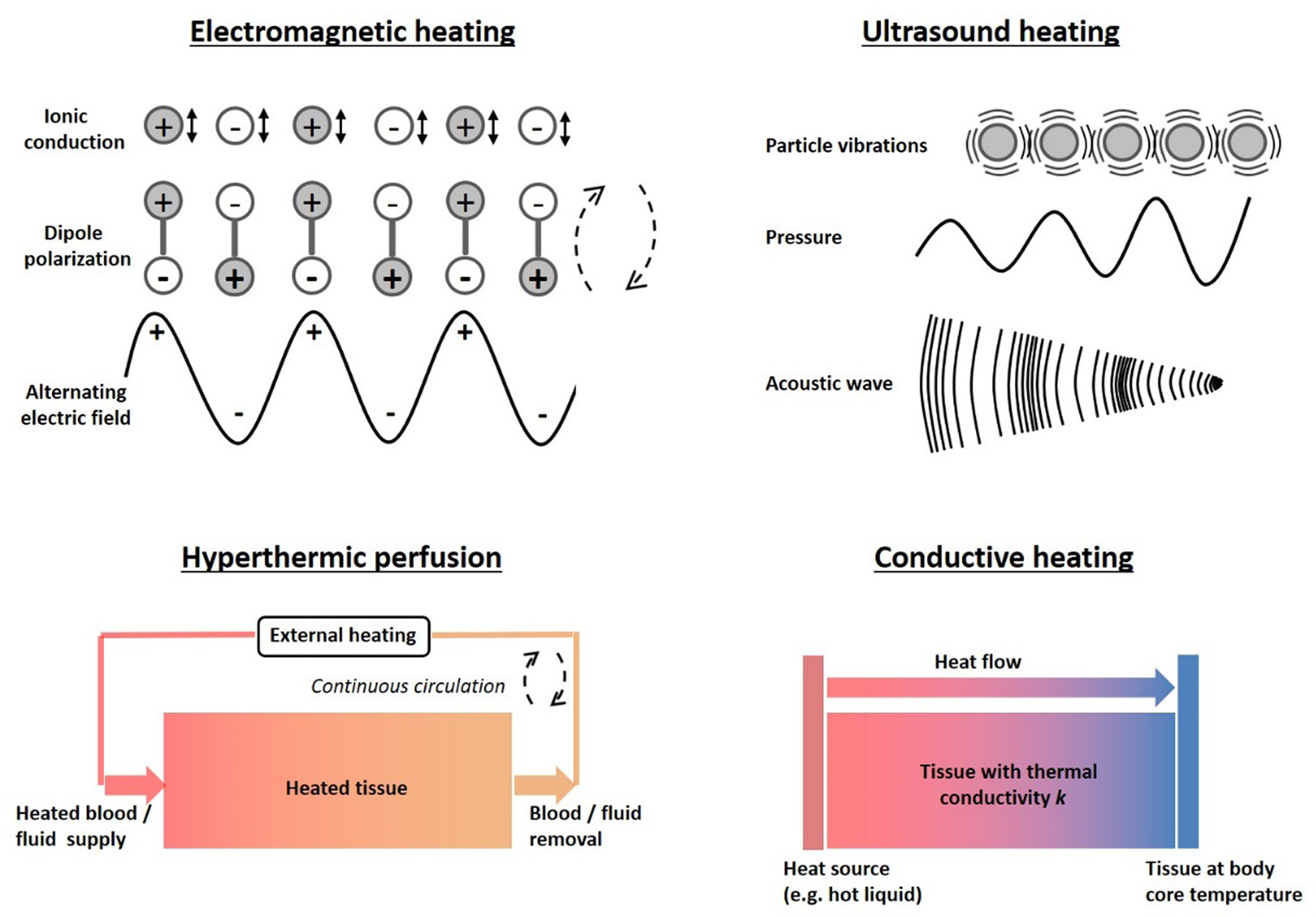
Schematic representation of the heating mechanisms of different clinical heating techniques: Electromagnetic (i.e. capacitive, radiofrequency, microwave, infrared and laser heating), focused ultrasound, hyperthermic perfusion and conductive heating.
2.1. Electromagnetic heating
Electromagnetic heating techniques apply a high frequency alternating sinusoidal electromagnetic (EM) field generated using one or more antennas. These EM fields cause dielectric heating by molecular dipole rotation/polarization/vibration in the MHz range and ionic conduction in the kHz range. Polar molecules (e.g. water) have an electric dipole moment and therefore these molecules align continuously with the alternating field. Electric forces cause rotating molecules to push, pull, and collide with other molecules, thereby distributing the energy to adjacent molecules and atoms, causing dielectric heating. In conduction, ions in tissue oscillate due to the forces exerted by the electric current. This current faces internal resistance because of the collisions of charged particles with neighboring molecules or atoms, causing dielectric heating. Tissue heating is dominated by ionic conduction in the extracellular fluid for lower frequencies (< 1 MHz) with the cell membranes acting as isolators, and by dipole polarization at higher frequencies (> 1 MHz). For frequencies > 1 MHz the cell membranes become permeable to the E-fields and the microscopic structure of tissues can be neglected[23].
Electromagnetic heating techniques can be subdivided into categories differing in frequency, wavelength and penetration depth. These include in ascending order of frequency (and descending penetration depth) radiofrequency (either capacitive or radiative), microwave heating, infrared and laser heating. Many microwave systems operate at an industrial, scientific and medical (ISM)-frequency which permits the use outside a Faraday cage for shielding.
2.1.1. Capacitive radiofrequency heating
Capacitive heating is achieved using metal electrodes that are coupled to a high power RF generator, operating at frequencies of 8, 13.56 or 27.12 MHz. Electrodes are usually applied in pairs with the target region positioned between the electrodes, coupled to water bolus bags or other suitable media to transfer the current into the body. These boluses are also applied for skin cooling. An electric field is applied between the electrodes and power is deposited in the tissue as a result of the alternating voltage field[24]. Power can be steered by using different sized electrodes, which concentrate current fields under the smallest electrode, such that both superficial and deep-seated tumor locations can be treated. The energy absorption in tissue by dielectric heating depends on the electrical conductivity and permittivity[25], which vary between different tissue types[26], resulting in an inhomogeneous power distribution. Therefore, excessive temperatures (hot spots) can occur at tissue interfaces, and due to the orientation of the main electric field component, which is perpendicular to fat layers, especially excessive heating of fat tissue can be treatment limiting when using this technique[27–29]. Therefore, capacitive heating becomes less effective with increasing thickness of the fat layer and heating of deep-seated tumors can be challenging[30–32].
Some variants of this heating technique exist, including coplanar electrodes and intraluminal/intracavitary electrodes placed in catheters, which create a capacitor between the electrode and tissue, with electrodes operating as balanced pairs or as individual applicators coupled to an external ground plane.
2.1.2. Radiative radiofrequency and microwave heating
Clinical radiative radiofrequency (RF) hyperthermia is applied using extracorporally placed antennas with operating frequencies ranging from 60 MHz to 150 MHz. RF hyperthermia is used for deep-seated tumor locations (e.g. pelvic tumors) because in this frequency range the wavelength is 25–60 cm in muscle tissue, yielding a large penetration depth of the electromagnetic energy. Microwave hyperthermia is usually applied for superficial or intraluminal/intracavitary tumor sites. Typical free ISM frequencies applied for microwave hyperthermia antennas are 433 MHz (Europe), 915 MHz (USA) and 2450 MHz. To couple the energy into tissue, a water bolus is usually applied between the antenna(s) and the tissue, for both radiofrequency and microwave heating; this water bolus can simultaneously be used for skin cooling when treating deep-seated tumors, or heating in the case of superficial tumor locations. As a result of inhomogeneous energy absorption in different tissues hot spots can occur at tissue interfaces.
Interstitial RF or microwave needles are used for minimally invasive ablation as an alternative to surgery, depending on the size and location of the tumor. Needle electrodes are inserted under image guidance (CT, ultrasound or MRI) into the target tissue. RF ablation applies an alternating current in the range of 350–500 kHz; microwave ablation frequencies are typically 915 MHz or 2.45 GHz. The main difference between RF and microwave ablation is that RF ablation induces areas of high current density mainly near the electrodes, while microwave ablation causes dielectric heating in a volume around the applicator. A rapid decrease of power density away from the applicator is a common trait for both RF and MW ablation techniques[33].
Radiofrequency and microwave hyperthermia and ablation devices can use a single electrode or antenna, an array or a phased array of antennas. With a 2D array of multiple antennas larger tumor areas can be heated. With a 3D phased array, in which multiple antennas are positioned in one or more rings around the patient, power steering and focusing of the electromagnetic energy into the tumor is possible (also at deep-seated locations) by adequate selection of phases and amplitudes to create constructive interference between the electromagnetic fields. As a result of the locoregional heating induced by this technique, some temperature rise in normal tissues is inevitable. However, this is usually not a problem, as long as excessive heating (hot spots) is avoided. This can be realized by adequate (destructive) interference[34].
2.1.3. Infrared and laser
Infrared (IR) heating applies infrared lamps (frequency > 300 GHz) to heat tissues. IR energy is strongly absorbed due to the presence of O-H bonds in water. As the penetration depth of this technique is typically less than 1 cm, it is suitable for very superficial heating. Infrared heating is also applied for whole body hyperthermia, using an isolated cabin to avoid heat loss[35]. Furthermore, optical fiber guided laser light is applied for local ablation of tissue. Interstitial laser ablation allows the delivery of laser energy directly into the tumor tissue, which maximizes penetration and effectiveness in the tumor, while minimizing damage to surrounding healthy tissues[36].
2.2. Ultrasound
Ultrasound (US) heating employs acoustic energy at frequencies 0.5–10 MHz to heat tissues and can be used to induce either hyperthermia or thermal ablation[37, 38]. In contrast to diagnostic US imaging, where fields are usually weakly focused, ultrasound transducers used for thermal therapy are often designed to focus pressure waves to converge on a specific focal zone. Heat is generated by frictional losses due to molecular collisions resulting from the applied US pressure wave. A variety of planar devices (with or without lenses), bowl-shaped sources, focused multi-transducer phased arrays and interstitial US applicators are used. For focused ultrasound (FUS), the ellipsoidal focal spot typically has dimensions of a few mm in diameter, up to ~1 cm in length, which decreases with increasing frequency and aperture. US technology enables excellent spatial and dynamic control of the power and temperature distribution[39] due to its relatively short wavelength (a few millimeters) and allows deep penetration in soft tissues, provided an US window is present. These properties enable conformal tumor heating for both ablation and hyperthermia by mechanically or electronically scanning a highly focused US beam through the target region[40–42].
In addition to thermal effects HIFU also damages cellular structures by cavitation, i.e. implosion of microbubbles formed during HIFU[43–45]. Due to the pressure waves, gas bubbles present in the blood stream start to oscillate in the sound field (stable cavitation) inducing mechanical effects such as the enhancement of the permeability of cell membranes or blood vessels. The latter can be used to open the blood brain barrier locally, allowing drug compounds to extravasate into the brain tissue[46]. Ultrasound-induced cavitation can actively transport and improve the distribution of therapeutic agents in tumors. Injection of microbubbles, polymeric nanocups or other nanoparticles can be used to realize stable and inertial cavitation, thereby offering a considerable potential for enhanced drug delivery and treatment monitoring in oncological and other biomedical applications[47–50].
Application of US for soft tissue heating requires clear acoustical access to the target tissue, which should not be obstructed by bone, air pockets or strongly absorbing material, such as, for example faeces in the bowels. As the absorption coefficient for ultrasound in bones is about fifty times higher than in soft tissue, any bone in the near field would lead to off-target heating. The acoustic impedance mismatch at the air/tissue interface leads to strong reflection and scattering of ultrasound at air pocket, which may cause local heating and unintentional ablation of adjacent tissue. On the other hand, the strong absorption of US in bone makes it a perfect modality for bone tumor ablation[51, 52], but other lesions may not be accessible for FUS, or require carefully planned applicator positioning to avoid normal tissue hot spots during treatment of deep seated pelvic and abdominal tumor sites.
2.3. Hyperthermic perfusion
Selected anatomical sites are suitable for perfusional hyperthermia techniques, generally combined with chemotherapy. One approach is the direct infusion of heated perfusate into the vascular system, for example for hyperthermic isolated limb perfusion (ILP). ILP involves temporarily connecting the circulatory system in the limb of the patient to an external pump to distribute both heat and drugs to the tumor target region, achieving relatively uniform heating and drug delivery in that region. Another approach is to circulate a hyperthermic carrier solution combined with chemotherapeutic agents inside body cavities to provide heating and drug delivery to tumors in the surface of the cavity. An example is Hyperthermic IntraPeritoneal Chemotherapy (HIPEC) which involves circulation of a heated chemotherapeutic solution in the peritoneal cavity to eradicate any microscopic tumors left behind after surgical removal of macroscopic tumor mass[53]. Hyperthermic intrathoracic chemotherapy (HITHOC) is a similar technique for intrathoracic lesions[54]. Hyperthermic Intra-Vesical Chemotherapy (HIVEC®) is another perfusion technique in which the bladder wall is treated by circulating a heated chemotherapy solution inside the bladder[55].
2.4. Conductive heating
Conductive heating was the earliest clinically applied hyperthermia technique, as it was used by ancient Greeks and Egyptians to treat superficial malignancies. One of the earliest post-industrial revolution hyperthermia techniques was developed by Westermark, using water heated metal coils at 42 – 44°C to treat cervical carcinoma in the late 19th century[56]. More recently, interstitial implants of metal needles with hot water and palladium-nickel thermoseeds have been developed and applied clinically[57]. These conductive heating techniques became less popular because the thermal penetration depth is governed by heat diffusion and perfusion, and therefore small in comparison to other heating techniques.
Magnetic nanoparticles (MNPs) were first proposed by Gilchrist et al in 1957[58]. Further developments have resulted in intriguing materials for clinical conductive heating[59–61]. When exposed to an external magnetic field, the magnetic materials will respond by aligning their atomic moments with the field, thus producing a magnetization that persists for a characteristic duration after the external magnetic field is removed[62, 63]. Heat is generated via magnetic hysteresis, a loss mechanism for which the amount of energy released depends on the frequency and amplitude of the applied magnetic field, as well as on the intrinsic magnetic properties of the nanoparticle. This dependency is complex and methodology for characterizing the heating is not standardized, making prediction of the amount of heat generated by MNPs challenging[64]. Generally, the relevant frequency range for MNP hysteresis heating is between 0.1 and 1 MHz. However, for clinical MNP hyperthermia a more limited range of about 0.1–0.2 MHz is used to limit excessive off-target tissue heating from induced eddy currents[64]. MNPs are typically suspended in a biocompatible fluid, which enables direct heating of the target tissue, thereby limiting contact with normal tissues[65, 66]. Due to their size (~100 nm) MNPs are colloids, which can be made stable in aqueous suspensions suitable for injection by using appropriate coatings[65–69].
3. Selection and use of equipment based on clinical requirements
Existing heat treatment approaches can be subdivided into three categories as noted earlier: local heating, locoregional heating and whole-body heating. The exposure time to achieve tumor cell death decreases with increasing temperatures (Figure 1). Hyperthermia treatment aims to provide synergistic effects with chemotherapy and/or radiotherapy in a typical 1h treatment, while thermal ablation is applied in a single short session lasting up to a few minutes. A general summary of heating techniques, goal temperatures, treatment scheme and duration is given in Table 1 and in this section an overview of clinical equipment for treatment of local disease (i.e. local and locoregional heating) and treatment of wide-spread disease (i.e. whole-body heating) is presented. At the end of each subsection a brief description of clinical applications is provided. These are summarized per tumor site in Table 2. Figure 3 presents a schematic overview of the heating depth from the skin and the tumor/target size that can be heated with various heating techniques. Figure 4 summarizes the principles of the different heating techniques graphically.
Table 1:
General summary of heating techniques, typical goal temperatures, treatment scheme and duration, as well as experimental (italic) and clinically proven applications.
| protocol | Synergizing with | Technique | Temperature range (⁰C) | Duration session (min) | Number of sessions & frequency | Tumor sites: Proven/routine experimental | refs | |
|---|---|---|---|---|---|---|---|---|
| Hyperthermia as sensitizer | Chemotherapy | Local | IHT | 39–45 | 60 | 1–12 sessions before, during or after chemo | ||
| SFUS | 39–45 | 30–60 | LI | [190] | ||||
| MNP | 39–45 | 60 | ||||||
| Intraluminal | 39–45 | 60 | BL, RE | [221–224, 400, 401] | ||||
| Capacitive | 39–45 | 60 | HN | [402] | ||||
| Radiative | 39–45 | 60 | ||||||
| Locoregional | Capacitive | 39–45 | 60 | BL, CE, LI, LU, OE, PA | [31, 295–297, 301, 305–308, 403–405] | |||
| Radiative | 39–45 | 60 | BL, CE, EX, LI, OE, PA, PE, PO | [311–315, 392, 406–412] | ||||
| HIPEC | 40–43 | 30–90 | 1 session during chemo | PE | [327–329, 332] | |||
| ILP | 38–39 | 60–90 | 1 session during chemo | EX | [335, 336] | |||
| WBH | 39–42 | 60–120 | LU, PA, PO, RE | [35] | ||||
| Radiotherapy | Local | IHT | 39–45 | 60 | 1–5 sessions, 1x/2x weekly during RT, before/after RT fraction | BR, HN, PR | [90, 117, 119, 413] | |
| SFUS | 39–45 | 60 | PR | |||||
| MNP | 39–45 | 60 | BR, PR | [214, 414] | ||||
| Intraluminal | 39–45 | 60 | OE, RE | [400, 415, 416] | ||||
| Capacitive | 39–45 | 60 | CW, HN, | [269, 417, 418] | ||||
| Radiative | 39–45 | 60 | CW, HN, IB, ME, | [253, 254, 257, 265, 267, 268, 419, 420] | ||||
| Locoregional | Capacitive | 39–45 | 60 | BL, BO, CE, LI, LU, OE, PA, PR, RE | [292–294, 296, 298, 299, 301–305, 309, 421–423] | |||
| radiative | 39–45 | 60 | BL, CE, OE, LI, PA, PR, RE | [272, 406, 410, 412, 424, 425] | ||||
| Thermal ablation | n.a. | Local | IHT | 50–100 | <5 | 1 session | BO, BL, BR, HN, IB, LI, KI, LU, OE, PA, PR, TH | [110, 121, 122, 124, 125, 127–142, 144, 145, 147, 149–151, 426] |
| SFUS | 50–100 | <5 | BO, BR, CE, IB, LI, KI, OE, PA, PO, PR, TH | [78, 81, 95, 138, 154, 167–169, 171–182, 184, 185, 187, 188] | ||||
| IRE | 50–100 | <10 | KI, LI, LU, PA, PR | [201–206] | ||||
BL: bladder, BO: bone (metastases, osteoid sarcoma), BR: brain, CE: cervix/uterus, CW: chest wall, EX: extremities, HN: head&neck, IB: intact breast, KI: kidney, LI: liver, LU: lung, ME: melanoma, OE: oesophagus, PA: pancreas, PO: pelvis other (soft tissue sarcoma/ pediatric tumors), PE: peritoneum, PR: prostate, RE: rectum, TH: thyroid.
Table 2:
Overview of clinical applications of local, locoregional and whole-body heating techniques. Open circles represent explorative (small/few phase I/II) use and solid black circles represent many/large phase I/II trials, retrospective analyses or phase III evidence.
| Local hyperthermia and ablation | Locoregional hyperthermia | WBH | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHT | SFUS | IRE | MNP | Intraluminal | Superficial | EM Locoregional | HIPEC | ILP | References | |||||||
| HT | ABL | HT | ABL | ABL | HT | ABL | cap | rad | cap | rad | ||||||
| BL: Bladder | ○ | ● | ○ | ○ | [55, 149, 216, 217, 221–224, 272, 300–302, 407, 408, 427] | |||||||||||
| BO: Bone (metastases, osteoid osteoma) | ○ | ● | ● | [121, 171, 309] | ||||||||||||
| BR: Brain | ● | ○ | ○ | ○ | [80, 118–120, 145, 147, 148, 154, 214] | |||||||||||
| CE: Cervix/Uterus | ● | ○/● | ● | [31, 167–170, 272, 307, 308, 310] | ||||||||||||
| CW: Chest wall | ○ | ● | [228, 232, 263–266, 417, 418] | |||||||||||||
| EX: Extremities | ● | ● | [311, 335, 336] | |||||||||||||
| HN: Head & Neck | ○ | ○ | ● | ●/○ | [130–133, 257, 269, 413, 419, 420] | |||||||||||
| IB: Intact breast | ○ | ○ | ○ | [136, 180, 253, 254] | ||||||||||||
| KI: Kidney | ○ | ○ | ○ | [134, 135, 184, 201, 428] | ||||||||||||
| LI: Liver | ● | ○ | ○ | ○ | ○ | ○ | [50, 104, 106, 107, 112, 124, 125, 139, 143, 144, 190, 201, 204, 305, 306, 412] | |||||||||
| LU: Lung | ● | ○ | ○ | ○ | [103, 108, 127–129, 140–142, 206, 292–297] | |||||||||||
| ME: Melanoma | ● | [267, 268] | ||||||||||||||
| OE: Oesophagus | ● | ○ | ○ | ○ | ○ | [122, 137, 185, 404, 405, 410, 415, 423, 426] | ||||||||||
| PA: Pancreas | ○ | ○ | ○ | ○ | ○ | ○ | [138, 179, 201–203, 315, 403, 406, 422] | |||||||||
| PE: Peritoneum | ○ | ● | [320, 322–325, 327–329, 392, 411] | |||||||||||||
| PO: Pelvis other: soft tissue sarcoma/ pediatric tumors | ○ | ● | ○ | [181, 182, 311–314] | ||||||||||||
| PR: Prostate | ○ | ○/● | ● | ○ | ○ | ○ | ○ | [78, 81, 90, 95, 117, 150, 151, 172–178, 205, 298, 299, 414, 424, 425] | ||||||||
| RE: Rectum | ○ | ○ | ● | ○ | [35, 272, 303, 304, 400, 401, 416] | |||||||||||
| TH: Thyroid | ● | ○ | [130–133, 187] | |||||||||||||
Figure 3:
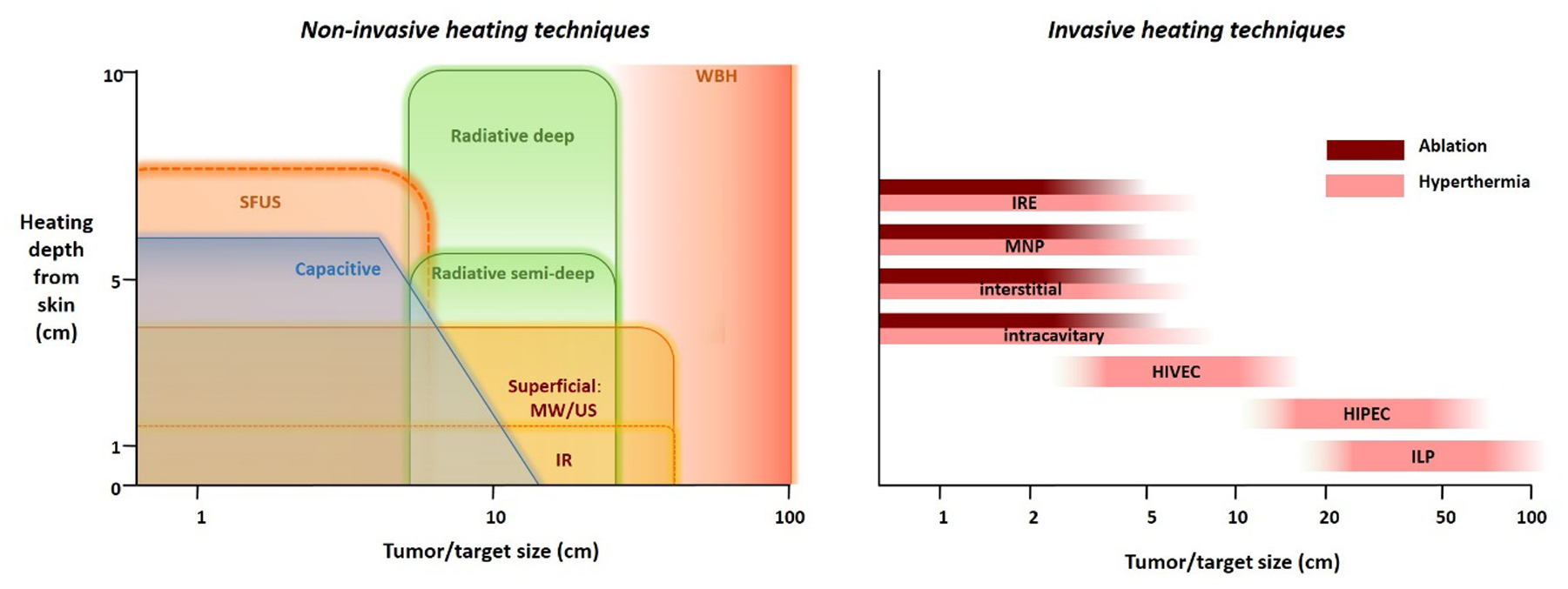
Left: schematic overview of the heating depth from the skin and the tumor/target size that can be heated with various non-invasive heating techniques. Right: Schematic overview of the tumor/target size that can be heated with various invasive heating techniques.
Figure 4:
Graphical representation of different heating techniques.
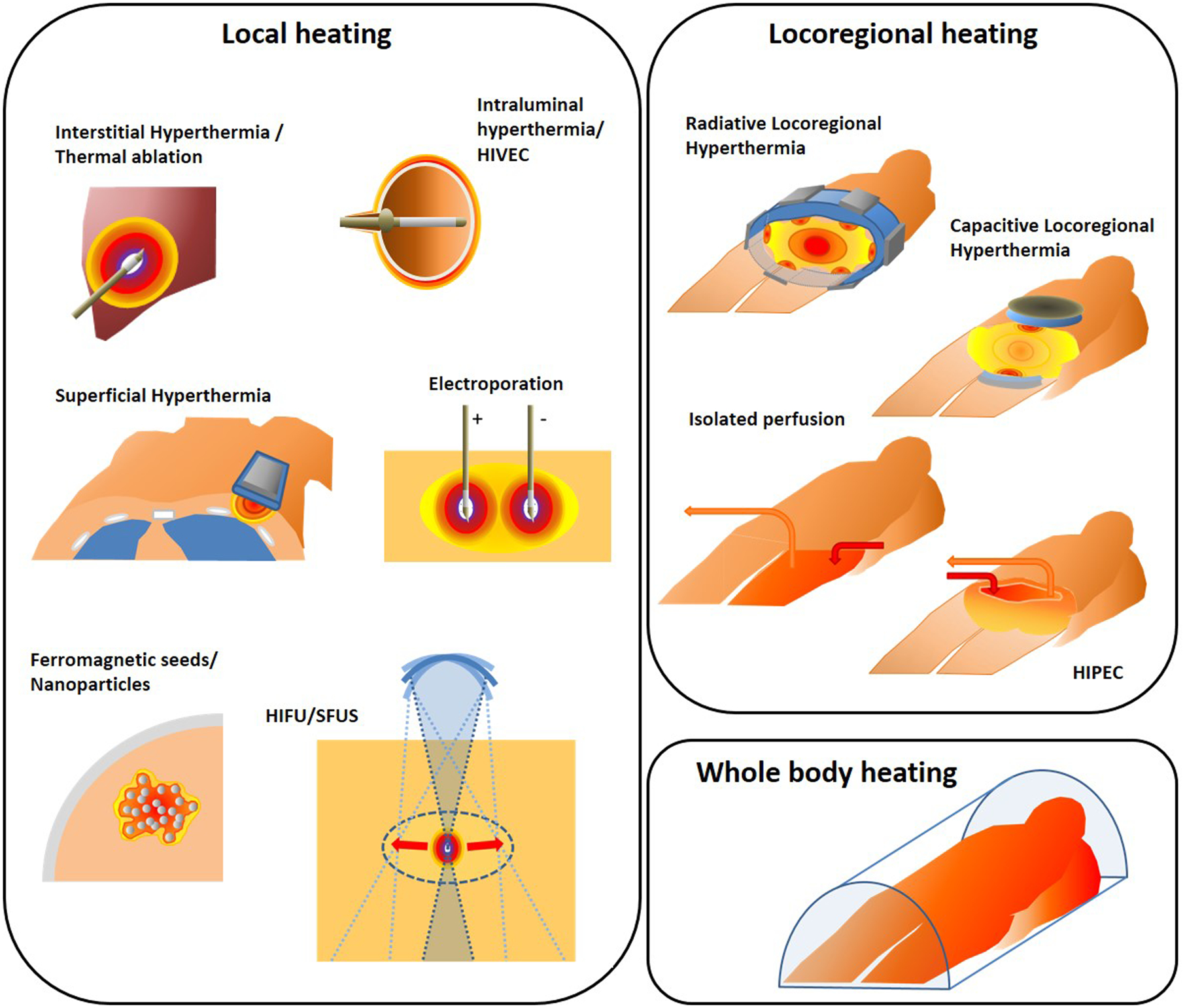
3.1. Local heating techniques
Local heating aims at heating the tumor target volume itself, with no (or minimal) heating of normal tissue surrounding the target. Tumor selective heating can be achieved with invasive applicators for deep seated locations, as used for interstitial heating, electroporation and nanoparticles, or with external applicators for more superficially located target regions. Scanned focused ultrasound with external applicators is the only non-invasive method capable of achieving local and conformal heating at deep seated locations. The different techniques and guiding rationale in each case are discussed in the following subsections.
3.1.1. Interstitial heating
Equipment:
Interstitial heating uses arrays of needle shaped applicators implanted into the target volume using catheters. Extensive research has been performed on interstitial heating techniques, resulting in several pre-clinical/research prototypes and commercially available products[70–74]. Investigations include hot sources[75–78], laser sources[79, 80], monopole, dipole, slot or helical coil microwave antennas[81–84], resistively-coupled radiofrequency local current field electrodes[85], capacitively-coupled radiofrequency catheter-based electrodes[86–88] and ultrasound transducers[89]. More detailed reviews of interstitial heating systems, including detailed system specifications, can be found in the literature[70–74].
Currently, ablative applications are more common, although initially applications of interstitial heating mainly focused on hyperthermia combined with brachytherapy or a brachytherapy boost, where the catheters used to deliver the radiation source can also be used for hyperthermia applicators and thermometry. Energy deposition for all techniques is confined to the close proximity of the individual applicators due to the line/point source nature of interstitial sources. The clinical challenge for interstitial hyperthermia is thus ensuring homogeneous hyperthermic temperatures. This requires close spacing of multiple small applicators within 1–2 cm apart to counterbalance the impact of heat loss due to (high) perfusion. This constrains the application to heating of relatively small tumor volumes[90].
When interstitial radiofrequency electrodes are used, the heating generated by the RF currents between electrodes is strongly influenced by local electrical tissue properties and the location and alignment of the other electrodes[91]. These drawbacks can be overcome by using electrodes operating from within low-loss catheters (e.g. Nylon or Teflon), creating high impedance capacitive coupling across the catheter wall, as in the 27 MHz multiple electrode current source (MECS) system[86–88]. A single probe consists of multiple independent electrodes, each 10–20 mm long. This enables 3D steering of the power deposition. Treatment monitoring and feedback is provided by multi-sensor thermocouple probes, integrated into the electrodes. The power and polarity of the electrodes can be modified to optimize the heating pattern.
Interstitial microwave antennas provide a better penetration depth than radiofrequency electrodes. The most clinically used microwave interstitial hyperthermia system is the commercially available BSD-500 interstitial/superficial system, involving a 915 MHz modified dipole antenna system from BSD – Pyrexar[92]. An 8-channel generator feeds into a 3- or 4-way power splitter to which up to 24 microwave antennas, inserted in standard Celcon or Nylon brachytherapy catheters, can be connected. Depending on the insertion depth, the effectively heated region can be up to ~5 cm in axial length. The antennas can operate in a conservative asynchronous mode or in a synchronous mode. In synchronous mode, the phase of each antenna can be modified with respect to the others to optimize the homogeneity of the power distribution. Up to 8 channels can be used for temperature monitoring using thermistor sensors[92].
The clinical challenge of interstitial ablation is to ensure complete tumor ablation, while avoiding thermal damage to surrounding healthy tissues. Catheter-mounted ultrasound transducers have been explored for interstitial ablation of tissue[93]. The radiating transducer portion can be segmented into separate tubular elements along the catheter length and even into separate sectors around the tube surface. Each sector or segment has individual power control, which provides precise control over both the angular and axial specific absorption rate (SAR) distribution[37, 72, 89]. This concept has been further explored to develop a probe for transurethral treatment of prostate cancer allowing full or focal gland ablation under MR-guidance[94]. The imaging is used for treatment planning as well as for near-real time temperature mapping during therapy with temperature maps being fed back to the system control for modulating power and device rotation speed[95]. The final CE and FDA approved device, TULSA-PRO was developed and marketed by Profound Medical (Mississauga, Canada). Transrectal US devices, which provide a high degree of spatial control have also been developed[96–98]. However, dwell times between sonications are required to protect the rectal wall from thermal damage, resulting in a relatively long treatment time. The advantage of transurethral US devices is that US is delivered from within the prostate gland, enabling continuous sonication and the use of unfocused beams for reduced treatment times[94].
Radiofrequency ablation (RFA) techniques have matured to facilitate ablation of larger tumors by introducing internal cooling, bipolar, straight and umbrella-shaped clustered probes and combinations of these design elements as strategies to maximize the size of the coagulation zone[99]. However, RFA at high power settings can result in charring of tissue immediately surrounding the electrode. This charring forms an effective insulator, thus limiting the size of the ablated region. Available monopolar RFA devices include the Cool‐tip Tyco (Tyco‐Valleylab, Boulder, CO, USA) and Radionics (Radionics, Burlington, MA, USA), the multitined RITA StarBurst (RITA Medical Systems, Inc., Mountain View, CA, USA) and RF 3000 Boston LeVeen needles (Boston Scientific Corporate, Natick, MA, USA). Celon Olympus (Medical and Industrial Equipment, Southend‐on‐Sea, Essex, UK) is a well-known producer of bipolar and multipolar devices. The circumferential HALO360+ bipolar RFA system and focal HALO90 (Covidien GI Solutions, formerly BÂRRX Medical) were developed for ablating the oesophagus.
Microwave ablation (MWA) is deemed capable of creating larger and more spherical ablation zones than RFA. Applicator cooling and an increase in the number of antennas are used to achieve a larger and a more spherical ablation zone[100]. For ablation of tissues with low electrical conductivity and poor thermal conduction, such as lung tumors, MWA is preferred to RFA. These characteristics do not degrade the volume heating of microwaves, allowing for larger ablation zones than RFA. MWA also allows more energy to be deposited more quickly than with RFA. Although this allows ablation of larger targets, the shape of the coagulation zone becomes less predictable, which increases the risk of adverse effects as intravascular steam burns outside the target. MWA devices operating at 2450 MHz and above are applied to create an ablation zone that is smaller and more spherical than the zone with devices operating at 915 MHz[101–103]. Available devices at 2450 MHz include the cooled 14 gauge mini-choked HS AMICA (HS Hospital Service, Rome, Italy)[104, 105], the Acculis (AngioDynamics, NY, USA)[106], the Solero (AngioDynamics, NY, USA), the Emprint Ablation System (Medtronic Inc, Minneapolis, MN, USA, formerly Covidien)[107, 108] and the NeuWave Certus Microwave Ablation System (NeuWave Medical, Madison, Wisconsin)[109]. For a more detailed overview of 915/2450 MHz devices the reader is referred to Lubner et al[110].
For both in RFA and MWA, the proximity of large blood vessels can cause cool tracks due to their long thermal equilibration length compared to the small lesion size[111]. This convective cooling effect counters the heat generation effects of the ablation devices and requires a modified ablation strategy, such as multi-applicator techniques, to optimize clinical outcome[112].
Besides RF, MW and US applicators, laser techniques are also used for thermal ablation. Laser Interstitial Thermal Therapy (LITT) uses laser light transmitted by an optical fiber which is converted into thermal energy and damages tissue in the target volume. The probe is often cooled to prevent tissue vaporization due to excessively high temperatures near the applicator[113]. An advantage of LITT is that it allows more accurate and safer ablation near critical structures than radiofrequency and microwave ablation. Commercially available systems include the Visualase Thermal Therapy System (Medtronic Inc, Minneapolis, MN) and the NeuroBlate LITT System (Monteris Medical Corporation, Plymouth, MN, USA). The Visualase Thermal Therapy System uses a diffusing fiber optic tip probe (980 nm at 15 W) combined with saline cooling and MRI temperature feedback[114]. Besides diffusing tip probes, the NeuroBlate LITT System provides a gas-cooled side-firing directional diode solid-state laser in the Nd-YAG range (1064 nm at 12 W). Control software-actuated rotational and linear movement is used to achieve spatially-controlled tumor ablation guided by real-time MRI temperature monitoring[80].
Clinical applications of interstitial heating:
Clinical use of interstitial hyperthermia includes mainly sites which are also considered suitable for brachytherapy such as prostate cancer, head and neck and brain tumors[71, 90, 115–120]. In a phase II/III randomized trial 79 glioblastoma patients were randomized to receive external beam radiotherapy (59.4Gy; 1.8Gy daily fractions), combined with a brachytherapy boost (60 Gy at 0.40–0.60 Gy/h) +/− 30 min interstitial hyperthermia at 915 MHz immediately before and after brachytherapy. Two year survival was 31% for the hyperthermia group vs. 15% for the brachytherapy alone group[119]. A retrospective analysis of 197 confirmed osteoid osteoma lesions treated with RFA showed this is a safe and effective technique. Two-year follow-up data were available for 126 procedures and showed 89% complete relief of symptoms. The success rate was 91% for initial treatments, and 60% for recurrences (p<0.001)[121]. The Surveillance versus Radiofrequency Ablation (SURF) trial randomized 136 patients with Barrett Oesophagus to RFA (n=68) or surveillance (n=68) and found that RFA reduced the risk of progression to high-grade dysplasia or adenocarcinoma to 1.5% for RFA vs 26.5% for control (95% CI, 14.1%−35.9%; P < 0.001) and the risk of progression to adenocarcinoma to 1.5% for RFA vs 8.8% for control (95% CI, 0%−14.7%; P = 0.03) [122]. A position paper by an international panel of ablation experts established the status of RFA in thermal ablation of colorectal liver metastases and recommended best practice based on 15 papers, each reporting on at least 50 patients, yielding a total of 1613 patients for evaluation. The data thus obtained allowed analysis of various relevant treatment parameters, including size of the tumor lesions that can be treated successfully, which is typically < 3 cm[123].
RFA and MWA are both widely used to ablate thoracic and gastrointenstinal tumors, e.g. liver[110, 124–126], lung[110, 127–129], thyroid[130–133], kidney[134, 135], breast[136], oesophagus[122, 137] and pancreas[138]. MWA is deemed capable of creating larger and more spherical ablation zones than RFA and thus clinical results of MWA are comparable to RFA for smaller lesions, but may be better for larger lesions[124, 125]. A randomized controlled trial involving 76 patients with hepatocellular carcinoma treated with RFA and 76 patients treated with MWA showed that at 2 years 6/98 lesions (6%) had local tumor progression in the MWA arm, versus 12/104 (12%) in the RFA arm (p=0.27)[139]. Better results for MWA versus RFA have also been reported for lung tumors[140]. Vogl et al. found in a retrospective analysis of 109 patients with colorectal lung metastases treated with LITT (21 patients, 31 ablations), RFA (41 patients, 75 ablations), and MWA (47 patients, 125 ablations) that MWA outperformed LITT and RFA in obtaining local tumor control[141]. Narsule et al found both RFA and MWA are a viable option for inoperable patients with early stage non-small cell lung cancer (NSCLC)[142]. MW ablation was also found to be safe, feasible, prevent hemorrhage and capable of eradicating tumors in treatment of hepatic adenomas[143]. A recent meta-analysis concluded that transarterial chemoembolization is more successful when combined with MWA for unresectable hepatocellular carcinoma with tumor size > 5 cm[144].
Clinical use of LITT in brain tumors appears to be safe and to result in better gross total resection combined with less neurologic morbidity than with surgical resection[145, 146]. A multicenter prospective study showed that LITT stabilized the Karnofsky score, preserved quality of life and cognition, and had a steroid-sparing effect in salvage treatment of patients showing progressive brain metastases after stereotactic radiosurgery[147]. LITT is thus currently frequently used as a salvage therapy for treatment of patients with recurrent malignant gliomas[148]. Laser ablation is feasible for bladder cancer as an alternative to transurethral bladder resection, although it remains unclear whether results are comparable due to the lack of randomized trials[149]. Laser ablation also provides a safe and effective treatment of obstructive benign prostatic disease, as demonstrated in a randomized study[150, 151]. Clinical outcome is equivalent to transurethral resection of the prostate (TURP), but with a more favorable toxicity profile; major intraoperative toxicity was absent[150, 151].
3.1.2. Scanned focused ultrasound
Equipment for ablation:
US and MR guided scanned HIFU is used for thermal ablation of tumors in a steadily growing number of applications[152]. During treatment, a piezoelectric ultrasound transducer is used to create a highly concentrated focal spot of acoustic energy. This HIFU focal spot is moved through the target region under US or MR guidance, with the aim of inducing temperatures exceeding ~60°C, which result in nearly instantaneous tissue coagulation in the focus while creating minimal thermal damage in the tissue around the focal volume. It is important that sufficient cooling time typically of a few minutes is ensured between sonication at two adjacent locations to prevent prefocal thermal damage. HIFU thus provides a noninvasive form of thermal tumor ablation with a 5–10% lower risk of side effects than for other methods[153]. The heating is localized to the focal volume, and, using long focal length transducers, also more deep-seated locations can be treated. Available CE or FDA approved devices include the Sonablate (SonaCare Medical, Charlotte, NC) and Ablatherm or Focal One® (EDAP TMS, Lyon, France) and the TULSA-PRO (Profound Medical Inc., Mississauga, Canada) for prostate tissue ablation, the Sonalleve system (Profound Medical Inc., Mississauga, Canada) and the ExAblate (InSighTec, Haifa, Israel) for both malignant and benign abdominal tumors and bone metastases. The ExAblate Neuro system uses dedicated phased-array transducers, which eliminates the need for a craniotomy when treating the brain. Up to 1024 ultrasound transducer elements mounted on a ‘helmet’ heat and ablate a deep brain target with no surgical incisions, guided by MR imaging. FDA approval was received for ablation of brain tissue for treatment of central tremor[154, 155].
Equipment for hyperthermia:
In principle MR guided HIFU is also capable of providing a temperature elevation in the mild hyperthermia range over an extended period of time (15–60 minutes), but this application is still experimental. HIFU guided by non-invasive MR thermometry is presently pursued primarily to induce local mild hyperthermia as a trigger for localized tumor-specific drug release from drugs encapsulated in thermosensitive liposomes[156]. Phantom tests with the ExAblate 2100 HIFU ablation system (InSightec Ltd, Haifa, Israel)[157, 158] and preclinical tests with the Sonalleve system (Profound Medical Inc., Mississauga, Canada) have demonstrated the feasibility of inducing a stable MR controlled temperature rise[159–161] resulting in significantly enhanced ThermoDox delivery in mouse[162], rat[163], rabbit[164] and pig models[165]. Using the electronic beam steering of a single HIFU focus limits the size of the maximum volume with a stable elevated temperature level to a few cubic centimeters. Larger volumes -as needed for human treatments- can be heated stably with multi-focus HIFU systems[166], or by a combination of electronic beam steering with a mechanical repositioning of the HIFU transducer[42].
Clinical applications for HIFU:
Good clinical results achieved in benign and malignant tumors include ablation of uterine fibroids[167–170], palliation of pain from bone metastases[171] and ablation of prostate tissue[172–177] have resulted in approval of both MR and US guided HIFU for the treatment of these sites in several countries. A prospective multicentre study of 625 patients undergoing HIFU for clinically significant nonmetastatic prostate cancer reported a 5 year failure-free survival, metastasis-free survival, cancer-specific survival, and overall survival of 88%, 98%, 100%, and 99%, respectively, with very few side effects compared to standard whole-gland therapy[173]. A review of 31 uncontrolled studies concluded that HIFU results in short- to medium-term tumor control in treatment of prostate cancer, with a low complication rate comparable with those of established therapies[178]. Successful use of HIFU mediated tumor ablation has been reported in a high number of explorative studies, among others for tumors of the pancreas[138, 179], the breast[180], soft tissue sarcoma[181], pediatric tumors[182, 183], liver[184], kidney[184], oesophagus[185], glioma[186] and other organs[187–189]. A recent pilot study, published in the Lancet Oncology, showed that highly targeted mild hyperthermia (≥39.5°C for at least 30 min) by ultrasound triggered delivery of doxorubicin to liver tumors that were refractory to standard chemotherapy is feasible, safe and able to enhance intratumoral drug delivery, providing targeted chemoablative response[190].
3.1.3. Electroporation
Equipment:
Irreversible Electroporation (IRE) is a relatively new interstitial local tumor ablation technique that creates pores in the tumor cell membrane by using a series of very short (e.g. ~100 μs), high voltage direct-current electrical pulses (ranging from hundreds to thousands of V/cm) between electrodes implanted around the tumor[191, 192]. The irreversible aspect is a result of the number of pores created such that a cell cannot recover from the collapse of cell membrane function. Though presented as non-thermal, inclusion of IRE in this review is justified since significant temperature rises in the hyperthermic and in the thermal ablation range are reported to occur during IRE, significantly contributing to the ablation effect[193–199]. These significant temperature increases are a result of the excessively high SAR generated in a short time (1 – 10 minutes) in the treatment area during IRE, particularly close to the electrodes. Careful placement of the electrodes is essential to manage these effects and prevent damage to surrounding healthy tissue, critical vascular structures and adjacent organs[200].
Clinical applications of IRE:
Clinical IRE is performed using the NanoKnife System of Angiodynamics (Latham, NY) and has been applied for treatment of tumors in lung, liver, kidney, pancreas and prostate, and has been proposed as a safer option than conventional ablation for tumors in close proximity to critical structures such as blood vessels and biliary ducts where thermal ablation would have caused serious complications[201–206]. Patients with persistent locally advanced disease after chemotherapy for whom surgical exploration is not feasible could benefit from this less invasive approach[202]. Note that general anesthesia is required with paralytic agents to prevent undesired muscular contractions, as well as careful synchronization with the electrical activity of the heart to avoid arrhythmias that could arise if the administered pulses stray outside of the refractory period of the cardiac cycle. Recently, high-frequency irreversible electroporation (H-FIRE) technology has been developed to enable electroporation of tumors without stimulation of the nearby skeletal muscle; this would avoid the need for general anesthesia[207]. Treatment efficacy decreases with increasing tumor size, and the procedure becomes more challenging with a large number of electrodes. Therefore, IRE should be limited to a maximum tumor diameter of 5 cm[208]. Phase III trials are ongoing, for example comparing chemotherapy plus stereotactic ablative radiotherapy with chemotherapy plus IRE for locally advanced pancreatic cancer.
3.1.4. Ferromagnetic seeds and nanoparticles
Equipment:
Ferromagnetic seeds provide a form of conductive interstitial hyperthermia developed for use in conjunction with brachytherapy. The advantage of using ferromagnetic seeds is the absence of a need for connecting wires since the heating is generated by exposing the patient to an external magnetic field, allowing for remotely controlled tissue heating from within the target[209]. Another advantage is the option to use self-regulating alloys, which heat only to the Curie temperature, i.e. the temperature at which a magnetic phase transition from ferromagnetic to paramagnetic occurs. The Curie temperature depends on the alloy composition and can be set to match the desired therapeutic level[210, 211].
Magnetic Fluid Hyperthermia (MFH) uses MNPs for heating the tumor while avoiding toxicity to the surrounding healthy tissue. The quality of the implantation of the nanoparticles in and around the target region, as pre-calculated in a planning system, is important. Nevertheless, by virtue of both variable tumor physical properties and complexities of nanofluid dynamics, nanoparticle distributions in tissue are often inhomogeneous[212], resulting in a heterogeneous temperature distribution. One method to compensate for this is to dynamically adjust the heating by adjusting the magnetic field amplitude[212]. Nanoparticle heat output is non-linear with magnetic field amplitude (i.e. specific loss power ∝ H2 or H3). The heat generated by nanoparticles will transfer away from the heat source(s) by conduction and convection, depending on tissue properties and cooling/heat sink effects of fluid (e.g. blood), respectively. On the other hand, tissue response to heating is also non-linear and dynamic vis a vis thermoregulatory response that changes the heat sink effect, and tissue damage resulting in collapse of perfusion-dependent cooling. Amplitude adjustment exploits these non-linear interactions to explicitly generate intense heat locally for a period of time (high amplitude), followed by a period of reduced heating (low amplitude) to allow time for heat generated to transfer throughout the target tissue. In the ideal scenario, a balance is struck between high/low amplitude cycling that deposits sufficient power to achieve a desired target thermal dose (e.g. CEM43T90 = 60 min). With this technique, the applied power is adjusted based on temperature feedback measured at the tumor-tissue boundary, in combination with a proportional-integral-derivative (PID) control system. Numerical simulations have recently shown that this is a promising strategy to realize a more homogeneous temperature distribution compared with straightforward constant power heating[212].
Clinical applications of ferromagnetic seeds and nanoparticles:
Clinical results of ferromagnetic seeds were encouraging; Mack et al. reported minimum and maximum temperatures of 41.0°C and 43.7°C and a 93% total response rate with only 7% grade 3 or 4 toxicity in a study of 44 patients with advanced primary or recurrent extra-cranial solid malignancies[213]. However, optimized performance of ferromagnetic seeds is impaired by the limited 3D temperature control and by the low thermal conductivity of the catheters or coatings, which are needed to implant the seeds or to ensure biocompatibility. This makes it difficult to ensure precision treatment.
Most MNPs are in a preclinical stage of development and clinical experience with MNPs is mainly limited to treating glioblastoma. The brain is a challenging tumor site where other hyperthermia methods are expected to cause major side effects. Results of a single-arm phase 2 study with 59 patients with small (< 4cm) recurrent glioblastoma lesions treated with MNP (median peak tumor temperature 51.2°C) combined with external beam radiotherapy (median biologically effective dose 30 Gy; 5×2 Gy/week) were encouraging with results suggesting a doubling of expected overall survival to 13.2 months, albeit in a favorable patient group[214]. For monitoring tumor progression the use of MRI is excluded due to the very high particle concentrations used, but good alternatives PET and SPECT are available[214]. NanoTherm® AS1 (MagForce Nanotechnologies AG, Berlin), the Iron-Oxide MNP used in this study, was approved for use combined with RT in patients with recurrent glioblastoma in Europe in 2010 and received FDA approval for a trial in prostate cancer patients in the USA.
3.1.5. Intraluminal hyperthermia / HIVEC
Equipment:
Intracavitary and intraluminal techniques apply local hyperthermia from within a cavity or lumen, e.g. vagina, oesophagus, urethra or bladder. Heating is generally limited to the lumen and its close proximity up to a depth of ≤1 cm. Both radiative and conductive techniques are available for intraluminal hyperthermia combined with chemotherapy for non-muscle invasive bladder cancer (NMIBC)[215]. Hyperthermic Intra-Vesical Chemotherapy (HIVEC®) involves recirculating a heated chemotherapy solution in the bladder using for example the Combat BRS system (Combat Medical, Wheathampstead, UK)[55]. Similar commercially available recirculating systems include the Unithermia system (Elmedical Ltd, Hod-Hasharon, Israel)[216] and the BR-TRG-I hyperthermic perfusion system (Guangzhou Bright Medical Technology Co., Ltd. Guangzhou, China)[217]. Recirculation of heated chemotherapy is a technically straightforward form of conductive heating and will give a fairly uniform temperature rise over the bladder wall provided the flow is sufficiently high. Its maximum penetration depth of ~0.5 cm in the bladder wall makes it suitable for early stage NMIBC[218]. Deeper tissue penetration up to ~1 cm is achieved using the Synergo® system (Medical Enterprises, Amsterdam, the Netherlands). This system uses an intracavitary 915MHz microwave antenna in a Foley catheter, which also includes up to five thermocouples to monitor the bladder wall temperature, and two channels for recirculation of the chemotherapy in the bladder[219, 220].
Clinical applications of intraluminal hyperthermia and HIVEC:
A recent trial in 161 intermediate and high risk NMIBC patients instilled with pirarubicin demonstrated a 24-month recurrence-free survival of 82.9% in the HIVEC group (n=76, three 45 min 45°C HT sessions 48h apart, 2nd session combined with pirarubicin instillation), versus 63.5% in the non-hyperthermia control group (n=85, p=0.008)[221].
A randomized controlled trial compared thermochemotherapy using the Synergo system (heating to 42±2°C) and chemotherapy alone with Mitomycin C for 83 patients with intermediate-/high-risk NMIBC. Eight weekly 40–60 min treatment sessions were followed by 4-monthly sessions. The 10-year disease-free survival rate was increased from 15% in the Mitomycin C arm to 53% in the Mitomycin C plus hyperthermia arm[222]. Two randomized trials demonstrated that Mitomycin C + Synergo based hyperthermia provides similar or possibly better results compared to standard Bacillus Calmette-Guérin (BCG) treatment for NMIBC patients[223, 224]. Disadvantages of this intracavitary heating approach are an uneven SAR distribution over the bladder wall, and that treatment of more deeply penetrating muscle invasive bladder cancer will require the use of a locoregional hyperthermia device[215, 218].
3.1.6. Superficial hyperthermia
Equipment:
In superficial heating, the energy is deposited in a limited volume of tissue close to the heating device. It is therefore applied only to tumors extending up to ~3–4 cm below the skin surface[225]. Several heating devices have been developed for both human[226] and veterinary clinical use[227]. Clinically used superficial heating equipment includes external infrared sources, microwave antennas, radiofrequency electrodes or ultrasound transducers[226].
Superficial tumors infiltrating up to 1.5–2 cm can be treated with infrared heating. The Hydrosun system (Hydrosun medizintechnik GmbH) provides contact free heating of large tumor areas. The penetration depth of infrared is normally less than 1 cm, but the Hydrosun applies water filtering of the radiation emitted by a halogen lamp of high color temperature to ensure effective heating up to 1.5–2 cm depth, and to avoid both overheating of the skin and tissue dehydration[228]. The Hydrosun system provides real-time infrared thermography measurement and control of superficial temperatures[228].
For superficial lesions with deeper infiltration, microwave heating is often applied. A water bolus with water circulating at a temperature typically around 40°C is used to couple the electromagnetic energy into the tissue. The selected exact water temperature influences the skin temperature and depends on the desired heating depth and whether the skin is target or not[229–231]. Many in-house developed prototypes have been introduced over the years and the first microwave systems usually applied a frequency of 2450 MHz[232, 233]. However, the penetration depth is relatively low at this high frequency, which means that tumors extending to 4 cm beneath the skin will not receive an adequate hyperthermia dose. Significant progress has been made in further development of systems with better penetration depth at 915 and 434 MHz. Furthermore, superficial antennas with various effective field sizes allowing adequate treatment of both small and larger tumor areas have been developed. This has resulted in clear progress in the quality of superficial heating equipment[234–239].
The 915 MHz BSD-500 system (Pyrexar Medical, Salt Lake City, USA), which delivers both interstitial and superficial hyperthermia, provides three different sizes of rigid waveguides: type MA-100, MA-120, and MA-151, with aperture sizes of 10 ×13 cm2, 18 × 24 cm2 and 4 × 5 cm2, respectively. The effective heating areas are somewhat smaller than the aperture size: 8 ×10 cm2, 12.5 × 19.5 cm2 and 2.5 × 2.5 cm2, respectively[225]. A water bolus couples electromagnetic fields into tissue. Additionally, two multi-element applicators are available with this system: an 8-element rigid array (SA-812) and a 24-element flexible array with spiral antennas (SA-24). The SA-812 applicator consists of a closely spaced array of 8 dual-armed Archimedean spiral antennas with a diameter of ~3.5 cm, 7 spirals surround a central spiral[240]. This allows adaptation of the heating pattern to irregularly shaped tumors by using appropriate amplitude settings for the individual antennas. The diameter of the applicator is 14 cm and spiral antennas are deposited on a plexiglass substrate with an integral water bolus. The SA-24 applicator consists of 24 dual-armed Archimedean spiral antennas mounted in a 4×6 array on a rectangular silicone carrier[241]. The applicator is flexible and can follow the body contour. The spiral antennas are connected in pairs of three to one of the eight RF power amplifiers of the BSD-500 system. These spiral antennas are considerably lighter in weight than waveguide applicators, and easier to position near complex patient anatomies. The heating depth of this 915 MHz system is 2–2.5 cm.
Deeper heating, up to 4 cm, can be achieved with 434 MHz antennas. The Yahta-4 system uses contact flexible microstrip applicator (CFMA; SRPC ‘Istok’, Fryazino, Russia) consisting of two coplanar active electrodes and a shield electrode, separated by a thin fluoroplastic substrate[242]. The applicator has an integrated water bolus and can be bent to follow the curvature of the body contour. Five different sizes are available to cover target regions of various dimensions. The smallest antenna, type 1H, has an aperture size of 7.2 × 19.7 cm2 and the largest, type 3H and 5H, have aperture sizes of 28.7 × 20.7 cm2 and 19.7 × 28.5 cm2, respectively, the difference being a 90° shift in field direction. Bending the applicator increases the effective heating depth[243, 244]. The water bolus thickness should not exceed 2 cm in order to avoid resonance effects[245]. The CFMA-SRH applicator has been developed with characteristics similar to the conventional CFMA, but allows simultaneous application of radiation and hyperthermia[246]. Simultaneous application of radiotherapy and hyperthermia yields comparable thermal enhancement in tumor tissue and normal tissue[247], which could lead to normal tissue toxicity. Therefore, tumor selective heating is important and new types of CFMA-SRH have been developed, consisting of an array of independent microstrip heating elements[238]. Four types of applicators are available, consisting of arrays of 1 × 3, 2 × 2, 3 × 3 and 3 × 4 heating elements. The sizes of the individual heating elements in the arrays range from 6 to 7.5 cm.
The ALBA ON 4000 (ALBA Hyperthermia, Rome, Italy) is a 434 MHz system for superficial hyperthermia, which uses antennas similar to the CFMA, but with a fixed curvature. The system has an integrated thermocouple thermometry system for continuous monitoring of 4–64 temperature sensors. The ALBA Double ON 4000 consists of two treatment units that can be used simultaneously. This allows coverage of larger treatment areas by placing two adjacent applicators, as well as treatment of two separate lesions at the same time.
The lucite cone applicator (LCA) is a 434 MHz water-filled horn applicator developed at Erasmus Medical Center[237]. An advantage of the LCA is the large effective field size, which approaches the aperture size and is ~2.5 times larger than for a conventional waveguide[236]. The aperture size of a single LCA is 10 × 10 cm2 and multiple antennas can be combined in an array configuration to cover the desired treatment area. In clinical practice, the size and rigidity of the LCA may compromise the treatment set-up and an applicator mounting system is required.
Superficial tumors can extend more than 4 cm deep and heating equipment operating in the microwave range does not provide sufficient penetration depth to heat these tumors effectively. Capacitive heating systems can penetrate to greater depths than 4 cm. Commercially available capacitive systems include the Thermotron RF8 (Yamamoto Vinita Co, Osaka, Japan), EHY-2000+ and EHY-2030 (Oncotherm Kft, Budapest, Hungary), Celsius TCS (Celsius42+ GmbH, Cologne, Germany), HY-DEEP 600WM (Andromedic srl, Velletri, Italy) and Synchrotherm (Synchrotherm, Vigevano, Italy). These systems all operate at a frequency of 8 or 13.56 MHz and because of the large wavelength at these frequencies, temperatures and treated volumes are comparable at 8 and 13.56 MHz. The devices use circular electrodes with an integrated water bolus bag and two opposing electrodes are positioned around the target region. The electrodes used clinically vary between 4 cm and 30 cm in diameter. When using two electrodes of differing size, the energy deposition is concentrated under the smallest electrode. Some systems apply added amplitude modulation in the kHz range to induce either additional non-thermal effects or very localized heating of cell structures. However, the latter has been demonstrated to be physically impossible[248], but research on the nonthermal effects is still ongoing, suggesting that the RF field might affect the mitochondrial function in cancer cells[249, 250] and change cell topography, morphology, motility, adhesion and division[251]. Capacitive variants of the CFMA and CFMA-SRH have also been developed, operating at 27, 40.68 or 70 MHz[238, 242], but clinical use of these antennas is sparse. A clinical limitation of capacitive heating in general may be excessive heating of fat tissue due to the orientation of the main electric field component[28, 252].
To realize the greater attainable penetration depth with radiative systems, either lower frequencies and/or multi-applicator arrangements are required. Radiative heating systems that provide sufficient penetration to heat deeply infiltrating superficial tumors are the 70 MHz AMC-2 system[253], the 70 MHz breast applicator developed at the Academic Medical Center Amsterdam[254] and the 140 MHz breast applicator developed at Duke University Medical Center[255]. The AMC-2 system has been developed for treatment of deep-seated advanced breast tumors and supraclavicular tumors[253]. The system consists of two horizontally revolving and height adjustable rectangular 70 MHz waveguides. The dorsal antenna is integrated in the treatment table and has an aperture size of 20×34 cm2. To enhance flexibility of this system. the top waveguide is interchangeable and aperture sizes of 8.5×34 cm2, 15×34 cm2 and 20×34 cm2 can be selected, depending on the treatment location and target dimension. Clinical results demonstrate adequate heating[253]. Both breast applicators have been developed for an intact breast. The 70 MHz AMC breast applicator has a treatment bed fitted with a 50×40×16 cm3 temperature controlled open water bolus[254]. The patient’s breast is immersed in the water and positioned in front of a 70 MHz waveguide in the bottom of the bolus, with aperture size 34×20 cm2. Clinical results demonstrate adequate heating, though the system is less comfortable for obese patients[254]. The 140 MHz Duke applicator uses a phased-array of four end-loaded dipole antennas, mounted on a Lexan water tank. Geometric focusing is employed, such that each antenna points in the direction of the target[255].
The HYPERcollar is a dedicated phased-array system for more challenging head & neck tumors and has been developed at the Erasmus Medical Center[256]. The system consists of multiple patch antennas, operating at 434 MHz. First clinical results show that the system is safe and feasible[257] and currently an MR-compatible system is under development[258].
Ultrasound energy has also been used to achieve superficial hyperthermia and there has been a similar evolution in technology as for microwave heating. Early devices applied single transducers operating at 0.5–3.5 MHz and only small superficial lesions could be treated[259]. The Sonotherm 1000 system (Labthermics Technologies Inc, Champaign, Il) provides two sizes of multi-sector applicators, combining multiple transducers in a non-focused planar array[260–263]. Each sector can be adapted individually to realize a uniform heating pattern. A 16-sector applicator of 15×15 cm2 has been developed to heat larger superficial tumor volumes with a depth up to 8 cm. A smaller 4-sector applicator of 7.5 × 7.5 cm2 allows treatment of anatomical sites difficult to reach with the large applicator, such as the head & neck. The treatment depth can be varied by changing the frequency (1 or 3.4 MHz). The system has a 16 channel thermometry system for thermocouple based thermometry and a therapy control algorithm controls the energy deposition, based on the information of the temperature measurement points.
Clinical applications of superficial heating:
Superficial heating is commonly applied to melanoma, head & neck cancer, and chest wall recurrences of breast cancer with good clinical results The importance of sufficient heating depth to achieve good tumor response was demonstrated for example by Van der Zee et al, who compared 2450 MHz and 434 MHz for treatment of recurrent breast cancer[232]. Re-irradiation (eight fractions of 4 Gy twice weekly) was combined with 60 min hyperthermia. The complete response rate (CR) was 74%, but for tumors with a diameter larger than 3 cm heating at 2450 MHz was less effective (65%) than for smaller tumors (CR 87%). Heating at 434 MHz performed equally well for tumors larger or smaller than 3 cm.
Randomized trials have demonstrated the effectiveness of radiation combined with microwave hyperthermia for recurrent breast cancer[264]. Vernon et al combined the results of five randomized trials for radiation +/− hyperthermia for superficial localized locally advanced and recurrent breast cancer and found an increase in overall CR rate from 41% with radiotherapy alone to 59% for combination with hyperthermia[265]. The greatest effect was observed in patients with recurrent lesions in previously irradiated areas, where the re- irradiation dose was limited. A randomized trial by Jones et al confirmed this good response and also showed that the effect was even better for previously irradiated patients[266]. For this group the CR was 23.5% for re-irradiation alone versus 68.2% in the re-irradiation plus hyperthermia arm. In a recent study very good results were also reported for water-filtered infrared heating using the Hydrosun system combined with hypofractionated re-irradiation (4 Gy once per week up to a total dose of 20 Gy, within 1–4 min after heating) for heavily pre-treated patients with large breast cancer recurrences[228]. A complete response rate of 61% was observed in patients with macroscopic disease with only grade 1 toxicity. This study suggests that even repeated re-irradiation could be considered as a treatment option when combined with hyperthermia.
The value of microwave superficial hyperthermia as an adjuvant to radiotherapy was also demonstrated in a multi-center randomized trial for patients with recurrent or metastatic malignant melanoma[267]. After a combined treatment of radiotherapy (three fractions of 8 or 9 Gy in 8 days) plus 60 min hyperthermia 2-year tumor control was 46%, compared with 28% with radiation alone[268].
Treatment of recurrent breast tumors with infiltration beyond 4 cm is more challenging. Capacitive heating yields sufficient penetration depth and allows therapeutic temperatures to be achieved, but temperature distributions can be very heterogeneous with treatment limiting hot spots [252]. The feasibility of adequate heating of these tumors with fewer hot spots has been demonstrated with the radiative 70 MHz AMC-2 system[253] and with the 70 MHz AMC breast applicator[254].
Capacitive hyperthermia has been demonstrated to be an effective technique for heating locally advanced head & neck tumors. A randomized trial comparing radiotherapy (66–70 Gy in 6.5–7 weeks) with radiotherapy plus weekly hyperthermia for 30 min showed that adding hyperthermia increased the complete response rate from 42.4% to 78.6% [269]. No excessive thermal toxicity was reported in this study.
Clinical results for ultrasound hyperthermia using the Sonotherm 1000 system have been reported for tumors in four sites: groin/trunk, axilla, breast or chest wall, and head & neck[263]. Therapeutic heating levels are feasible and of these categories groin and trunk tumors were most effectively heated. Head & neck tumors proved more difficult to heat with only 8% of the measurement points exceeding 42°C.
3.2. Locoregional heating techniques
Locoregional hyperthermia aims at heating the tumor target volume plus an additional margin of normal tissue. Locoregional heating techniques include electromagnetic heating, Hyperthermic IntraPeritoneal Chemotherapy (HIPEC) and isolated perfusion. Extending heating to an additional normal tissue margin is effective for pre-heating of blood supply to the tumor or to treat microscopic spread outside the macroscopic tumor.
3.2.1. Electromagnetic locoregional heating
Equipment:
Electromagnetic locoregional heating uses external heating devices with antennas positioned around the patient and is typically applied for deep-seated tumors, for example in the pelvic or abdominal region. In contrast to more focused techniques such as HIFU, with locoregional heating the region around the tumor is also heated to some extent and the temperature distribution is largely determined by the individual anatomy. In the pelvis tumor heating is sometimes restricted by hot spots near bony and fatty tissues. Compared with local heating techniques, locoregional heating allows a more homogeneous temperature distribution in the target region to be achieved, since the heated volume is larger, including a large normal tissue margin, and the inflowing blood will be slightly preheated and thus cause less cooling[111]. Mild heating of normal tissue around the tumor will usually not increase normal tissue toxicity when radiotherapy and hyperthermia are applied sequentially with a short time interval[270–272]. However, hot spots in normal tissue should be avoided. These are manifested as complaints of pain when the temperature exceeds 45°C. These hot spots typically occur at tissue interfaces associated with a large contrast in dielectric and thermal tissue properties, e.g. going from muscle to fat or bone, particularly if the E-field is perpendicular to these interfaces. Pace-makers and metallic implants in the treatment region are usually a contra-indication for treatment.
Electromagnetic locoregional heating can be performed using capacitive heating devices or radiative phased-array systems. Commercially available capacitive heating systems have already been discussed in the section “Superficial Hyperthermia”. For deep heating of centrally located tumors equally sized electrodes with a diameter ≥ 25 cm are usually used. These double-electrode systems have no power steering potential to avoid hot spots. The choice of bolus and its specific design is important for capacitive heating to reduce heating of the superficial fat layers, which is a serious risk because the main E-Field direction is perpendicular to the superficial fat layers. Overlay boluses can be used for more aggressive skin cooling[273], but achievable temperatures at deep-seated tumor locations decrease for increasing fat layer thickness[29].
Radiative phased array systems consist of multiple antennas organized in one or more rings and allow power steering by adapting phase amplitude settings of the individual antennas or antenna pairs. The dominant E-field direction is parallel to the superficial fat layers, i.e. in cranial-caudal direction. Focusing of the electromagnetic energy on the tumor location, as well as suppression of severe hot spots in normal tissue can be realized by adjusting phase and amplitude settings for power steering to create constructive interference in the tumor and destructive interference at hot spot locations among the electromagnetic fields radiated by the antennas[274–276]. In the applied frequency range, i.e. between 60 and 150 MHz, the heating focus is typically 10–15 cm in diameter, which allows heating of larger tumors compared to other techniques (Figure 3). Commercially available phased-array systems are the BSD-2000 systems (Pyrexar Medical, Salt Lake City, USA) [275, 277] and the ALBA 4D system (ALBA Hyperthermia, Rome, Italy)[278]. Because of the large number of degrees of freedom of these systems, optimization of antenna settings by treatment planning is becoming increasingly common (see section Treatment planning) and has been validated for both systems[279–282].
The BSD-2000 systems include the Sigma-60 system and the smaller bore Sigma-30, with 8 paired dipoles organized in one ring and the Sigma-Eye system, with 24 paired dipoles distributed over three rings, which allows improved power steering properties. Cross-coupling between antennas in the dipole array can affect phase-amplitude control during clinical applications[283, 284], but online adjustments can overcome this problem[285]. A hybrid version of the Sigma-30 and Sigma-Eye system has been integrated in suitable MR-scanners to enable non-invasive MR-thermometry. The first prototype of this MR-HT hybrid system was installed and evaluated at Charité Berlin using a Siemens Symphony 1.5 T scanner[286–288]. Other versions employ a 1.5 T Philips Ingenia MR system and a 1.5 T GE MR450w MR system[289]. More details on the status of MR thermography are given in the thermometry section.
The ALBA-4D system consists of four rectangular waveguides organized in one ring and is similar to the AMC-4 system, developed and built by the Academic Medical Center Amsterdam[290]. The AMC-8 system, with 8 rectangular waveguides in two rings, has also been developed and built by the Academic Medical Center Amsterdam[291]. This system has a greater heating length and improved power steering compared with the single ring AMC-4 system.
Clinical applications of electromagnetic locoregional heating:
Capacitive locoregional heating has been investigated for several tumor locations, such as lung[292–297], prostate[298, 299], bladder[300–302], rectum[303, 304] and liver[305, 306], and treatments were generally deemed feasible. Degrees of clinical success are usually varying, but good clinical results can be achieved with capacitive hyperthermia, provided that the patient has only a thin superficial fat layer[29]. Increasing temperatures to 38–39°C have shown a significant increase in tumor blood perfusion for cervical cancer patients[30]. Early results of an ongoing phase III trial using the EHY-2000+ device report improved 6 month local disease control for cervical cancer patients treated with radiochemotherapy combined with capacitive hyperthermia (45.5%) compared to radiochemotherapy alone (24.1%)[307]. A multicenter randomized study in cervical cancer patients showed that cisplatin-based radiochemotherapy plus weekly hyperthermia for 60 min using capacitive heating (Thermotron RF8) resulted in a higher 5-year complete response rate (88%) than with radiochemotherapy alone (77.6%)[308]. Clinical outcome appeared to depend on the thermal dose achieved; a successive analysis showed that a thermal dose of at least one cumulative equivalent minute at 43°C (CEM43T90 ≥1) is required for a significant effect of hyperthermia[31]. A phase III prospective, randomized controlled trial demonstrated the effectiveness of capacitive hyperthermia in patients with painful bony metastases[309]. Radiotherapy (10×3Gy) plus twice weekly hyperthermia for 40 min showed a significant increase in pain control rate and an extended response duration compared to radiotherapy alone. The accumulated complete response rate within 3 months after treatment was 58.6% in the radiotherapy+hyperthermia group versus 32.1% in the radiotherapy-alone group[309].
Excellent clinical results have been achieved with radiative locoregional heating of deep-seated tumors in the pelvis, including cervix, bladder, rectum, sarcoma and pediatric tumors. The Dutch deep hyperthermia trial showed the beneficial effect of combining standard radiotherapy with 60 min weekly hyperthermia for pelvic tumors (cervix, rectum, bladder)[272]. The treatment effect did not differ significantly with tumor site, but adding hyperthermia seemed to be most effective for cervical cancer, for which 3-year overall survival was 27% in the radiotherapy group and 51% in the radiotherapy plus hyperthermia group[272]. After 12 years the survival of the patients in the radiotherapy plus hyperthermia group was still twice as high as survival in the radiotherapy-arm (37% vs 20%)[310]. A randomized phase III trial for soft-tissue sarcoma demonstrated that adding 60 min hyperthermia to chemotherapy (etoposide, ifosfamide and doxorubicin) on day 1 and 4 for each cycle significantly improves long-term outcome, with a 10-year survival of 52.6% for chemotherapy plus hyperthermia versus 42.7% for chemotherapy alone[311]. The combination of hyperthermia plus chemotherapy is also very important in treatment of pediatric sarcoma or germ cell tumors that respond poorly to chemotherapy, or recur after chemotherapy[312–314]. A prospective study reported a 5-year survival of 72% and the long-term prognosis was almost similar to those for patients receiving first-line chemotherapy treatment[312]. Ongoing clinical trials evaluate the addition of radiative locoregional hyperthermia to chemotherapy in treatment of e.g. pancreatic cancer[315], or to chemoradiation in treatment of e.g. rectum, pancreatic and anal cancer[316–318].
3.2.2. HIPEC
Equipment:
Hyperthermic IntraPeritoneal Chemotherapy (HIPEC) is a procedure during which a solution containing chemotherapeutic drugs is maintained at an elevated temperature level (41–43°C) and circulated in the peritoneal cavity (abdomen) for a period of 30–90 minutes, aiming at eradication of microscopic tumor lesions of peritoneal carcinomatosis (PC). As drug penetration is limited to a few millimeters[319]. HIPEC is generally preceded by cytoreductive surgery (CRS), surgical removal of macroscopic tumor[53]. Commercially available HIPEC devices consist of a peristaltic or roller pump, a temperature controlled container for the chemotherapy, tubing to transport chemotherapy to and from the patient and temperature probes to be used in the peritoneal cavity. Systems used include the ThermoChem™ HT-1000 and HT-2000 (ThermaSolutions, White Bear Lake, MN, USA), Performer (RAND Biotech, Medolla, Italy) and other devices. The chemoperfusion technique is either ‘closed’ (with temporary skin closure during chemoperfusion) or ‘open’ (the coliseum technique, referring to the skin being pulled upward around the open peritoneal cavity) during HIPEC[53]. Both approaches have specific advantages in terms of flow and temperature management during HIPEC.
Clinical applications of HIPEC:
Clinical application of HIPEC has gained much popularity in the last decade[320–326], with promising results in stage III ovarian cancer and in gastric cancer with peritoneal metastases. A phase III study comparing cytoreductive surgery with surgery plus 90 min HIPEC with cisplatin at 40°C for ovarian cancer reported that HIPEC increased the median overall survival from 33.9 months to 45.7 months after a median follow-up of 4.7 years, with no significant difference in side-effects[327]. In gastric cancer with peritoneal metastases, a recent retrospective multicenter comparison with propensity score based matching of CRS alone versus CRS combined with HIPEC showed that the addition of HIPEC improved overall survival from 12 to 19 months (hazard ratio 0.42 – 0.86; p = 0.005)[328]. In colorectal cancer with peritoneal metastases (PM), the place of HIPEC remains uncertain. A Dutch randomized trial comparing IV chemotherapy alone (only fluorouracil with leucovorin) with CRS and 90 min mitomycin C-based HIPEC at 41–42°C inflow temperature showed that the median survival doubled from 12.6 months with standard therapy to 22.3 months with CRS and HIPEC, after a median follow up of 21.6 months[329]. However, recent randomized trials of surgery alone versus surgery combined with oxaliplatin based HIPEC for 30 min at 42°C failed to show efficacy of the addition of HIPEC, either in the treatment or in the prevention of PM[330–332]. As a result, the value of HIPEC for colorectal cancer regarding optimal choice of drug, HIPEC temperature and treatment duration are under debate[333], even including the role of the raised temperature[334].
3.2.3. Isolated perfusion
Equipment:
Prophylactic hyperthermic isolated limb perfusion (ILP) is applied for treatment of localized high-risk melanoma and soft tissue sarcoma in extremities[335, 336]. ILP involves placement of intraluminal vascular catheters in the inflow and outflow vessels of the extremity, which are then connected to an extracorporeal flow circuit to circulate heated chemotherapeutics for 60–90 min. An upstream tourniquet compresses collateral vessels to fully isolate the extremity. An advantage of ILP is the ability to use higher drug doses, thus locally enhancing efficacy of chemotherapy in the limb while avoiding systemic side effects. By using the vascular system for heat delivery, the temperature rise is inherently uniform. All the equipment required consists of standard equipment already in use for standard surgical care (tourniquet, catheters, heart-lung machine).
Clinical applications of isolated perfusion:
This very effective treatment approach may avoid the need for amputations. A multicenter non-randomized trial combining tumor necrosis factor (TNF) with 60–90 min melphalan-based ILP for treatment of locally advanced soft tissue sarcomas in extremities reported response rates above 70% and limb salvage rates above 80%[336]. For ILP mild temperatures of 38–39°C are essential to obtain a good response without damage to the normal tissues in the limb. Higher temperatures (42–43°C) increase the response rate, but are also associated with very severe normal tissue toxicity[336].
3.3. Whole body hyperthermia
Equipment:
During whole body hyperthermia (WBH) the body core temperature is increased to 39–40°C for 6 hours (fever range WBH)[337] or to 41–42°C for 60 min (extreme WBH) [35, 338]. WBH is usually applied in combination with systemic therapy, for treatment of metastatic disease. Insulation of the body is required to avoid heat loss. During extreme WBH the patient is under sedation or even general anaesthesia. Several systems have been developed to apply WBH using radiant heat. Current commercially available WBH systems use infrared radiation and include the Iratherm1000 (Von Ardenne GmbH, Dresden, Germany) and the Heckel HT3000 (Hydrosun Medizintechik GmbH, Müllheim, Germany) systems. Both devices use water-filtered infrared A and are capable of providing a safe, well controlled and fairly homogeneous temperature elevation[338]. Extra-corporal perfusion and convective WBH techniques have also been applied, but these tended to be associated with higher levels of toxicity and have been abandoned in favor of radiative WBH techniques[338].
Clinical applications of whole body hyperthermia:
Phase I/II clinical trials have been performed for e.g. ovarian cancer, colorectal adenocarcinoma, lung cancer and sarcoma and studies report serious grade 3/4 toxicity, of which myelosuppression is most common[35]. Absence of phase III trial results makes the additive value of WBH as part of palliative or curative care unclear. Less demanding treatment options are usually preferred. Ongoing research is investigating the potential benefit of WBH in combination with immunotherapy[13] as well as for non-malignant diseases[339].
4. Treatment control
Treatment control is important for ensuring high quality hyperthermia treatments. For all non-conductive hyperthermia techniques the steady state tumor temperature distribution achieved during hyperthermia depends on the balance between heat generated in the tissue by the hyperthermia device, and heat removed by thermal conduction, blood flow in discrete large vessels and perfusion in small vessels. Heat generation and removal are both difficult to predict accurately due to uncertainty in the dynamic and patient dependent tissue and perfusion properties. At hyperthermic temperatures, perfusion is significantly increased in the heated region as a result of physiological temperature regulation mechanisms in the body. The magnitude of this increase is dynamic and on forehand unknown and depends on the local temperature and other factors[340]. Therefore temperature feedback combined with treatment planning are needed to generate the desired temperature distribution. In this section we briefly discuss the role of thermometry and treatment planning for achieving treatment control for the devices presented in this review.
4.1. Thermometry
Adequate temperature control is crucial in clinical hyperthermia in view of the strong dose-effect relationship of hyperthermia both for achieving optimal therapeutic gain[3, 268, 341] as well as for avoiding side effects in normal tissue[342, 343]. Thermometry is performed to this end with either single or multi-sensor probes in or near the target volume, or non-invasively by external thermometry methods. Invasive temperature probes provide the gold standard (except with ultrasound where viscous artefacts give unpredictable results) and are based on different measurement principles, including thermocouples, thermistors and fiber-optic sensors. A method is selected based on its appropriateness, including compatibility with the hyperthermia methods, used as outlined in reviews and guidelines[344, 345]. The metal leads of thermocouple probes require attention as they may cause local interference and erroneous read-outs when combined with EM and US based hyperthermia devices[346–348]. When thermocouple thermometry is combined with MR thermometry the metal leads may cause field inhomogeneities resulting in critical MR signal voids. Fiber-optic probes are generally insensitive to electromagnetic interference, but are mechanically fragile and can be disturbed when used during US or laser-based hyperthermia. A major limitation of probes is their invasive nature, which is not always feasible.
Non-invasive thermal monitoring would be ideal, and for very superficial skin tumors real-time non-invasive infrared thermography can provide 2-D temperature maps, this thermometry method measures the infrared energy of the patient and converts this to temperature. Infrared thermometry is standardly used in combination with the contact-free infrared hyperthermia treatment using the Hydrosun system (Hydrosun medizintechnik GmbH)[228]. For 3D thermal monitoring, CT, MR and US based non-invasive thermometry methods have been developed[345, 349–351], but presently these do not provide the same accuracy as thermometry probes. A major drawback of CT thermometry is the ionizing radiation issue, which makes that MR and US-based methods are generally preferred. When applying MR-thermometry, proton resonance frequency shift (PRFS) methods are preferred because of the excellent linearity and temperature dependence of the PRF[349]. Qualitative US ablation monitoring, which targets tissue coagulation and bubble formation is feasible and robust US thermometry exists for temperatures above 50°C[345]. Several ultrasound acquisition strategies are employed in ultrasound thermometry and ablation monitoring. For example, changes in stiffness monitored with US shear wave imaging are indicative of cellular necrosis[352].
Non-invasive thermometry is less challenging and often clinically used for thermal ablation monitoring, where a relatively low temperature resolution suffices to detect a temperature rise as this rise is much higher (10 – 40 °C) than for hyperthermia devices (1 – 6 °C). Therefore, applications for mild hyperthermia are still limited and particularly preferred for tumor sites where placement of invasive probes bears significant clinical risks, bearing in mind the pitfalls of the presently available MR or US based non-invasive thermometry methods when used in the mild hyperthermia temperature range. Although US methods have made significant progress for thermometry in this range[345], noninvasive PRFS-based MR-thermometry is most frequently used[353]. This method can provide reference-based temperature maps in near-real time. Under favorable, low blood flow conditions an accuracy of ~1°C can be achieved, but the method is sensitive to field drifts and susceptibilities and significant artefacts can occur due to movement, e.g. in the presence of high blood flow, breathing, cardiac and bowel motion, limiting the number of appropriate tumor locations[345, 349, 354]. These issues all need to be compensated for, for accurate temperature mapping. MR-guided hyperthermia works well for large fixated tumors (especially soft tissue sarcoma)[355]. Since MR-thermometry requires a trade-off between spatial and temporal resolution, acquisition is often limited to representative tissue slices to reduce acquisition times and allow online control. Practical methods for on-line optimization of the temperature distribution achieved with locoregional hyperthermia with MR-thermometry feed-back are still under development[285].
For scanned focused ultrasound treatments, US guidance offers fast imaging with the benefit of immediately revealing obstacles in the acoustic beam path, while MR provides high contrast soft tissue images suitable for planning. Furthermore, since MR allows the acquisition of near-real time temperature information, this can be used to establish a feedback system to the ultrasound driving electronics[356, 357]. The closed-loop feedback can be used either to achieve or to maintain a given target temperature or to deliver a predefined thermal dose. This provides better lesion control as it can compensate real-time for local differences in ultrasound absorption or heat loss due to perfusion. Recently, a model predictive control algorithm in combination with MR temperature mapping was introduced for a voxel-based heating strategy that allows compensation for local heat losses e.g. due to perfusion with a high spatial resolution[39]. MR thermometry finds now routine use in all MR-HIFU applications[358, 359].
Based on these useful clinical applications, heating devices such as HIFU or RF antennas have been integrated into MR scanners to allow temperature mapping[285, 360]. Commercially available MR guided devices which incorporate MR-thermometry sequences include the BSD-2000 sigma-eye which can be integrated into the MR systems of various vendors (see section “Locoregional heating techniques”), as well as the Sonalleve HIFU system integrated into the 3T Achieva MR system (Philips) and the ExAblate FUS system integrated into the Discovery MR450w 1.5T wide bore and Discovery MR750w 3.0T wide bore MR systems (GE). US guidance is clinically used in combination with ablative SFUS devices; the Sonablate[361] and Ablatherm[362] systems, where feedback is based on changes in echogenicity rather than temperature mapping[363] (see also section ”Local heating techniques: scanned focused ultrasound”).
4.2. Treatment planning
Treatment planning simulates SAR distributions and/or temperature patterns in the patient to visualize the effect of different treatment strategies[364]. This can be very helpful in assisting in treatment control. The prediction of SAR and temperature distributions is clinically helpful, even though well-known dependencies on patient models and tissue properties remain. Therefore, quantitatively reliable prospective treatment planning of SAR distributions is challenging and quantitative prediction of temperature is even more challenging because of the uncertainties in tissue properties, and a lack a priori in knowledge of the dynamic changes in perfusion levels which occur during heating[364–367].
Treatment planning is therefore still a research tool for most heating techniques, or is under development for relatively new techniques such as MNP, IRE and HIPEC[368–372]. For thermal ablation, treatment planning can be especially helpful to predict the size of the ablation zone when the target region is located close to critical structures or large cooling blood vessels which can affect the ablation zone[373]. Recent research focused on 3D planning of image-guided RFA by means of interactive access path determination based on optimization[374]. A first retrospective study indicated that this approach has the potential to improve the classical RFA planning based on inspection of 2D images alone[374]. Software-guided RFA of primary and secondary liver tumors is currently evaluated in the ClinicIMPPACT study; a multicenter-, prospective-, non-randomized clinical trial to evaluate the accuracy and efficiency of innovative planning and simulation software[375]. MWA has the potential of creating larger ablation zones compared to RFA, but at the same time this yields a greater risk of thermal damage in surrounding healthy tissues, which explains the research interest in development of personalized MWA treatment planning[376]. However, the ultimate planning of MWA is still based on clinical experience of the physician. Characterization of dielectric and thermal property changes and tissue shrinkage are essential for the development of numerical predictive tools.
An additional challenge for reliable hyperthermia treatment planning is the fact that dielectric tissue properties change significantly during treatment in the ablative temperature range[377]. To improve the robustness of treatment planning predictions for ablation, application of probabilistic modelling techniques is presently investigated[378]. For most anatomical locations (with exception of the brain) treatment planning for ultrasound is very challenging since full-wave 3D simulations are extremely computationally intensive, and thus it is mostly used to optimize or adapt the aperture of US phased arrays to the tumor location or shape[379, 380]. Treatment planning for HIFU has to some extent become obsolete because of the use of on-line MR-thermometry for treatment guidance.
Significant progress has been made in the development of treatment planning for classical electromagnetic superficial and locoregional hyperthermia, where the integration into the clinical workflow is becoming common practice[364]. Treatment planning can be very useful for applicator selection to determine a treatment strategy[27, 381] or to evaluate potential for heating[280, 382]. Despite the limited quantitative reliability, treatment planning can help to optimize heating patterns for locoregional phased array systems. A cross-over trial comparing treatment planning-guided steering and steering with empirical steering guidelines demonstrated that steering guided by treatment planning is feasible[383]. Since measured and simulated changes in SAR and temperature resulting from phase and amplitude adjustments during hyperthermia treatments correlate[384, 385], adaptive treatment planning was further evaluated to be used on-line to determine alternative antenna settings that suppress hot spots, or to improve tumor heating during treatment. The practical use of treatment planning to improve treatment control has been demonstrated during clinical hyperthermia treatments[34, 355, 386, 387]. Furthermore, ongoing research aims to quantify the radiosensitizing effect of hyperthermia in terms of equivalent radiation dose, i.e. the radiation dose yielding the same biological effect as the combination of radiotherapy and hyperthermia. Results from biological modelling applied to prostate cancer[388], cervical cancer[389], and recurrent paediatric sarcoma[390] patients demonstrated substantial dose escalations of typically 10 Gy by adding hyperthermia. Biological modelling can also be useful to answer clinically relevant research questions such as the optimal timing between radiotherapy and hyperthermia[271]. Thermoradiotherapy planning is feasible and important to optimize hyperthermia combined with radiotherapy in individual patients[391].
5. Future perspectives
As demonstrated in this review, a large number of hyperthermia and thermal ablation devices have been developed, which are routinely and successfully used for a variety of tumor indications. Progress has resulted in many dedicated devices designed to meet specific heating requirements for specific tumor sites. The hyperthermia field has progressed from single source hyperthermia applicators to sophisticated multiple source instruments. Successful clinical trials have been conducted which combined hyperthermia with radiotherapy or chemotherapy. In some cases, the hyperthermia devices possessed limited spatial control of the SAR distribution. Nevertheless, results demonstrated that the degree of control was sufficient for the tumor sites involved, which included bladder, superficial tumors and deep-seated pelvic tumors. Unmet clinical needs are progressing towards more challenging tumor sites such as brain, deep-seated head and neck tumors and peritoneal metastases[392–395]. As a result, there is a trend towards device designs providing greater spatial control of the energy deposition to ensure that SAR and temperature distributions in tissues reliably match with rigorous treatment plans to achieve effective treatment of challenging tumor sites, while sparing adjacent critical structures. Real-time 3-D temperature feedback data combined with reliable treatment planning to guide the applicator settings is also required to achieve the desired tumor target temperature distribution. Examples of this trend include development of dedicated MR guided hyperthermia applicators for brain tumors[393] and H&N tumors[256–258] based on MW technology, as well as US or MR guided HIFU devices, both for hyperthermia and for thermal ablation. A design challenge for most of these devices is matching the technical and clinical performance of the device with the oncological requirements for the specific site, e.g. the target volume size for gastrointestinal tumors and the need to spare surrounding normal tissue for brain tumors. For treatment planning, the use of validated and robust algorithms is important, which could possibly be standardized by defining established modelling and validation guidelines for development of software tools.
As shown in Figure 5, another clear trend over the past two decades is the steadily growing interest in almost all hyperthermic applications, but especially applications of thermal ablation techniques and the more recent rise of HIPEC. This trend is also evident in the increasing clinical application of specific forms of thermal ablation and hyperthermia as shown in Table 2. Thermal ablation is now more popular than application of hyperthermia as radiosensitizer or chemosensitizer, and the rapid rise of HIPEC and hyperthermic chemotherapy for NMIBC testify that combination of hyperthermia with chemotherapy is growing faster than combination of hyperthermia with radiotherapy, which is also associated with a different focus in device development. A breakthrough in heat-triggered tumor targeted drug delivery is one trend that would further strengthen popularity of the combination with chemotherapy[156, 162–165, 190]. Finally, the recent trend to combine thermal therapies with immunotherapies has demonstrated significant potential to address recurrence and metastasis in preclinical and clinical pilot studies[396–399]. When this is supported by further clinical evidence, a further growing interest in hyperthermic applications can be expected.
Figure 5:
Trends in heating technologies, represented by a rough estimate of the number of publications from 1995 till present on whole body hyperthermia (WBH), hyperthermia combined with radiotherapy (HT+RT), hyperthermia combined with chemotherapy (HT+CHT), hyperthermic intraperitoneal chemotherapy (HIPEC) and thermal ablation. Search terms used in Web of Science were: (WBH OR whole body hyperthermia; (hyperthermia AND radiotherapy) OR thermoradiotherapy; (hyperthermia AND chemotherapy) OR thermochemotherapy; HIPEC OR Hyperthermic Intraperitoneal Chemotherapy; thermal ablation OR RFA OR MWA). NB: Although this search did probably not retrieve all relevant publications and some overlap is likely between topics (e.g. HT+CHT and HIPEC), this graph is indicative for the trends in interest for different heating techniques.
Funding
Funding support for R. Ivkov was provided by the National Cancer Institute (5R01CA194574-02 and 5R01CA247290), and the Jayne Koskinas Ted Giovanis Foundation for Health and Policy.
Footnotes
Disclosure statement
R. Ivkov is an inventor on nanoparticle patents. All patents are assigned to either The Johns Hopkins University or Aduro BioTech, Inc. R. Ivkov consults for, and is a member of the Scientific Advisory Board for Imagion Biosystems, a company developing imaging with magnetic iron oxide nanoparticles. R. Ivkov also consults for Magnetic Insight, a company developing magnetic particle imaging. All other authors report no conflicts of interest. The contents of this paper are solely the responsibility of the authors.
6. References
- [1].Coley WB, The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- [2].Rau B, Wust P, Tilly W, Gellermann J, Harder C, Riess H, Budach V, Felix R, and Schlag PM, Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys. 2000;48:381–91. [DOI] [PubMed] [Google Scholar]
- [3].Kroesen M, Mulder HT, Van Holthe JML, Aangeenbrug AA, Mens JWM, Van Doorn HC, Paulides MM, Oomen-de Hoop E, Vernhout RM, Lutgens LC, Van Rhoon GC, and Franckena M, Confirmation of thermal dose as a predictor of local control in cervical carcinoma patients treated with state-of-the-art radiation therapy and hyperthermia. Radiother Oncol. 2019;140:150–158. [DOI] [PubMed] [Google Scholar]
- [4].Bakker A, Van der Zee J, van tienhoven G, Kok HP, Rasch CRN, and Crezee H, Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: A systematic review. Int J Hyperthermia. 2019;36:1024–1039. [DOI] [PubMed] [Google Scholar]
- [5].van Rhoon GC, Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int J Hyperthermia. 2016;32:50–62. [DOI] [PubMed] [Google Scholar]
- [6].Sapareto SA and Dewey WC, Thermal dose determination in cancer therapy. Int.J.Radiat.Oncol.Biol.Phys 1984;10:787–800. [DOI] [PubMed] [Google Scholar]
- [7].Dewey WC, Hopwood LE, Sapareto SA, and Gerweck LE, Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977;123:463–74. [DOI] [PubMed] [Google Scholar]
- [8].Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, Zelensky A, Van Bree C, Stalpers LJ, Buist MR, Soullie T, Rens J, Verhagen HJ, O’Connor MJ, Franken NA, Ten Hagen TL, Kanaar R, and Aten JA, Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc.Natl.Acad.Sci.U.S.A 2011;108:9851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kampinga HH and Dikomey E, Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399–408. [DOI] [PubMed] [Google Scholar]
- [10].Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, Brizel DM, and Vujaskovic Z, Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res. 2004;10:4287–93. [DOI] [PubMed] [Google Scholar]
- [11].Vujaskovic Z and Song CW, Physiological mechanisms underlying heat-induced radiosensitization. Int.J.Hyperthermia 2004;20:163–174. [DOI] [PubMed] [Google Scholar]
- [12].Issels RD, Hyperthermia adds to chemotherapy. European Journal of Cancer. 2008;44:2546–2554. [DOI] [PubMed] [Google Scholar]
- [13].Bull JMC, A review of immune therapy in cancer and a question: can thermal therapy increase tumor response? International Journal of Hyperthermia. 2018;34:840–852. [DOI] [PubMed] [Google Scholar]
- [14].Dewhirst MW, Vujaskovic Z, Jones E, and Thrall D, Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia. 2005;21:779–90. [DOI] [PubMed] [Google Scholar]
- [15].van den Tempel N, Laffeber C, Odijk H, van Cappellen WA, van Rhoon GC, Franckena M, and Kanaar R, The effect of thermal dose on hyperthermia-mediated inhibition of DNA repair through homologous recombination. Oncotarget. 2017;8:44593–44604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prakash P and Diederich CJ, Considerations for theoretical modelling of thermal ablation with catheter-based ultrasonic sources: implications for treatment planning, monitoring and control. Int J Hyperthermia. 2012;28:69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Calderwood SK, Theriault JR, and Gong J, How is the immune response affected by hyperthermia and heat shock proteins? Int J Hyperthermia. 2005;21:713–6. [DOI] [PubMed] [Google Scholar]
- [18].Skitzki JJ, Repasky EA, and Evans SS, Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs. 2009;10:550–8. [PMC free article] [PubMed] [Google Scholar]
- [19].Chu KF and Dupuy DE, Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. [DOI] [PubMed] [Google Scholar]
- [20].Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, and Hoopes PJ, Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19:267–94. [DOI] [PubMed] [Google Scholar]
- [21].Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, Beck M, Nadobny J, Budach V, Wust P, and Ghadjar P, Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia. 2015;31:609–614. [DOI] [PubMed] [Google Scholar]
- [22].Stauffer PR, Evolving technology for thermal therapy of cancer. Int.J.Hyperthermia 2005;21:731–744. [DOI] [PubMed] [Google Scholar]
- [23].Foster KR and Schwan HP, Dielectric properties of tissues and biological materials: a critical review. Crit Rev Biomed Eng. 1989;17:25–104. [PubMed] [Google Scholar]
- [24].Wust P, Seebass M, Nadobny J, and Felix R, “Electromagnetic deep heating technology.,” in Thermoradiotherapy and thermochemotherapy: Volume 1, Biology, Physiology and Physics. Berlin, New York: Springer Verlag, Seegenschmiedt MH, Fessenden P, and Vernon CC, Eds., ed, 1995, pp. 219–251. [Google Scholar]
- [25].Griffiths DJ, Introduction to Electrodynamics, 2nd edition, 2 ed: Prentice Hall International, 1989. [Google Scholar]
- [26].Gabriel S, Lau RW, and Gabriel C, The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys.Med.Biol 1996;41:2251–2269. [DOI] [PubMed] [Google Scholar]
- [27].Kok HP, Navarro F, Strigari L, Cavagnaro M, and Crezee J, Locoregional hyperthermia of deep-seated tumours applied with capacitive and radiative systems: a simulation study. Int J Hyperthermia. 2018;34:714–730. [DOI] [PubMed] [Google Scholar]
- [28].Kok HP and Crezee J, A comparison of the heating characteristics of capacitive and radiative superficial hyperthermia. Int.J.Hyperthermia 2017;33:378–386. [DOI] [PubMed] [Google Scholar]
- [29].Hiraoka M, Jo S, Akuta K, Nishimura Y, Takahashi M, and Abe M, Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer. 1987;60:121–7. [DOI] [PubMed] [Google Scholar]
- [30].Lee SY, Kim JH, Han YH, and Cho DH, The effect of modulated electro-hyperthermia on temperature and blood flow in human cervical carcinoma. Int J Hyperthermia. 2018;34:953–960. [DOI] [PubMed] [Google Scholar]
- [31].Ohguri T, Harima Y, Imada H, Sakurai H, Ohno T, Hiraki Y, Tuji K, Tanaka M, and Terashima H, Relationships between thermal dose parameters and the efficacy of definitive chemoradiotherapy plus regional hyperthermia in the treatment of locally advanced cervical cancer: data from a multicentre randomised clinical trial. Int J Hyperthermia. 2018;34:461–468. [DOI] [PubMed] [Google Scholar]
- [32].Ho JC, Nguyen L, Law JJ, Ware MJ, Keshishian V, Lara NC, Nguyen T, Curley SA, and Corr SJ, Non-Invasive Radiofrequency Field Treatment to Produce Hepatic Hyperthermia: Efficacy and Safety in Swine. IEEE J Transl Eng Health Med. 2017;5:1500109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vrba J, Franconi C, and Lapes M, Theoretical limits for the penetration depth of intracavitary applicators. Int.J.Hyperthermia 1996;12:737–742. [DOI] [PubMed] [Google Scholar]
- [34].Kok HP, Korshuize - van Straten L, Bakker A, De Kroon-Oldenhof R, Geijsen ED, Stalpers LJA, and Crezee J, On-line adaptive hyperthermia treatment planning during locoregional heating to suppress treatment limiting hot spots. Int J Radiat Oncol Biol Phys. 2017;99:1039–1047. [DOI] [PubMed] [Google Scholar]
- [35].Lassche G, Crezee J, and Van Herpen CML, Whole-body hyperthermia in combination with systemic therapy in advanced solid malignancies. Critical Reviews in Oncology Hematology. 2019;139:67–74. [DOI] [PubMed] [Google Scholar]
- [36].Missios S, Bekelis K, and Barnett GH, Renaissance of laser interstitial thermal ablation. Neurosurg Focus. 2015;38:E13. [DOI] [PubMed] [Google Scholar]
- [37].Diederich CJ and Hynynen K, Ultrasound technology for hyperthermia. Ultrasound Med Biol. 1999;25:871–87. [DOI] [PubMed] [Google Scholar]
- [38].Zhu L, Altman MB, Laszlo A, Straube W, Zoberi I, Hallahan DE, and Chen H, Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med Biol. 2019;45:1025–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sebeke L, Deenen DA, Maljaars E, Heijman E, de Jager B, Heemels W, and Grull H, Model predictive control for MR-HIFU-mediated, uniform hyperthermia. Int J Hyperthermia. 2019;36:1040–1050. [DOI] [PubMed] [Google Scholar]
- [40].Salomir R, Vimeux FC, de Zwart JA, Grenier N, and Moonen CT, Hyperthermia by MR-guided focused ultrasound: accurate temperature control based on fast MRI and a physical model of local energy deposition and heat conduction. Magn Reson Med. 2000;43:342–7. [DOI] [PubMed] [Google Scholar]
- [41].Quesson B, Vimeux F, Salomir R, de Zwart JA, and Moonen CT, Automatic control of hyperthermic therapy based on real-time Fourier analysis of MR temperature maps. Magn Reson Med. 2002;47:1065–72. [DOI] [PubMed] [Google Scholar]
- [42].Tillander M, Hokland S, Koskela J, Dam H, Andersen NP, Pedersen M, Tanderup K, Ylihautala M, and Kohler M, High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med Phys. 2016;43:1539–49. [DOI] [PubMed] [Google Scholar]
- [43].Coussios CC, Farny CH, Haar GT, and Roy RA, Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int J Hyperthermia. 2007;23:105–20. [DOI] [PubMed] [Google Scholar]
- [44].Ebbini ES and ter Haar G, Ultrasound-guided therapeutic focused ultrasound: current status and future directions. Int J Hyperthermia. 2015;31:77–89. [DOI] [PubMed] [Google Scholar]
- [45].Ter Haar G HIFU Tissue Ablation: Concept and Devices. Adv Exp Med Biol. 2016;880:3–20. [DOI] [PubMed] [Google Scholar]
- [46].Bing C, Ladouceur-Wodzak M, Wanner CR, Shelton JM, Richardson JA, and Chopra R, Trans-cranial opening of the blood-brain barrier in targeted regions using a stereotaxic brain atlas and focused ultrasound energy. J Ther Ultrasound. 2014;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kwan JJ, Myers R, Coviello CM, Graham SM, Shah AR, Stride E, Carlisle RC, and Coussios CC, Ultrasound-Propelled Nanocups for Drug Delivery. Small. 2015;11:5305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Treat LH, McDannold N, Zhang Y, Vykhodtseva N, and Hynynen K, Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38:1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rahim AA, Taylor SL, Bush NL, ter Haar GR, Bamber JC, and Porter CD, Spatial and acoustic pressure dependence of microbubble-mediated gene delivery targeted using focused ultrasound. J Gene Med. 2006;8:1347–57. [DOI] [PubMed] [Google Scholar]
- [50].Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, and Cranston D, High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42:931–5. [DOI] [PubMed] [Google Scholar]
- [51].Rodrigues DB, Stauffer PR, Vrba D, and Hurwitz MD, Focused ultrasound for treatment of bone tumours. Int J Hyperthermia. 2015;31:260–71. [DOI] [PubMed] [Google Scholar]
- [52].ten Eikelder HM, Bosnacki D, Elevelt A, Donato K, Di Tullio A, Breuer BJ, van Wijk JH, van Dijk EV, Modena D, Yeo SY, and Grull H, Modelling the temperature evolution of bone under high intensity focused ultrasound. Phys Med Biol. 2016;61:1810–28. [DOI] [PubMed] [Google Scholar]
- [53].Helderman RFCPA Loke DR, Kok HP Oei AL, Tanis PJ Franken NAP, and Crezee J, Variation in Clinical Application of Hyperthermic Intraperitoneal Chemotherapy: A Review. Cancers (Basel). 2019;11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].de Bree E, van Ruth S, Baas P, Rutgers EJ, van Zandwijk N, Witkamp AJ, and Zoetmulder FA, Cytoreductive surgery and intraoperative hyperthermic intrathoracic chemotherapy in patients with malignant pleural mesothelioma or pleural metastases of thymoma. Chest. 2002;121:480–7. [DOI] [PubMed] [Google Scholar]
- [55].Sousa A, Pineiro I, Rodriguez S, Aparici V, Monserrat V, Neira P, Carro E, Murias C, and Uribarri C, Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate-high-risk non-muscle-invasive bladder cancer. International Journal of Hyperthermia. 2016;32:374–380. [DOI] [PubMed] [Google Scholar]
- [56].Westermark F, Uber die behandlung des ulzerierenden cervixkarzinoms mittels konstanter warme. Zentralbl Gynakol. 1898;22:1335–1339. [Google Scholar]
- [57].van Wieringen N, van Dijk JD, Nieuwenhuys GJ, Snel CE, and Cetas TC, Power absorption and temperature control of multi-filament palladium-nickel thermoseeds for interstitial hyperthermia. Phys Med Biol. 1996;41:2367–80. [DOI] [PubMed] [Google Scholar]
- [58].Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, and Taylor CB, Selective inductive heating of lymph nodes. Ann Surg. 1957;146:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wust P, Gneveckow U, Johannsen M, Bohmer D, Henkel T, Kahmann F, Sehouli J, Felix R, Ricke J, and Jordan A, Magnetic nanoparticles for interstitial thermotherapy--feasibility, tolerance and achieved temperatures. Int J Hyperthermia. 2006;22:673–85. [DOI] [PubMed] [Google Scholar]
- [60].Lanier OL, Korotych OI, Monsalve AG, Wable D, Savliwala S, Grooms NWF, Nacea C, Tuitt OR, and Dobson J, Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int J Hyperthermia. 2019;36:687–701. [DOI] [PubMed] [Google Scholar]
- [61].Jordan A, Wust P, Fahling H, John W, Hinz A, and Felix R, Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia. 1993;9:51–68. [DOI] [PubMed] [Google Scholar]
- [62].Eggeman AS, Majetich SA, Farrell D, and Pankhurst QA, Size and concentration effects on high frequency hysteresis of iron oxide nanoparticles. IEEE Transactions on Magnetics. 2007;43:2451–2453. [Google Scholar]
- [63].Dennis CL and Ivkov R, Physics of heat generation using magnetic nanoparticles for hyperthermia. Int J Hyperthermia. 2013;29:715–29. [DOI] [PubMed] [Google Scholar]
- [64].Dennis CL, Krycka KL, Borchers JA, Desautels RD, van Lierop J, Huls NF, Jackson AJ, Gruettner C, and Ivkov R, Internal Magnetic Structure of Nanoparticles Dominates Time-Dependent Relaxation Processes in a Magnetic Field. Advanced Functional Materials. 2015;25:4300–4311. [Google Scholar]
- [65].Mahmoudi K, Bouras A, Bozec D, Ivkov R, and Hadjipanayis C, Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy and application in humans. International Journal of Hyperthermia. 2018;34:1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sharma A, Cornejo C, Mihalic J, Geyh A, Bordelon DE, Korangath P, Westphal F, Gruettner C, and Ivkov R, Physical characterization and in vivo organ distribution of coated iron oxide nanoparticles. Scientific Reports. 2018;8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gruttner C, Muller K, Teller J, Westphal F, Foreman A, and Ivkov R, Synthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapy. Journal of Magnetism and Magnetic Materials. 2007;311:181–186. [Google Scholar]
- [68].Dennis CL, Jackson AJ, Borchers JA, Hoopes PJ, Strawbridge R, Foreman AR, van Lierop J, Gruttner C, and Ivkov R, Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology. 2009;20: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gruttner C, Muller K, Teller J, and Westphal F, Synthesis and functionalisation of magnetic nanoparticles for hyperthermia applications. International Journal of Hyperthermia. 2013;29:777–789. [DOI] [PubMed] [Google Scholar]
- [70].Ryan TP and Brace CL, Interstitial microwave treatment for cancer: historical basis and current techniques in antenna design and performance. Int J Hyperthermia. 2017;33:3–14. [DOI] [PubMed] [Google Scholar]
- [71].Salgaonkar VA and Diederich CJ, Catheter-based ultrasound technology for image-guided thermal therapy: current technology and applications. Int J Hyperthermia. 2015;31:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dobsicek Trefna H, Schmidt M, van Rhoon GC, Kok HP, Gordeyev SS, Lamprecht U, Marder D, Nadobny J, Ghadjar P, Abdel-Rahman S, Kukielka AM, Strnad V, Hurwitz MD, Vujaskovic Z, Diederich CJ, Stauffer PR, and Crezee J, Quality assurance guidelines for interstitial hyperthermia. Int J Hyperthermia. 2019;36:277–294. [DOI] [PubMed] [Google Scholar]
- [73].Stauffer PR, Diederich CJ, and Seegenschmiedt MH, “Interstitial heating technologies,” in Thermoradiotherapy and thermochemotherapy: Volume 1, Biology, Physiology and Physics. Berlin, New York: Springer Verlag, Seegenschmiedt MH, Fessenden P, and Vernon CC, Eds., ed, 1995, pp. 279–320. [Google Scholar]
- [74].Fallahi H and Prakash P, Antenna Designs for Microwave Tissue Ablation. Crit Rev Biomed Eng. 2018;46:495–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schreier K, Budihna M, Lesnicar H, Handlzeller L, Hand JW, Prior MV, Clegg ST, and Brezovich IA, Preliminary Studies of Interstitial Hyperthermia Using Hot Water. International Journal of Hyperthermia. 1990;6:431–444. [DOI] [PubMed] [Google Scholar]
- [76].Deford JA, Babbs CF, Patel UH, Bleyer MW, Marchosky JA, and Moran CJ, Effective Estimation and Computer Control of Minimum Tumor Temperature during Conductive Interstitial Hyperthermia. International Journal of Hyperthermia. 1991;7:441–453. [DOI] [PubMed] [Google Scholar]
- [77].Stauffer PR, Cetas TC, Fletcher AM, Deyoung DW, Dewhirst MW, Oleson JR, and Roemer RB, Observations on the Use of Ferromagnetic Implants for Inducing Hyperthermia. Ieee Transactions on Biomedical Engineering. 1984;31:76–90. [DOI] [PubMed] [Google Scholar]
- [78].Tucker RD, Platz CE, Huidobro C, and Larson T, Interstitial thermal therapy in patients with localized prostate cancer: Histologic analysis. Urology. 2002;60:166–169. [DOI] [PubMed] [Google Scholar]
- [79].Steger AC, Lees WR, Walmsley K, and Bown SG, Interstitial laser hyperthermia: a new approach to local destruction of tumours. BMJ. 1989;299:362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mohammadi AM and Schroeder JL, Laser interstitial thermal therapy in treatment of brain tumors - the NeuroBlate System. Expert Review of Medical Devices. 2014;11:109–119. [DOI] [PubMed] [Google Scholar]
- [81].Sherar MD, Trachtenberg J, Davidson SR, McCann C, Yue CK, Haider MA, and Gertner MR, Interstitial microwave thermal therapy for prostate cancer. J Endourol. 2003;17:617–25. [DOI] [PubMed] [Google Scholar]
- [82].Satoh T, Stauffer PR, and Fike JR, Thermal Distribution Studies of Helical-Coil Microwave Antennas for Interstitial Hyperthermia. International Journal of Radiation Oncology Biology Physics. 1988;15:1209–1218. [DOI] [PubMed] [Google Scholar]
- [83].Bertram JM, Yang D, Converse MC, Webster JG, and Mahvi DM, A review of coaxial-based interstitial antennas for hepatic microwave ablation. Crit Rev Biomed Eng. 2006;34:187–213. [DOI] [PubMed] [Google Scholar]
- [84].O’Rourke AP, Haemmerich D, Prakash P, Converse MC, Mahvi DM, and Webster JG, Current status of liver tumor ablation devices. Expert Rev Med Devices. 2007;4:523–37. [DOI] [PubMed] [Google Scholar]
- [85].Astrahan MA and Norman A, A localized current field hyperthermia system for use with 192-iridium interstitial implants. Med Phys. 1982;9:419–24. [DOI] [PubMed] [Google Scholar]
- [86].Visser AG, Deurloo IKK, Levendag PC, Ruifrok ACC, Cornet B, and Van Rhoon GC, An Interstitial Hyperthermia System at 27-Mhz. Int J Hyperthermia. 1989;5:265–276. [DOI] [PubMed] [Google Scholar]
- [87].Kaatee RSJP, Crezee H, Kanis BP, Lagendijk JJW, Levendag PC, and Visser AG, Spatial temperature control with a 27 MHz current source interstitial hyperthermia system. International Journal of Radiation Oncology Biology Physics. 1997;37:189–197. [DOI] [PubMed] [Google Scholar]
- [88].van der Koijk JF, Lagendijk JJW, Crezee J, de Bree J, Kotte ANTJ, van Leeuwen GMJ, and Battermann JJ, The influence of vasculature on temperature distributions in MECS interstitial hyperthermia: importance of longitudinal control. Int.J.Hyperthermia 1997;13:365–385. [DOI] [PubMed] [Google Scholar]
- [89].Diederich CJ, Ultrasound applicators with integrated catheter-cooling for interstitial hyperthermia: theory and preliminary experiments. Int J Hyperthermia. 1996;12:279–97; discussion 299–300. [DOI] [PubMed] [Google Scholar]
- [90].Van Vulpen M, Raaymakers BW, Lagendijk JJW, Crezee J, De Leeuw AAC, Van Moorselaar JRA, Ligtvoet CM, and Battermann JJ, Three-dimensional controlled interstitial hyperthermia combined with radiotherapy for locally advanced prostate carcinoma--a feasibility study. Int.J.Radiat.Oncol.Biol.Phys 2002;53:116–126. [DOI] [PubMed] [Google Scholar]
- [91].Emami B, “Interstitial thermoradiotherapy in the treatment of malignant tumors.,” in Interstitial Hyperthermia: Physics, Biology and Clinical Aspects. Vol. 3, VSP, Utrecht, The Netherlands., Urano Mand Douple E, Eds., ed, 1992, pp. 199–220. [Google Scholar]
- [92].Pyrexar Medical Inc. (2019). “BSD-500 Features”. Available from: http://www.pyrexar.com/hyperthermia/bsd-500.
- [93].Nau WH, Diederich CJ, and Stauffer PR, Directional power deposition from direct-coupled and catheter-cooled interstitial ultrasound applicators. Int J Hyperthermia. 2000;16:129–44. [DOI] [PubMed] [Google Scholar]
- [94].Chopra R, Burtnyk M, N’Djin WA, and Bronskill M, MRI-controlled transurethral ultrasound therapy for localised prostate cancer. Int J Hyperthermia. 2010;26:804–21. [DOI] [PubMed] [Google Scholar]
- [95].Chopra R, Colquhoun A, Burtnyk M, N’Djin WA, Kobelevskiy I, Boyes A, Siddiqui K, Foster H, Sugar L, Haider MA, Bronskill M, and Klotz L, MR imaging-controlled transurethral ultrasound therapy for conformal treatment of prostate tissue: initial feasibility in humans. Radiology. 2012;265:303–13. [DOI] [PubMed] [Google Scholar]
- [96].Bihrle R, Foster RS, Sanghvi NT, Fry FJ, and Donohue JP, High-intensity focused ultrasound in the treatment of prostatic tissue. Urology. 1994;43:21–6. [DOI] [PubMed] [Google Scholar]
- [97].Gelet A, Chapelon JY, Margonari J, Theillere Y, Gorry F, Souchon R, and Bouvier R, High-intensity focused ultrasound experimentation on human benign prostatic hypertrophy. Eur Urol. 1993;23 Suppl 1:44–7. [DOI] [PubMed] [Google Scholar]
- [98].Hutchinson EB and Hynynen K, Intracavitary ultrasound phased arrays for prostate thermal therapies: MRI compatibility and in vivo testing. Med Phys. 1998;25:2392–9. [DOI] [PubMed] [Google Scholar]
- [99].Ahmed M, Brace CL, Lee FT Jr., and Goldberg SN, Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hoffmann R, Rempp H, Erhard L, Blumenstock G, Pereira PL, Claussen CD, and Clasen S, Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology. 2013;268:89–97. [DOI] [PubMed] [Google Scholar]
- [101].Sun Y, Cheng Z, Dong L, Zhang G, Wang Y, and Liang P, Comparison of temperature curve and ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine livers. Eur J Radiol. 2012;81:553–7. [DOI] [PubMed] [Google Scholar]
- [102].Sawicki JF, Shea JD, Behdad N, and Hagness SC, The impact of frequency on the performance of microwave ablation. Int J Hyperthermia. 2017;33:61–68. [DOI] [PubMed] [Google Scholar]
- [103].Vogl TJ, Roman A, Nour-Eldin NA, Hohenforst-Schmidt W, Bednarova I, and Kaltenbach B, A comparison between 915 MHz and 2450 MHz microwave ablation systems for the treatment of small diameter lung metastases. Diagn Interv Radiol. 2018;24:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Amabile C, Ahmed M, Solbiati L, Meloni MF, Solbiati M, Cassarino S, Tosoratti N, Nissenbaum Y, Ierace T, and Goldberg SN, Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia. 2017;33:34–42. [DOI] [PubMed] [Google Scholar]
- [105].Cavagnaro M, Amabile C, Bernardi P, Pisa S, and Tosoratti N, A minimally invasive antenna for microwave ablation therapies: design, performances, and experimental assessment. IEEE Trans Biomed Eng. 2011;58:949–59. [DOI] [PubMed] [Google Scholar]
- [106].Filippiadis DK, Spiliopoulos S, Konstantos C, Reppas L, Kelekis A, Brountzos E, and Kelekis N, Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: safety and efficacy of high-power microwave platforms. Int J Hyperthermia. 2018;34:863–869. [DOI] [PubMed] [Google Scholar]
- [107].Imajo K, Tomeno W, Kanezaki M, Honda Y, Kessoku T, Ogawa Y, Yoshida K, Yoneda M, Kirikoshi H, Ono M, Kaneta T, Inoue T, Teratani T, Saito S, and Nakajima A, New microwave ablation system for unresectable liver tumors that forms large, spherical ablation zones. J Gastroenterol Hepatol. 2018;33:2007–2014. [DOI] [PubMed] [Google Scholar]
- [108].Ierardi AM, Coppola A, Lucchina N, and Carrafiello G, Treatment of lung tumours with high-energy microwave ablation: a single-centre experience. Med Oncol. 2017;34:5. [DOI] [PubMed] [Google Scholar]
- [109].Al-Hakim RA, Abtin FG, Genshaft SJ, Kutay E, and Suh RA, Defining New Metrics in Microwave Ablation of Pulmonary Tumors: Ablation Work and Ablation Resistance Score. Journal of Vascular and Interventional Radiology. 2016;27:1380–1386. [DOI] [PubMed] [Google Scholar]
- [110].Lubner MG, Brace CL, Hinshaw JL, and Lee FT Jr., Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Crezee J and Lagendijk JJ, Temperature uniformity during hyperthermia: the impact of large vessels. Phys.Med.Biol 1992;37:1321–1337. [DOI] [PubMed] [Google Scholar]
- [112].Loriaud A, Denys A, Seror O, Vietti Violi N, Digklia A, Duran R, Trillaud H, and Hocquelet A, Hepatocellular carcinoma abutting large vessels: comparison of four percutaneous ablation systems. Int J Hyperthermia. 2018;34:1171–1178. [DOI] [PubMed] [Google Scholar]
- [113].Bredlau AL, McCrackin MA, Motamarry A, Helke K, Chen C, Broome AM, and Haemmerich D, Thermal Therapy Approaches for Treatment of Brain Tumors in Animals and Humans. Critical Reviews in Biomedical Engineering. 2016;44:443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Carpentier A, McNichols RJ, Stafford RJ, Guichard JP, Reizine D, Delaloge S, Vicaut E, Payen D, Gowda A, and George B, Laser thermal therapy: Real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers in Surgery and Medicine. 2011;43:943–950. [DOI] [PubMed] [Google Scholar]
- [115].Goffinet DR, Prionas SD, Kapp DS, Samulski TV, Fessenden P, Hahn GM, Lohrbach AW, Mariscal JM, and Bagshaw MA, Interstitial 192Ir flexible catheter radiofrequency hyperthermia treatments of head and neck and recurrent pelvic carcinomas. Int J Radiat Oncol Biol Phys. 1990;18:199–210. [DOI] [PubMed] [Google Scholar]
- [116].Prionas SD, Kapp DS, Goffinet DR, Ben Yosef R, Fessenden P, and Bagshaw MA, Thermometry of interstitial hyperthermia given as an adjuvant to brachytherapy for the treatment of carcinoma of the prostate. Int.J.Radiat.Oncol.Biol.Phys 1994;28:151–162. [DOI] [PubMed] [Google Scholar]
- [117].Kukielka AM, Hetnal M, and Bereza K, Evaluation of tolerance and toxicity of high-dose-rate brachytherapy boost combined with interstitial hyperthermia for prostate cancer. Int J Hyperthermia. 2016;32:324–30. [DOI] [PubMed] [Google Scholar]
- [118].Sneed PK, Gutin PH, Stauffer PR, Phillips TL, Prados MD, Weaver KA, Suen S, Lamb SA, Ham B, and Ahn DK, Thermoradiotherapy of recurrent malignant brain tumors. Int.J.Radiat.Oncol.Biol.Phys 1992;23:853–861. [DOI] [PubMed] [Google Scholar]
- [119].Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, Lamb SA, Voss B, Davis RL, Wara WM, Larson DA, Phillips TL, and Gutin PH, Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287–95. [DOI] [PubMed] [Google Scholar]
- [120].Hulshof MC, Raaymakers BW, Lagendijk JJ, Koot RW, Crezee H, Stalpers LJ, and Gonzalez Gonzalez D, A feasibility study of interstitial hyperthermia plus external beam radiotherapy in glioblastoma multiforme using the Multi ELectrode Current Source (MECS) system. Int J Hyperthermia. 2004;20:451–63. [DOI] [PubMed] [Google Scholar]
- [121].Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, and Mankin HJ, Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229:171–5. [DOI] [PubMed] [Google Scholar]
- [122].Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, Fullarton G, Di Pietro M, Ravi N, Visser M, Offerhaus GJ, Seldenrijk CA, Meijer SL, ten Kate FJ, Tijssen JG, and Bergman JJ, Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–17. [DOI] [PubMed] [Google Scholar]
- [123].Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, Chen MH, Choi BI, de Baere T, Dupuy D, Gangi A, Gervais D, Helmberger T, Jung EM, Lee F, Lencioni R, Liang P, Livraghi T, Lu D, Meloni F, Pereira P, Piscaglia F, Rhim H, Salem R, Sofocleous C, Solomon SB, Soulen M, Tanaka M, Vogl T, Wood B, and Solbiati L, Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontieres meeting 2013. Eur Radiol. 2015;25:3438–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tan W, Deng Q, Lin S, Wang Y, and Xu G, Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:264–272. [DOI] [PubMed] [Google Scholar]
- [125].Luo W, Zhang Y, He G, Yu M, Zheng M, Liu L, and Zhou X, Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Weiss J, Winkelmann MT, Gohla G, Kubler J, Clasen S, Nikolaou K, and Hoffmann R, MR-guided microwave ablation in hepatic malignancies: clinical experiences from 50 procedures. Int J Hyperthermia. 2020;37:349–355. [DOI] [PubMed] [Google Scholar]
- [127].Bi N, Shedden K, Zheng X, and Kong FS, Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Pooled Analysis. Int J Radiat Oncol Biol Phys. 2016;95:1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Tsakok MT, Jones D, MacNeill A, and Gleeson FV, Is microwave ablation more effective than radiofrequency ablation in achieving local control for primary pulmonary malignancy? Interact Cardiovasc Thorac Surg. 2019; [DOI] [PubMed] [Google Scholar]
- [129].Sidoff L and Dupuy DE, Clinical experiences with microwave thermal ablation of lung malignancies. Int J Hyperthermia. 2017;33:25–33. [DOI] [PubMed] [Google Scholar]
- [130].Ben Hamou A, Ghanassia E, Espiard S, Abi Rached H, Jannin A, Correas JM, Do Cao C, Kyheng M, Vantyghem MC, and Monpeyssen H, Safety and efficacy of thermal ablation (radiofrequency and laser): should we treat all types of thyroid nodules? (dagger). Int J Hyperthermia. 2019;36:666–676. [DOI] [PubMed] [Google Scholar]
- [131].Wang L, Xu D, Yang Y, Li M, Zheng C, Qiu X, and Huang B, Safety and efficacy of ultrasound-guided percutaneous thermal ablation in treating low-risk papillary thyroid microcarcinoma: A pilot and feasibility study. J Cancer Res Ther. 2019;15:1522–1529. [DOI] [PubMed] [Google Scholar]
- [132].Cho SJ, Baek JH, Chung SR, Choi YJ, and Lee JH, Thermal Ablation for Small Papillary Thyroid Cancer: A Systematic Review. Thyroid. 2019;29:1774–1783. [DOI] [PubMed] [Google Scholar]
- [133].Papini E, Pacella CM, Solbiati LA, Achille G, Barbaro D, Bernardi S, Cantisani V, Cesareo R, Chiti A, Cozzaglio L, Crescenzi A, De Cobelli F, Deandrea M, Fugazzola L, Gambelunghe G, Garberoglio R, Giugliano G, Luzi L, Negro R, Persani L, Raggiunti B, Sardanelli F, Seregni E, Sollini M, Spiezia S, Stacul F, Van Doorne D, Sconfienza LM, and Mauri G, Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36:376–382. [DOI] [PubMed] [Google Scholar]
- [134].Filippiadis D, Mauri G, Marra P, Charalampopoulos G, Gennaro N, and De Cobelli F, Percutaneous ablation techniques for renal cell carcinoma: current status and future trends. Int J Hyperthermia. 2019;36:21–30. [DOI] [PubMed] [Google Scholar]
- [135].Higgins LJ and Hong K, Renal Ablation Techniques: State of the Art. AJR Am J Roentgenol. 2015;205:735–41. [DOI] [PubMed] [Google Scholar]
- [136].Garcia-Tejedor A, Guma A, Soler T, Valdivieso A, Petit A, Contreras N, Chappuis CG, Falo C, Pernas S, Amselem A, Pla MJ, Fernandez-Montoli E, Burdio F, and Ponce J, Radiofrequency Ablation Followed by Surgical Excision versus Lumpectomy for Early Stage Breast Cancer: A Randomized Phase II Clinical Trial. Radiology. 2018;289:317–324. [DOI] [PubMed] [Google Scholar]
- [137].Pouw RE, Klaver E, Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Pech O, Manner H, Ragunath K, Fernandez-Sordo JO, Fullarton G, Di Pietro M, Januszewicz W, O’Toole D, and Bergman JJ, Radiofrequency ablation for low-grade dysplasia in Barrett’s esophagus: long-term outcome of a randomized trial. Gastrointest Endosc. 2020; [DOI] [PubMed] [Google Scholar]
- [138].Saccomandi P, Lapergola A, Longo F, Schena E, and Quero G, Thermal ablation of pancreatic cancer: A systematic literature review of clinical practice and pre-clinical studies. Int J Hyperthermia. 2018;35:398–418. [DOI] [PubMed] [Google Scholar]
- [139].Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aube C, Digklia A, Pache I, Deltenre P, Knebel JF, and Denys A, Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–325. [DOI] [PubMed] [Google Scholar]
- [140].Macchi M, Belfiore MP, Floridi C, Serra N, Belfiore G, Carmignani L, Grasso RF, Mazza E, Pusceddu C, Brunese L, and Carrafiello G, Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34:96. [DOI] [PubMed] [Google Scholar]
- [141].Vogl TJ, Eckert R, Naguib NN, Beeres M, Gruber-Rouh T, and Nour-Eldin NA, Thermal Ablation of Colorectal Lung Metastases: Retrospective Comparison Among Laser-Induced Thermotherapy, Radiofrequency Ablation, and Microwave Ablation. AJR Am J Roentgenol. 2016;207:1340–1349. [DOI] [PubMed] [Google Scholar]
- [142].Narsule CK, Sridhar P, Nair D, Gupta A, Oommen RG, Ebright MI, Litle VR, and Fernando HC, Percutaneous thermal ablation for stage IA non-small cell lung cancer: long-term follow-up. J Thorac Dis. 2017;9:4039–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Smolock AR, Cristescu MM, Potretzke TA, Ziemlewicz TJ, Lubner MG, Hinshaw JL, Brace CL, and Lee FT Jr., Microwave Ablation for the Treatment of Hepatic Adenomas. J Vasc Interv Radiol. 2016;27:244–9. [DOI] [PubMed] [Google Scholar]
- [144].Wang L, Ke Q, Lin N, Huang Q, Zeng Y, and Liu J, The efficacy of transarterial chemoembolization combined with microwave ablation for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:1288–1296. [DOI] [PubMed] [Google Scholar]
- [145].Barnett GH, Voigt JD, and Alhuwalia MS, A Systematic Review and Meta-Analysis of Studies Examining the Use of Brain Laser Interstitial Thermal Therapy versus Craniotomy for the Treatment of High-Grade Tumors in or near Areas of Eloquence: An Examination of the Extent of Resection and Major Complication Rates Associated with Each Type of Surgery. Stereotactic and Functional Neurosurgery. 2016;94:164–173. [DOI] [PubMed] [Google Scholar]
- [146].Alattar AA, Bartek J, Chian VL, Mohammadi AM, Barnett GH, Sloan A, and Chen CC, Stereotactic Laser Ablation as Treatment of Brain Metastases Recurring after Stereotactic Radiosurgery: A Systematic Literature Review. World Neurosurgery. 2019;128:134–142. [DOI] [PubMed] [Google Scholar]
- [147].Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM, Leuthardt E, Chamoun R, Judy K, Asher A, Essig M, Dietrich J, and Chiang VL, Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. Journal of Neurosurgery. 2019;130:804–811. [DOI] [PubMed] [Google Scholar]
- [148].Rodriguez A and Tatter SB, Laser Ablation of Recurrent Malignant Gliomas: Current Status and Future Perspective. Neurosurgery. 2016;79 Suppl 1:S35–S39. [DOI] [PubMed] [Google Scholar]
- [149].Kramer MW, Wolters M, Cash H, Jutzi S, Imkamp F, Kuczyk MA, Merseburger AS, and Herrmann TR, Current evidence of transurethral Ho:YAG and Tm:YAG treatment of bladder cancer: update 2014. World J Urol. 2015;33:571–9. [DOI] [PubMed] [Google Scholar]
- [150].Woods E, Laser ablation of the prostate: a safe effective treatment of obstructive benign prostatic disease. Can Urol Assoc J. 2010;4:344–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Al-Ansari A, Younes N, Sampige VP, Al-Rumaihi K, Ghafouri A, Gul T, and Shokeir AA, GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia: a randomized clinical trial with midterm follow-up. Eur Urol. 2010;58:349–55. [DOI] [PubMed] [Google Scholar]
- [152]. https://www.fusfoundation.org/the-technology/state-of-the-technology.
- [153].Peek MCL, Ahmed M, Napoli A, Usiskin S, Baker R, and Douek M, Minimally invasive ablative techniques in the treatment of breast cancer: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33:191–202. [DOI] [PubMed] [Google Scholar]
- [154].Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, Schwartz M, Hynynen K, Lozano AM, Shah BB, Huss D, Dallapiazza RF, Gwinn R, Witt J, Ro S, Eisenberg HM, Fishman PS, Gandhi D, Halpern CH, Chuang R, Butts Pauly K, Tierney TS, Hayes MT, Cosgrove GR, Yamaguchi T, Abe K, Taira T, and Chang JW, A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med. 2016;375:730–9. [DOI] [PubMed] [Google Scholar]
- [155]. https://www.insightec.com.
- [156].Hijnen N, Langereis S, and Grull H, Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv Drug Deliv Rev. 2014;72:65–81. [DOI] [PubMed] [Google Scholar]
- [157].Salgaonkar VA, Prakash P, Rieke V, Ozhinsky E, Plata J, Kurhanewicz J, Hsu IC, and Diederich CJ, Model-based feasibility assessment and evaluation of prostate hyperthermia with a commercial MR-guided endorectal HIFU ablation array. Med Phys. 2014;41:033301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Ozhinsky E, Salgaonkar VA, Diederich CJ, and Rieke V, MR thermometry-guided ultrasound hyperthermia of user-defined regions using the ExAblate prostate ablation array. Journal of Therapeutic Ultrasound. 2018;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Partanen A, Yarmolenko PS, Viitala A, Appanaboyina S, Haemmerich D, Ranjan A, Jacobs G, Woods D, Enholm J, Wood BJ, and Dreher MR, Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia. 2012;28:320–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Hijnen NM, Heijman E, Kohler MO, Ylihautala M, Ehnholm GJ, Simonetti AW, and Grull H, Tumour hyperthermia and ablation in rats using a clinical MR-HIFU system equipped with a dedicated small animal set-up. International Journal of Hyperthermia. 2012;28:141–155. [DOI] [PubMed] [Google Scholar]
- [161].Lam MK, Oerlemans C, Froeling M, Deckers R, Barten-Van Rijbroek AD, Viergever MA, Moonen CTW, Bos C, and Bartels LW, DCE-MRI and IVIM-MRI of rabbit Vx2 tumors treated with MR-HIFU-induced mild hyperthermia. Journal of Therapeutic Ultrasound. 2016;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Farr N, Wang YN, D’Andrea S, Starr F, Partanen A, Gravelle KM, McCune JS, Risler LJ, Whang SG, Chang A, Hingorani SR, Lee D, and Hwang JH, Hyperthermia-enhanced targeted drug delivery using magnetic resonance-guided focussed ultrasound: a pre-clinical study in a genetic model of pancreatic cancer. International Journal of Hyperthermia. 2018;34:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Hijnen N, Kneepkens E, de Smet M, Langereis S, Heijman E, and Grull H, Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E4802–E4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Bing CC, Patel P, Staruch RM, Shaikh S, Nofiele J, Staruch MW, Szczepanski D, Williams NS, Laetsch T, and Chopra R, Longer heating duration increases localized doxorubicin deposition and therapeutic index in Vx2 tumors using MR-HIFU mild hyperthermia and thermosensitive liposomal doxorubicin. International Journal of Hyperthermia. 2019;36:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Chu W, Staruch RM, Pichardo S, Tillander M, Kohler MO, Huang Y, Ylihautala M, McGuffin M, Czarnota G, and Hynynen K, Magnetic Resonance-Guided High-Intensity Focused Ultrasound Hyperthermia for Recurrent Rectal Cancer: MR Thermometry Evaluation and Preclinical Validation. Int J Radiat Oncol Biol Phys. 2016;95:1259–67. [DOI] [PubMed] [Google Scholar]
- [166].Partanen A, Tillander M, Yarmolenko PS, Wood BJ, Dreher MR, and Kohler MO, Reduction of peak acoustic pressure and shaping of heated region by use of multifoci sonications in MR-guided high-intensity focused ultrasound mediated mild hyperthermia. Medical Physics. 2013;40: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ikink ME, Voogt MJ, Verkooijen HM, Lohle PN, Schweitzer KJ, Franx A, Mali WP, Bartels LW, and van den Bosch MA, Mid-term clinical efficacy of a volumetric magnetic resonance-guided high-intensity focused ultrasound technique for treatment of symptomatic uterine fibroids. Eur Radiol. 2013;23:3054–61. [DOI] [PubMed] [Google Scholar]
- [168].Łoziński T, Filipowska J, Gurynowicz G, Zgliczyńska M, Kluz T, Jędra R, Skowyra A, and Ciebiera M, The effect of high-intensity focused ultrasound guided by magnetic resonance therapy on obstetrical outcomes in patients with uterine fibroids - experiences from the main Polish center and a review of current data. Int J Hyperthermia. 2019;36:582–590. [DOI] [PubMed] [Google Scholar]
- [169].Pron G, Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MRgHIFU) Treatment of Symptomatic Uterine Fibroids: An Evidence-Based Analysis. Ont Health Technol Assess Ser. 2015;15:1–86. [PMC free article] [PubMed] [Google Scholar]
- [170].He M, Jacobson H, Zhang C, Setzen R, and Zhang L, A retrospective study of ultrasound-guided high intensity focussed ultrasound ablation for multiple uterine fibroids in South Africa. Int J Hyperthermia. 2018;34:1304–1310. [DOI] [PubMed] [Google Scholar]
- [171].Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, Kuten A, Meyer JE, LeBlang SD, Roberts A, Choi J, Larner JM, Napoli A, Turkevich VG, Inbar Y, Tempany CM, and Pfeffer RM, Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Golan R, Bernstein AN, McClure TD, Sedrakyan A, Patel NA, Parekh DJ, Marks LS, and Hu JC, Partial Gland Treatment of Prostate Cancer Using High-Intensity Focused Ultrasound in the Primary and Salvage Settings: A Systematic Review. Journal of Urology. 2017;198:1000–1009. [DOI] [PubMed] [Google Scholar]
- [173].Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, Hosking-Jervis F, Hindley RG, Lewi H, McCartan N, Moore CM, Nigam R, Ogden C, Persad R, Shah K, van der Meulen J, Virdi J, Winkler M, Emberton M, and Ahmed HU, A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. Eur Urol. 2018;74:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Rischmann P, Gelet A, Riche B, Villers A, Pasticier G, Bondil P, Jung JL, Bugel H, Petit J, Toledano H, Mallick S, Rouviere O, Rabilloud M, Tonoli-Catez H, and Crouzet S, Focal High Intensity Focused Ultrasound of Unilateral Localized Prostate Cancer: A Prospective Multicentric Hemiablation Study of 111 Patients. Eur Urol. 2017;71:267–273. [DOI] [PubMed] [Google Scholar]
- [175].Ghai S, Perlis N, Lindner U, Hlasny E, Haider MA, Finelli A, Zlotta AR, Kulkarni GS, van der Kwast TH, McCluskey SA, Kucharczyk W, and Trachtenberg J, Magnetic resonance guided focused high frequency ultrasound ablation for focal therapy in prostate cancer - phase 1 trial. Eur Radiol. 2018;28:4281–4287. [DOI] [PubMed] [Google Scholar]
- [176].Tay KJ, Cheng CWS, Lau WKO, Khoo J, Thng CH, and Kwek JW, Focal Therapy for Prostate Cancer with In-Bore MR-guided Focused Ultrasound: Two-Year Follow-up of a Phase I Trial-Complications and Functional Outcomes. Radiology. 2017;285:620–628. [DOI] [PubMed] [Google Scholar]
- [177].Crouzet S, Blana A, Murat FJ, Pasticier G, Brown SCW, Conti GN, Ganzer R, Chapet O, Gelet A, Chaussy CG, Robertson CN, Thuroff S, and Ward JF, Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. BJU Int. 2017;119:896–904. [DOI] [PubMed] [Google Scholar]
- [178].Cordeiro ER, Cathelineau X, Thuroff S, Marberger M, Crouzet S, and de la Rosette JJ, High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int. 2012;110:1228–42. [DOI] [PubMed] [Google Scholar]
- [179].Dababou S, Marrocchio C, Rosenberg J, Bitton R, Pauly KB, Napoli A, Hwang JH, and Ghanouni P, A meta-analysis of palliative treatment of pancreatic cancer with high intensity focused ultrasound. J Ther Ultrasound. 2017;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Peek MCL, Ahmed M, Scudder J, Baker R, Charalampoudis P, Pinder SE, Douek M, and Collaborators H-F, High-intensity focused ultrasound in the treatment of breast fibroadenomata (HIFU-F trial). International Journal of Hyperthermia. 2018;34:1002–1009. [DOI] [PubMed] [Google Scholar]
- [181].Yu W, Tang L, Lin F, Jiang L, and Shen Z, Significance of HIFU in local unresectable recurrence of soft tissue sarcoma, a single-center, respective, case series in China. Surg Oncol. 2019;30:117–121. [DOI] [PubMed] [Google Scholar]
- [182].Sharma KV, Yarmolenko PS, Eranki A, Partanen A, Celik H, Kim A, Oetgen M, and Kim PCW, Magnetic Resonance Imaging-guided High-intensity Focused Ultrasound Applications in Pediatrics: Early Experience at Children’s National Medical Center. Top Magn Reson Imaging. 2018;27:45–51. [DOI] [PubMed] [Google Scholar]
- [183].Yarmolenko PS, Eranki A, Partanen A, Celik H, Kim A, Oetgen M, Beskin V, Santos D, Patel J, Kim PCW, and Sharma K, Technical aspects of osteoid osteoma ablation in children using MR-guided high intensity focussed ultrasound. Int J Hyperthermia. 2018;34:49–58. [DOI] [PubMed] [Google Scholar]
- [184].Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, Gleeson FV, Cranston DW, Phillips RR, and Middleton MR, The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Melodelima D, Prat F, Fritsch J, Theillere Y, and Cathignol D, Treatment of esophageal tumors using high intensity intraluminal ultrasound: first clinical results. Journal of Translational Medicine. 2008;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Alkins RD and Mainprize TG, High-Intensity Focused Ultrasound Ablation Therapy of Gliomas. Prog Neurol Surg. 2018;32:39–47. [DOI] [PubMed] [Google Scholar]
- [187].Maloney E and Hwang JH, Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015;31:302–9. [DOI] [PubMed] [Google Scholar]
- [188].Zhao WP, Zhang J, Han ZY, Yao JP, Zhou X, and Liang P, A clinical investigation treating different types of fibroids identified by MRI-T2WI imaging with ultrasound guided high intensity focused ultrasound. Sci Rep. 2017;7:10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Izadifar Z, Izadifar Z, Chapman D, and Babyn P, An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J Clin Med. 2020;9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Lyon PC, Gray MD, Mannaris C, Folkes LK, Stratford M, Campo L, Chung DYF, Scott S, Anderson M, Goldin R, Carlisle R, Wu F, Middleton MR, Gleeson FV, and Coussios CC, Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): a single-centre, open-label, phase 1 trial. Lancet Oncol. 2018;19:1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Jiang CL, Davalos RV, and Bischof JC, A Review of Basic to Clinical Studies of Irreversible Electroporation Therapy. Ieee Transactions on Biomedical Engineering. 2015;62:4–20. [DOI] [PubMed] [Google Scholar]
- [192].Davalos RV, Mir IL, and Rubinsky B, Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–31. [DOI] [PubMed] [Google Scholar]
- [193].Dunki-Jacobs EM, Philips P, and Martin RCG, Evaluation of thermal injury to liver, pancreas and kidney during irreversible electroporation in an in vivo experimental model. British Journal of Surgery. 2014;101:1113–1121. [DOI] [PubMed] [Google Scholar]
- [194].van Gemert MJC, Wagstaff PGK, de Bruin DM, van Leeuwen TG, van der Wal AC, Heger M, and van der Geld CWM, Irreversible Electroporation: Just Another Form of Thermal Therapy? Prostate. 2015;75:332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].van den Bos W, Scheffer HJ, Vogel JA, Wagstaff PG, de Bruin DM, de Jong MC, van Gemert MJ, de la Rosette JJ, Meijerink MR, Klaessens JH, and Verdaasdonk RM, Thermal Energy during Irreversible Electroporation and the Influence of Different Ablation Parameters. J Vasc Interv Radiol. 2016;27:433–43. [DOI] [PubMed] [Google Scholar]
- [196].Faroja M, Ahmed M, Appelbaum L, Ben-David E, Moussa M, Sosna J, Nissenbaum I, and Goldberg SN, Irreversible Electroporation Ablation: Is All the Damage Nonthermal? Radiology. 2013;266:462–470. [DOI] [PubMed] [Google Scholar]
- [197].Wagstaff PG, de Bruin DM, van den Bos W, Ingels A, van Gemert MJ, Zondervan PJ, Verdaasdonk RM, van Lienden KP, van Leeuwen TG, de la Rosette JJ, and Laguna Pes MP, Irreversible electroporation of the porcine kidney: Temperature development and distribution. Urol Oncol. 2015;33:168 e1–7. [DOI] [PubMed] [Google Scholar]
- [198].Agnass P, Van Veldhuisen E, Van Gemert MJ, van der Geld CWM, Van Lienden KP, Van Gulik TM, Meijerink MR, Besselink MG, Kok HP, and Crezee J, Mathematical Modeling of the Thermal Effects of Irreversible Electroporation for In Vitro, In Vivo and Clinical Use: A Systematic Review. Int J Hyperthermia (in press). 2020; [DOI] [PubMed] [Google Scholar]
- [199].Agnass P, van Veldhuisen E, Vogel JA, Kok HP, de Keijzer MJ, Schooneveldt G, De Haan LR, Klaessens JH, Scheffer HJ, Meijerink MR, Van Lienden KP, Van Gulik TM, Heger M, Crezee J, and Besselink MG, Thermodynamic profiling during irreversible electroporation in porcine liver and pancreas: a case study series. J Clin Transl Res 2020;5:4. [PMC free article] [PubMed] [Google Scholar]
- [200].Garcia PA, Davalos RV, and Miklavcic D, A Numerical Investigation of the Electric and Thermal Cell Kill Distributions in Electroporation-Based Therapies in Tissue. Plos One. 2014;9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Scheffer HJ, Nielsen K, de Jong MC, van Tilborg AA, Vieveen JM, Bouwman AR, Meijer S, van Kuijk C, van den Tol PM, and Meijerink MR, Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25:997–1011; quiz 1011. [DOI] [PubMed] [Google Scholar]
- [202].Scheffer HJ, Vroomen LGPH, de Jong MC, Melenhorst MCAM, Zonderhuis BM, Daams F, Vogel JA, Besselink MGH, van Kuijk C, Witvliet J, de van der Schueren MAE, de Gruijl TD, Stam AGM, van den Tol PMP, van Delft F, Kazemier G, and Meijerink MR, Ablation of Locally Advanced Pancreatic Cancer with Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology. 2017;282:585–597. [DOI] [PubMed] [Google Scholar]
- [203].Vogel JA, Rombouts SJ, de Rooij T, van Delden OM, Dijkgraaf MG, van Gulik TM, van Hooft JE, van Laarhoven HW, Martin RC, Schoorlemmer A, Wilmink JW, van Lienden KP, Busch OR, and Besselink MG, Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Annals of Surgical Oncology. 2017;24:2734–2743. [DOI] [PubMed] [Google Scholar]
- [204].Kourounis G, Tabet PP, Moris D, Papalambros A, Felekouras E, Georgiades F, Astras G, and Petrou A, Irreversible electroporation (Nanoknife (R) treatment) in the field of hepatobiliary surgery: Current status and future perspectives. Journal of Buon. 2017;22:141–149. [PubMed] [Google Scholar]
- [205].Guenther E, Klein N, Zapf S, Weil S, Schlosser C, Rubinsky B, and Stehling K, Prostate cancer treatment with Irreversible Electroporation (IRE): Safety, efficacy and clinical experience in 471 treatments. Plos One. 2019;14: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ricke J, Jurgens JHW, Deschamps F, Tselikas L, Uhde K, Kosiek O, and De Baere T, Irreversible Electroporation (IRE) Fails to Demonstrate Efficacy in a Prospective Multicenter Phase II Trial on Lung Malignancies: The ALICE Trial. Cardiovascular and Interventional Radiology. 2015;38:401–408. [DOI] [PubMed] [Google Scholar]
- [207].Byron CR, DeWitt MR, Latouche EL, Davalos RV, and Robertson JL, Treatment of Infiltrative Superficial Tumors in Awake Standing Horses Using Novel High-Frequency Pulsed Electrical Fields. Frontiers in Veterinary Science. 2019;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Martin RC 2nd, Durham AN, Besselink MG, Iannitti D, Weiss MJ, Wolfgang CL, and Huang KW, Irreversible electroporation in locally advanced pancreatic cancer: A call for standardization of energy delivery. J Surg Oncol. 2016;114:865–871. [DOI] [PubMed] [Google Scholar]
- [209].Brezovich IA, Atkinson WJ, and Lilly MB, Local Hyperthermia with Interstitial Techniques. Cancer Research. 1984;44:4752–4756. [PubMed] [Google Scholar]
- [210].Brezovich IA, Lilly MB, Meredith RF, Weppelmann B, Henderson RA, Brawner W Jr., and Salter MM, Hyperthermia of pet animal tumours with self-regulating ferromagnetic thermoseeds. Int J Hyperthermia. 1990;6:117–30. [DOI] [PubMed] [Google Scholar]
- [211].van Wieringen N, van Dijk JD, van Veldhuizen J, and Nieuwenhuys GJ, The effect of catheters and coatings on the performance of palladium-nickel thermoseeds: evaluation and design of implantation techniques. Int J Hyperthermia. 1997;13:187–204. [DOI] [PubMed] [Google Scholar]
- [212].Kandala SK, Liapi E, Whitcomb LL, Attaluri A, and Ivkov R, Temperature-controlled power modulation compensates for heterogeneous nanoparticle distributions: a computational optimization analysis for magnetic hyperthermia. Int J Hyperthermia. 2019;36:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Mack CF, Stea B, Kittelson JM, Shimm DS, Sneed PK, Phillips TL, Swift PS, Luk K, Stauffer PR, Chan KW, Steeves R, Cassady JR, and Cetas TC, Interstitial Thermoradiotherapy with Ferromagnetic Implants for Locally Advanced and Recurrent Neoplasms. International Journal of Radiation Oncology Biology Physics. 1993;27:109–115. [DOI] [PubMed] [Google Scholar]
- [214].Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, and Jordan A, Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. Journal of Neuro-Oncology. 2011;103:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Stauffer PR and van Rhoon GC, Overview of bladder heating technology: matching capabilities with clinical requirements. International Journal of Hyperthermia. 2016;32:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Soria F, Milla P, Fiorito C, Pisano F, Sogni F, Di Marco M, Pagliarulo V, Dosio F, and Gontero P, Efficacy and safety of a new device for intravesical thermochemotherapy in non-grade 3 BCG recurrent NMIBC: a phase I-II study. World Journal of Urology. 2016;34:189–195. [DOI] [PubMed] [Google Scholar]
- [217].Ba M, Cui S, Wang B, Long H, Yan Z, Wang S, Wu Y, and Gong Y, Bladder intracavitary hyperthermic perfusion chemotherapy for the prevention of recurrence of non-muscle invasive bladder cancer after transurethral resection. Oncol Rep. 2017;37:2761–2770. [DOI] [PubMed] [Google Scholar]
- [218].Schooneveldt G, Bakker A, Balidemaj E, Chopra R, Crezee J, Geijsen ED, Hartmann J, Hulshof MC, Kok HP, Paulides MM, Sousa-Escandon A, Stauffer PR, and Maccarini PF, Thermal dosimetry for bladder hyperthermia treatment. An overview. Int J Hyperthermia. 2016;32:417–33. [DOI] [PubMed] [Google Scholar]
- [219].Colombo R, Lev A, Dapozzo LF, Freschi M, Gallus G, and Rigatti P, A New Approach Using Local Combined Microwave Hyperthermia and Chemotherapy in Superficial Transitional Bladder-Carcinoma Treatment. Journal of Urology. 1995;153:959–963. [PubMed] [Google Scholar]
- [220].Rath-Wolfson L, Moskovitz B, Dekel Y, Kugel V, and Koren R, Combined intravesical hyperthermia and mitomycin chemotherapy: a preliminary in vivo study. International Journal of Experimental Pathology. 2003;84:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Zhou J, Li L, Li X, Yu Q, Cui S, Shu K, Liu J, Liu J, Ding D, and Du T, Efficacy analysis of a novel thermochemotherapy scheme with pirarubicin for intermediate- and high-risk nonmuscle-invasive bladder cancer: a singleinstitution nonrandomized concurrent controlled trial. International Journal of Hyperthermia. 2019;36:868–875. [DOI] [PubMed] [Google Scholar]
- [222].Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, and Engelstein D, Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU.Int 2011;107:912–918. [DOI] [PubMed] [Google Scholar]
- [223].Arends TJH, Nativ O, Maffezzini M, de Cobelli O, Canepa G, Verweij F, Moskovitz B, van der Heijden AG, and Witjes JA, Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guerin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. European Urology. 2016;69:1046–1052. [DOI] [PubMed] [Google Scholar]
- [224].Tan WS, Panchal A, Buckley L, Devall AJ, Loubiere LS, Pope AM, Feneley MR, Cresswell J, Issa R, Mostafid H, Madaan S, Bhatt R, McGrath J, Sangar V, Griffiths TRL, Page T, Hodgson D, Datta SN, Billingham LJ, and Kelly JD, Radiofrequency-induced Thermo-chemotherapy Effect Versus a Second Course of Bacillus Calmette-Guerin or Institutional Standard in Patients with Recurrence of Non-muscle-invasive Bladder Cancer Following Induction or Maintenance Bacillus Calmette-Guerin Therapy (HYMN): A Phase III, Open-label, Randomised Controlled Trial. European Urology. 2019;75:63–71. [DOI] [PubMed] [Google Scholar]
- [225].Van Rhoon GC, “External Electromagnetic Methods and Devices,” in Physics of Thermal Therapy: Fundamentals and Clinical Applications, Moros EG; and Hendee WR, Eds., ed: CRC Press, Taylor & Francis Group, Boca Raton, FL, 2013, pp. 139–158. [Google Scholar]
- [226].Trefna HD, Crezee J, Schmidt M, Marder D, Lamprecht U, Ehmann M, Nadobny J, Hartmann J, Lomax N, Abdel-Rahman S, Curto S, Bakker A, Hurwitz MD, Diederich CJ, Stauffer PR, and Van Rhoon GC, Quality assurance guidelines for superficial hyperthermia clinical trials : II. Technical requirements for heating devices. Strahlentherapie Und Onkologie. 2017;193:351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Dressel S, Gosselin MC, Capstick MH, Carrasco E, Weyland MS, Scheidegger S, Neufeld E, Kuster N, Bodis S, and Rohrer Bley C, Novel hyperthermia applicator system allows adaptive treatment planning: Preliminary clinical results in tumour-bearing animals. Vet Comp Oncol. 2018;16:202–213. [DOI] [PubMed] [Google Scholar]
- [228].Notter M, Piazena H, and Vaupel P, Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: a retrospective study of 73 patients. Int J Hyperthermia. 2016;33:227–236. [DOI] [PubMed] [Google Scholar]
- [229].Van der Gaag ML, de Bruijne M, Samaras T, Van der Zee J, and Van Rhoon GC, Development of a guideline for the water bolus temperature in superficial hyperthermia. Int.J.Hyperthermia 2006;22:637–656. [DOI] [PubMed] [Google Scholar]
- [230].Arunachalam K, Maccarini PF, Craciunescu OI, Schlorff JL, and Stauffer PR, Thermal characteristics of thermobrachytherapy surface applicators for treating chest wall recurrence. Phys Med Biol. 2010;55:1949–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Kok HP, Groen J, Bakker A, and Crezee J, Modelling Curved Contact Flexible Microstrip Applicators for Patient-Specific Superficial Hyperthermia Treatment Planning. Cancers (Basel). 2020;12: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].van der Zee J, van der Holt B, Rietveld PJ, Helle PA, Wijnmaalen AJ, van Putten WL, and van Rhoon GC, Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation. Br J Cancer. 1999;79:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Lindholm CE, Kjellen E, Nilsson P, Weber L, and Hill S, Prognostic factors for tumour response and skin damage to combined radiotherapy and hyperthermia in superficial recurrent breast carcinomas. Int J Hyperthermia. 1995;11:337–55. [DOI] [PubMed] [Google Scholar]
- [234].Stauffer PR, Maccarini P, Arunachalam K, Craciunescu O, Diederich C, Juang T, Rossetto F, Schlorff J, Milligan A, Hsu J, Sneed P, and Vujaskovic Z, Conformal microwave array (CMA) applicators for hyperthermia of diffuse chest wall recurrence. Int J Hyperthermia. 2010;26:686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Lee WM, Gelvich EA, van der Baan P, Mazokhin VN, and van Rhoon GC, Assessment of the performance characteristics of a prototype 12-element capacitive contact flexible microstrip applicator (CFMA-12) for superficial hyperthermia. Int J Hyperthermia. 2004;20:607–24. [DOI] [PubMed] [Google Scholar]
- [236].Rietveld PJ, van Putten WL, Van der Zee J, and Van Rhoon GC, Comparison of the clinical effectiveness of the 433 MHz Lucite cone applicator with that of a conventional waveguide applicator in applications of superficial hyperthermia. Int.J.Radiat.Oncol.Biol.Phys 1999;43:681–687. [DOI] [PubMed] [Google Scholar]
- [237].van Rhoon GC, Rietveld PJ, and van der Zee J, A 433 MHz Lucite cone waveguide applicator for superficial hyperthermia. Int J Hyperthermia. 1998;14:13–27. [DOI] [PubMed] [Google Scholar]
- [238].Kosterev VV, Kramer-Ageev EA, Mazokhin VN, van Rhoon GC, and Crezee J, Development of a novel method to enhance the therapeutic effect on tumours by simultaneous action of radiation and heating. Int J Hyperthermia. 2015;31:443–52. [DOI] [PubMed] [Google Scholar]
- [239].Maccarini PF, Arunachalam K, Juang T, De Luca V, Rangarao S, Neumann D, Martins CD, Craciunescu O, and Stauffer PR, Shaping and resizing of multifed slot radiators used in conformal microwave antenna arrays for hyperthermia treatment of large superficial diseases. Int Conf Electromagn Adv Appl. 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Johnson JE, Neuman DG, Maccarini PF, Juang T, Stauffer PR, and Turner P, Evaluation of a dual-arm Archimedean spiral array for microwave hyperthermia. Int J Hyperthermia. 2006;22:475–90. [DOI] [PubMed] [Google Scholar]
- [241].Lee ER, Wilsey TR, Tarczy-Hornoch P, Kapp DS, Fessenden P, Lohrbach A, and Prionas SD, Body conformable 915 MHz microstrip array applicators for large surface area hyperthermia. IEEE Trans.Biomed.Eng 1992;39:470–483. [DOI] [PubMed] [Google Scholar]
- [242].Gelvich EA and Mazokhin VN, Contact flexible microstrip applicators (CFMA) in a range from microwaves up to short waves. IEEE Trans.Biomed.Eng 2002;49:1015–1023. [DOI] [PubMed] [Google Scholar]
- [243].Lamaitre G, Van Dijk JDP, Gelvich EA, Wiersma J, and Schneider CJ, SAR characteristics of three types of Contact Flexible Microstrip Applicators for superficial hyperthermia. Int.J.Hyperthermia 1996;12:255–269. [DOI] [PubMed] [Google Scholar]
- [244].Kok HP, Correia D, De Greef M, Van Stam G, Bel A, and Crezee J, SAR deposition by curved CFMA-434 applicators for superficial hyperthermia: Measurements and simulations. Int.J.Hyperthermia 2010;26:171–184. [DOI] [PubMed] [Google Scholar]
- [245].Gelvich EA and Mazokhin VN, Resonance effects in applicator water boluses and their influence on SAR distribution patterns. Int.J.Hyperthermia 2000;16:113–128. [DOI] [PubMed] [Google Scholar]
- [246].Gelvich EA, Klimanov VA, Kramer-Ageev EA, and Mazokhin VN, Computational evaluation of changes in ionizing radiation dose distribution in tissues caused by EM applicators when external radiation and hyperthermia act simultaneously. Int.J.Hyperthermia 2006;22:343–352. [DOI] [PubMed] [Google Scholar]
- [247].Overgaard J, Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int.J.Radiat.Oncol.Biol.Phys 1980;6:1507–1517. [DOI] [PubMed] [Google Scholar]
- [248].Wust P, Ghadjar P, Nadobny J, Beck M, Kaul D, Winter L, and Zschaeck S, Physical analysis of temperature-dependent effects of amplitude-modulated electromagnetic hyperthermia. Int J Hyperthermia. 2019;36:1246–1254. [DOI] [PubMed] [Google Scholar]
- [249].Curley SA, Palalon F, Lu X, and Koshkina NV, Noninvasive radiofrequency treatment effect on mitochondria in pancreatic cancer cells. Cancer. 2014;120:3418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Curley SA, Palalon F, Sanders KE, and Koshkina NV, The effects of non-invasive radiofrequency treatment and hyperthermia on malignant and nonmalignant cells. Int J Environ Res Public Health. 2014;11:9142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Ware MJ, Tinger S, Colbert KL, Corr SJ, Rees P, Koshkina N, Curley S, Summers HD, and Godin B, Radiofrequency treatment alters cancer cell phenotype. Sci Rep. 2015;5:12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Van Wieringen N, Wiersma J, Zum Vörde Sive Vörding PJ, Oldenborg S, Gelvich EA, Mazokhin VN, Van Dijk JDP, and Crezee H, Characteristics and performance evaluation of the capacitive Contact Flexible Microstrip Applicator operating at 70 MHz for external hyperthermia. Int.J.Hyperthermia 2009;25:542–553. [DOI] [PubMed] [Google Scholar]
- [253].van Stam G, Kok HP, Hulshof M, Kolff MW, van Tienhoven G, Sijbrands J, Bakker A, Zum Vorde Sive Vording PJ, Oldenborg S, de Greef M, Rasch CRN, and Crezee H, A flexible 70 MHz phase-controlled double waveguide system for hyperthermia treatment of superficial tumours with deep infiltration. Int J Hyperthermia. 2017;33:796–809. [DOI] [PubMed] [Google Scholar]
- [254].Crezee J, Van Tienhoven G, Kolff MW, Sijbrands J, Van Stam G, Oldenborg S, Geijsen ED, Hulshof MCCM, and Kok HP, Development of a 70 MHz unit for hyperthermia treatment of deep-seated breast tumors. International Journal of Microwave and Wireless Technologies. 2017;9:1317–1324. [Google Scholar]
- [255].Wu L, McGough RJ, Arabe OA, and Samulski TV, An RF phased array applicator designed for hyperthermia breast cancer treatments. Phys Med Biol. 2006;51:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Paulides MM, Bakker JF, Neufeld E, van der Zee J, Jansen PP, Levendag PC, and van Rhoon GC, The HYPERcollar: a novel applicator for hyperthermia in the head and neck. Int J Hyperthermia. 2007;23:567–76. [DOI] [PubMed] [Google Scholar]
- [257].Verduijn GM, de Wee EM, Rijnen Z, Togni P, Hardillo JAU, Ten Hove I, Franckena M, van Rhoon GC, and Paulides MM, Deep hyperthermia with the HYPERcollar system combined with irradiation for advanced head and neck carcinoma - a feasibility study. Int J Hyperthermia. 2018;34:994–1001. [DOI] [PubMed] [Google Scholar]
- [258].Paulides MM, Bakker JF, Hofstetter LW, Numan WC, Pellicer R, Fiveland EW, Tarasek M, Houston GC, van Rhoon GC, Yeo DT, and Kotek G, Laboratory prototype for experimental validation of MR-guided radiofrequency head and neck hyperthermia. Phys Med Biol. 2014;59:2139–54. [DOI] [PubMed] [Google Scholar]
- [259].Corry PM, Barlogie B, Tilchen EJ, and Armour EP, Ultrasound-induced hyperthermia for the treatment of human superficial tumors. Int J Radiat Oncol Biol Phys. 1982;8:1225–9. [DOI] [PubMed] [Google Scholar]
- [260].Underwood HR, Burdette EC, Ocheltree KB, and Magin RL, A multi-element ultrasonic hyperthermia applicator with independent element control. Int J Hyperthermia. 1987;3:257–67. [DOI] [PubMed] [Google Scholar]
- [261].Moros EG, Fan X, and Straube WL, Experimental assessment of power and temperature penetration depth control with a dual frequency ultrasonic system. Med Phys. 1999;26:810–7. [DOI] [PubMed] [Google Scholar]
- [262].Novak P, Moros EG, Straube WL, and Myerson RJ, SURLAS: a new clinical grade ultrasound system for sequential or concomitant thermoradiotherapy of superficial tumors: applicator description. Med Phys. 2005;32:230–40. [DOI] [PubMed] [Google Scholar]
- [263].Samulski TV, Grant WJ, Oleson JR, Leopold KA, Dewhirst MW, Vallario P, and Blivin J, Clinical experience with a multi-element ultrasonic hyperthermia system: analysis of treatment temperatures. Int J Hyperthermia. 1990;6:909–22. [DOI] [PubMed] [Google Scholar]
- [264].Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR, and Jones EL, Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: a review of the randomised data. Int J Hyperthermia. 2010;26:612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, Van der Zee J, van Putten WLJ, Van Rhoon GC, van Dijk JDP, González González D, Liu FF, Goodman P, and Sherar M, Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int.J.Radiat.Oncol.Biol.Phys 1996;35:731–744. [DOI] [PubMed] [Google Scholar]
- [266].Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, and Dewhirst MW, Randomized trial of hyperthermia and radiation for superficial tumors. J.Clin.Oncol 2005;23:3079–3085. [DOI] [PubMed] [Google Scholar]
- [267].Overgaard J, González González D, Hulshof MCCM, Arcangeli G, Dahl O, Mella O, and Bentzen SM, Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995;345:540–543. [DOI] [PubMed] [Google Scholar]
- [268].Overgaard J, Gonzalez DG, Hulshof MCCH, Arcangeli G, Dahl O, Mella O, and Bentzen SM, Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. International Journal of Hyperthermia. 1996;12:3–20. [DOI] [PubMed] [Google Scholar]
- [269].Huilgol NG, Gupta S, and Sridhar CR, Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: a report of randomized trial. J.Cancer Res.Ther 2010;6:492–496. [DOI] [PubMed] [Google Scholar]
- [270].Van Leeuwen CM, Oei AL, Chin KWTK, Crezee, Bel A, Westermann AM, Buist MR, Franken NAP, Stalpers LJA, and Kok HP, A short time interval between radiotherapy and hyperthermia reduces in-field recurrence and mortality in women with advanced cervical cancer. Radiation Oncology. 2017;12:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].van Leeuwen CM, Crezee J, Oei AL, Franken NAP, Stalpers LJA, Bel A, and Kok HP, The effect of time interval between radiotherapy and hyperthermia on planned equivalent radiation dose. Int J Hyperthermia. 2018;34:901–909. [DOI] [PubMed] [Google Scholar]
- [272].Van der Zee J, González González D, Van Rhoon GC, van Dijk JDP, van Putten WLJ, and Hart AA, Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119–1125. [DOI] [PubMed] [Google Scholar]
- [273].Kato H, Hyodo K, Akassa N, Nishimura K, Uchida H, Kasai T, and Sugimura K, Optimization of bolus for capacitive type heating. Japanese Journal of Hyperthermic Oncology. 1997;13:10–17. [Google Scholar]
- [274].Paulsen KD, Geimer S, Tang J, and Boyse WE, Optimization of pelvic heating rate distributions with electromagnetic phased arrays. Int.J.Hyperthermia 1999;15:157–186. [DOI] [PubMed] [Google Scholar]
- [275].Wust P, Seebass M, Nadobny J, Deuflhard P, Monich G, and Felix R, Simulation studies promote technological development of radiofrequency phased array hyperthermia. Int.J.Hyperthermia 1996;12:477–494. [DOI] [PubMed] [Google Scholar]
- [276].Kok HP, De Greef M, Borsboom PP, Bel A, and Crezee J, Improved power steering with double and triple ring waveguide systems: the impact of the operating frequency. Int.J.Hyperthermia 2011;27:224–239. [DOI] [PubMed] [Google Scholar]
- [277].Turner PF, Tumeh A, and Schaefermeyer T, BSD-2000 approach for deep local and regional hyperthermia: physics and technology. Strahlenther.Onkol 1989;165:738–741. [PubMed] [Google Scholar]
- [278].Zweije R, Kok HP, Bakker A, Bel A, and Crezee J, Technical and Clinical Evaluation of the ALBA-4D 70MHz Loco-Regional Hyperthermia System. Proceedings of the 48th European Microwave Conference. 2018;328–331. [Google Scholar]
- [279].Gellermann J, Wust P, Stalling D, Seebass M, Nadobny J, Beck R, Hege H, Deuflhard P, and Felix R, Clinical evaluation and verification of the hyperthermia treatment planning system hyperplan. Int.J.Radiat.Oncol.Biol.Phys 2000;47:1145–1156. [DOI] [PubMed] [Google Scholar]
- [280].Sreenivasa G, Gellermann J, Rau B, Nadobny J, Schlag P, Deuflhard P, Felix R, and Wust P, Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int.J.Radiat.Oncol.Biol.Phys 2003;55:407–419. [DOI] [PubMed] [Google Scholar]
- [281].Van Haaren PMA, Kok HP, Van den Berg CAT, Zum Vorde Sive Vording PJ, Oldenborg S, Stalpers LJ, Schilthuis MS, De Leeuw AAC, and Crezee J, On verification of hyperthermia treatment planning for cervical carcinoma patients. Int.J.Hyperthermia 2007;23:303–314. [DOI] [PubMed] [Google Scholar]
- [282].Van Haaren P, Kok P, Van Stam G, Wiersma J, van de Kamer JB, van Dijk J, and Crezee H, SAR measurements and FDTD calculations in inhomogeneous phantom models. 9th International Congress on Hyperthermic Oncology, St.Louis, USA, 2004.Abstracts. 2004;pp. 167. [Google Scholar]
- [283].Wust P, Beck R, Berger J, Fahling H, Seebass M, Wlodarczyk W, Hoffmann W, and Nadobny J, Electric field distributions in a phased-array applicator with 12 channels: measurements and numerical simulations. Med.Phys 2000;27:2565–2579. [DOI] [PubMed] [Google Scholar]
- [284].Wust P, Fahling H, Wlodarczyk W, Seebass M, Gellermann J, Deuflhard P, and Nadobny J, Antenna arrays in the SIGMA-eye applicator: interactions and transforming networks. Med Phys. 2001;28:1793–805. [DOI] [PubMed] [Google Scholar]
- [285].Weihrauch M, Wust P, Weiser M, Nadobny J, Eisenhardt S, Budach V, and Gellermann J, Adaptation of antenna profiles for control of MR guided hyperthermia (HT) in a hybrid MR-HT system. Med.Phys 2007;34:4717–4725. [DOI] [PubMed] [Google Scholar]
- [286].Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Budach V, Felix R, Tunn PU, Reichardt P, and Wust P, Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: correlation with response and direct thermometry. Cancer. 2006;107:1373–1382. [DOI] [PubMed] [Google Scholar]
- [287].Gellermann J, Wlodarczyk W, Hildebrandt B, Ganter H, Nicolau A, Rau B, Tilly W, Fahling H, Nadobny J, Felix R, and Wust P, Noninvasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 Tesla hybrid system. Cancer Res. 2005;65:5872–5880. [DOI] [PubMed] [Google Scholar]
- [288].Gellermann J, Wlodarczyk W, Ganter H, Nadobny J, Fahling H, Seebass M, Felix R, and Wust P, A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: validation in a heterogeneous phantom. Int J Radiat Oncol Biol Phys. 2005;61:267–77. [DOI] [PubMed] [Google Scholar]
- [289].Mulder HT, Curto S, Paulides MM, Franckena M, and van Rhoon GC, Systematic quality assurance of the BSD2000–3D MR-compatible hyperthermia applicator performance using MR temperature imaging. Int J Hyperthermia. 2018;35:305–313. [DOI] [PubMed] [Google Scholar]
- [290].van Dijk JDP, Schneider CJ, van Os RM, Blank LE, and González González D, Results of deep body hyperthermia with large waveguide radiators. Adv.Exp.Med.Biol 1990;267:315–319. [DOI] [PubMed] [Google Scholar]
- [291].Crezee J, Van Haaren PMA, Westendorp H, De Greef M, Kok HP, Wiersma J, Van Stam G, Sijbrands J, Zum Vörde Sive Vörding PJ, JDP Van Dijk, Hulshof MCCM, and Bel A, Improving locoregional hyperthermia delivery using the 3-D controlled AMC-8 phased array hyperthermia system : A preclinical study. Int.J.Hyperthermia 2009;25:581–592. [DOI] [PubMed] [Google Scholar]
- [292].Ohguri T, Imada H, Yahara K, Morioka T, Nakano K, Terashima H, and Korogi Y, Radiotherapy with 8-MHz radiofrequency-capacitive regional hyperthermia for stage III non-small-cell lung cancer: the radiofrequency-output power correlates with the intraesophageal temperature and clinical outcomes. Int J Radiat Oncol Biol Phys. 2009;73:128–35. [DOI] [PubMed] [Google Scholar]
- [293].Ohguri T, Imada H, Yahara K, Moon SD, Yamaguchi S, Yatera K, Mukae H, Hanagiri T, Tanaka F, and Korogi Y, Re-irradiation plus regional hyperthermia for recurrent non-small cell lung cancer: a potential modality for inducing long-term survival in selected patients. Lung Cancer. 2012;77:140–5. [DOI] [PubMed] [Google Scholar]
- [294].Ohguri T, Yahara K, Moon SD, Yamaguchi S, Imada H, Terashima H, and Korogi Y, Deep regional hyperthermia for the whole thoracic region using 8 MHz radiofrequency-capacitive heating device: relationship between the radiofrequency-output power and the intra-oesophageal temperature and predictive factors for a good heating in 59 patients. Int J Hyperthermia. 2011;27:20–6. [DOI] [PubMed] [Google Scholar]
- [295].Ohguri T, Imada H, Narisada H, Yahara K, Morioka T, Nakano K, Miyaguni Y, and Korogi Y, Systemic chemotherapy using paclitaxel and carboplatin plus regional hyperthermia and hyperbaric oxygen treatment for non-small cell lung cancer with multiple pulmonary metastases: preliminary results. Int J Hyperthermia. 2009;25:160–7. [DOI] [PubMed] [Google Scholar]
- [296].Moon SD, Ohguri T, Imada H, Yahara K, Yamaguchi S, Hanagiri T, Yasumoto K, Yatera K, Mukae H, Terashima H, and Korogi Y, Definitive radiotherapy plus regional hyperthermia with or without chemotherapy for superior sulcus tumors: a 20-year, single center experience. Lung Cancer. 2011;71:338–43. [DOI] [PubMed] [Google Scholar]
- [297].Kodama K, Doi O, Higashiyama M, Yokouchi H, and Tatsuta M, Long-term results of postoperative intrathoracic chemo-thermotherapy for lung cancer with pleural dissemination. Cancer. 1993;72:426–31. [DOI] [PubMed] [Google Scholar]
- [298].Tomura K, Ohguri T, Mulder HT, Murakami M, Nakahara S, Yahara K, and Korogi Y, The usefulness of mobile insulator sheets for the optimization of deep heating area for regional hyperthermia using a capacitively-coupled heating method: Phantom, simulation and clinical prospective studies. Int J Hyperthermia. 2018;34:1092–1103. [DOI] [PubMed] [Google Scholar]
- [299].Yahara K, Ohguri T, Yamaguchi S, Imada H, Narisada H, Ota S, Tomura K, Sakagami M, Fujimoto N, and Korogi Y, Definitive radiotherapy plus regional hyperthermia for high-risk and very high-risk prostate carcinoma: Thermal parameters correlated with biochemical relapse-free survival. Int J Hyperthermia. 2015;31:600–8. [DOI] [PubMed] [Google Scholar]
- [300].Okamura T, Ueda K, Hashimoto Y, Tozawa K, and Kohri K, Immunohistochemical evaluation of p53 and retinoblastoma proteins in relation to hyperthermia treatment: results in human urothelial carcinomas. Int J Hyperthermia. 1996;12:813–24. [DOI] [PubMed] [Google Scholar]
- [301].Ohguri T, Imada H, Nomoto S, Kato F, Yahara K, Morioka T, Nakano K, and Korogi Y, Initial Experience of Bladder Preservation Therapy Using Chemoradiotherapy with Regional Hyperthermia for Muscle-invasive Bladder Cancer Jpn. J. Hyperthermic Oncol. 2005;21:151–157. [Google Scholar]
- [302].Masunaga SI, Hiraoka M, Akuta K, Nishimura Y, Nagata Y, Jo S, Takahashi M, Abe M, Terachi T, Oishi K, and et al. , Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int J Hyperthermia. 1994;10:31–40. [DOI] [PubMed] [Google Scholar]
- [303].Ohguri T, Imada H, Kato F, Yahara K, Morioka T, Nakano K, and Korogi Y, Radiotherapy with 8 MHz radiofrequency-capacitive regional hyperthermia for pain relief of unresectable and recurrent colorectal cancer. Int J Hyperthermia. 2006;22:1–14. [DOI] [PubMed] [Google Scholar]
- [304].Ohguri T, Imada H, Yahara K, Kakeda S, Tomimatsu A, Kato F, Nomoto S, Terashima H, and Korogi Y, Effect of 8-MHz radiofrequency-capacitive regional hyperthermia with strong superficial cooling for unresectable or recurrent colorectal cancer. Int J Hyperthermia. 2004;20:465–75. [DOI] [PubMed] [Google Scholar]
- [305].Nagata Y, Hiraoka M, Nishimura Y, Masunaga S, Mitumori M, Okuno Y, Fujishiro M, Kanamori S, Horii N, Akuta K, Sasai K, Abe M, and Fukuda Y, Clinical results of radiofrequency hyperthermia for malignant liver tumors. Int J Radiat Oncol Biol Phys. 1997;38:359–65. [DOI] [PubMed] [Google Scholar]
- [306].Murata T, Akagi K, Imamura M, Nasu R, Kimura H, Nagata K, and Tanaka Y, Studies on hyperthermia combined with arterial therapeutic blockade for treatment of tumors: (Part III) effectiveness of hyperthermia combined with arterial chemoembolization using degradable starch microspheres on advanced liver cancer. Oncol Rep. 1998;5:709–12. [DOI] [PubMed] [Google Scholar]
- [307].Minnaar CA, Kotzen JA, Ayeni OA, Naidoo T, Tunmer M, Sharma V, Vangu MD, and Baeyens A, The effect of modulated electro-hyperthermia on local disease control in HIV-positive and -negative cervical cancer women in South Africa: Early results from a phase III randomised controlled trial. PLoS One. 2019;14:e0217894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Harima Y, Ohguri T, Imada H, Sakurai H, Ohno T, Hiraki Y, Tuji K, Tanaka M, and Terashima H, A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int J Hyperthermia. 2016;32:801–8. [DOI] [PubMed] [Google Scholar]
- [309].Chi MS, Yang KL, Chang YC, Ko HL, Lin YH, Huang SC, Huang YY, Liao KW, Kondo M, and Chi KH, Comparing the Effectiveness of Combined External Beam Radiation and Hyperthermia Versus External Beam Radiation Alone in Treating Patients With Painful Bony Metastases: A Phase 3 Prospective, Randomized, Controlled Trial. Int J Radiat Oncol Biol Phys. 2018;100:78–87. [DOI] [PubMed] [Google Scholar]
- [310].Franckena M, Stalpers LJ, Koper PC, Wiggenraad RG, Hoogenraad WJ, van Dijk JD, Warlam-Rodenhuis CC, Jobsen JJ, Van Rhoon GC, and Van der Zee J, Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the Dutch Deep Hyperthermia Trial. Int.J.Radiat.Oncol.Biol.Phys 2008;70:1176–1182. [DOI] [PubMed] [Google Scholar]
- [311].Issels RD, Lindner LH, Verweij J, Wessalowski R, Reichardt P, Wust P, Ghadjar P, Hohenberger P, Angele M, Salat C, Vujaskovic Z, Daugaard S, Mella O, Mansmann U, Durr HR, Knosel T, Abdel-Rahman S, Schmidt M, Hiddemann W, Jauch KW, Belka C, Gronchi A, European Organization for the R, Treatment of Cancer-Soft T, Bone Sarcoma G, and the European Society for Hyperthermic O, Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Wessalowski R, Schneider DT, Mils O, Friemann V, Kyrillopoulou O, Schaper J, Matuschek C, Rothe K, Leuschner I, Willers R, Schonberger S, Gobel U, Calaminus G, and group Ms, Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: an open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol. 2013;14:843–52. [DOI] [PubMed] [Google Scholar]
- [313].Wessalowski R, Schneider DT, Mils O, Hannen M, Calaminus G, Engelbrecht V, Pape H, Willers R, Engert J, Harms D, and Gobel U, An approach for cure: PEI-chemotherapy and regional deep hyperthermia in children and adolescents with unresectable malignant tumors. Klin Padiatr. 2003;215:303–9. [DOI] [PubMed] [Google Scholar]
- [314].Seifert G, Budach V, Keilholz U, Wust P, Eggert A, and Ghadjar P, Regional hyperthermia combined with chemotherapy in paediatric, adolescent and young adult patients: current and future perspectives. Radiat Oncol. 2016;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C, and Issels RD, Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29:8–16. [DOI] [PubMed] [Google Scholar]
- [316].Ott OJ, Schmidt M, Semrau S, Strnad V, Matzel KE, Schneider I, Raptis D, Uter W, Grutzmann R, and Fietkau R, Chemoradiotherapy with and without deep regional hyperthermia for squamous cell carcinoma of the anus. Strahlenther Onkol. 2019;195:607–614. [DOI] [PubMed] [Google Scholar]
- [317].Datta NR, Pestalozzi B, Clavien PA, Siebenhuner A, Puric E, Khan S, Mamot C, Riesterer O, Knuchel J, Reiner CS, Bodis S, and members of the HTG, “HEATPAC” - a phase II randomized study of concurrent thermochemoradiotherapy versus chemoradiotherapy alone in locally advanced pancreatic cancer. Radiat Oncol. 2017;12:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Gani C, Bonomo P, Zwirner K, Schroeder C, Menegakis A, Rodel C, and Zips D, Organ preservation in rectal cancer - Challenges and future strategies. Clin Transl Radiat Oncol. 2017;3:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Carlier C, Mathys A, De Jaeghere E, Steuperaert M, De Wever O, and Ceelen W, Tumour tissue transport after intraperitoneal anticancer drug delivery. Int J Hyperthermia. 2017;33:534–542. [DOI] [PubMed] [Google Scholar]
- [320].Klaver CEL, Stam R, Sloothaak DAM, Crezee J, Bemelman WA, Punt CJA, and Tanis PJ, Colorectal cancer at high risk of peritoneal metastases; long term outcomes of a pilot study on adjuvant laparoscopic HIPEC and future perspectives. Oncotarget. 2017;8:51200–51209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Klaver CEL, Musters GD, Bemelman WA, Punt CJA, Verwaal VJ, Dijkgraaf MGW, Aalbers AGJ, van der Bilt JDW, Boerma D, Bremers AJA, Burger JWA, Buskens CJ, Evers P, van Ginkel RJ, van Grevenstein WMU, Hemmer PHJ, de Hingh IHJT, Lammers LA, van Leeuwen BL, Meijerink WJHJ, Nienhuijs SW, Pon J, Radema SA, van Ramshorst B, Snaebjornsson P, Tuynman JB, te Velde EA, Wiezer MJ, de Wilt JHW, and Tanis PJ, Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. Bmc Cancer. 2015;15: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Cowan RA, O’Cearbhaill RE, Zivanovic O, and Chi DS, Current status and future prospects of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) clinical trials in ovarian cancer. Int J Hyperthermia. 2017;33:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Pinto A, Eveno C, and Pocard M, Update on clinical trials in colorectal cancer peritoneal metastasis. Int J Hyperthermia. 2017;33:543–547. [DOI] [PubMed] [Google Scholar]
- [324].Goere D, Passot G, Gelli M, Levine EA, Bartlett DL, Sugarbaker PH, and Glehen O, Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: results from a worldwide analysis issue of the Peritoneal Surface Oncology Group International (PSOGI). Int J Hyperthermia. 2017;33:520–527. [DOI] [PubMed] [Google Scholar]
- [325].Rovers KP, de Bree E, Yonemura Y, and de Hingh IH, Treatment of peritoneal metastases from small bowel adenocarcinoma. Int J Hyperthermia. 2017;33:571–578. [DOI] [PubMed] [Google Scholar]
- [326].Sugarbaker PH, Strategies to improve local control of resected pancreas adenocarcinoma. Surg Oncol. 2017;26:63–70. [DOI] [PubMed] [Google Scholar]
- [327].van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh I, van der Velden J, Arts HJ, Massuger L, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, and Sonke GS, Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018;378:230–240. [DOI] [PubMed] [Google Scholar]
- [328].Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Gagniere J, Lefevre JH, Courvoisier T, Kianmanesh R, Vaudoyer D, Rivoire M, Meeus P, Passot G, Glehen O, Fregat, and Networks B-R, Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019;37:2028–2040. [DOI] [PubMed] [Google Scholar]
- [329].Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, and Zoetmulder FA, Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–43. [DOI] [PubMed] [Google Scholar]
- [330].Klaver CEL, Wisselink DD, Punt CJA, Snaebjornsson P, Crezee J, Aalbers AGJ, Brandt A, Bremers AJA, Burger JWA, Fabry HFJ, Ferenschild F, Festen S, van Grevenstein WMU, Hemmer PHJ, de Hingh I, Kok NFM, Musters GD, Schoonderwoerd L, Tuynman JB, van de Ven AWH, van Westreenen HL, Wiezer MJ, Zimmerman DDE, van Zweeden AA, Dijkgraaf MGW, Tanis PJ, and group Cc, Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4:761–770. [DOI] [PubMed] [Google Scholar]
- [331].Goere D, Glehen O, Quenet F, Ducreux M, Guilloit JM, and Texier M, Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-NTC01226394). Journal of Clinical Oncology. 2018;36: [Google Scholar]
- [332].Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, Marchal F, Loi V, Meeus P, De Forges H, Stanbury T, Paineau J, and Glehen O, A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. Journal of Clinical Oncology. 2018;36: [Google Scholar]
- [333].Ceelen W, HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur J Surg Oncol. 2019;45:400–402. [DOI] [PubMed] [Google Scholar]
- [334].Rau B, Brandl A, Piso P, Pelz J, Busch P, Demtroder C, Schule S, Schlitt HJ, Roitman M, Tepel J, Sulkowski U, Uzunoglu F, Hunerbein M, Horbelt R, Strohlein M, Beckert S, Konigsrainer I, Konigsrainer A, Peritoneum Surface Oncology G, members of the StuDo Q, Peritoneum Registry of the German Society for G, and Visceral S, Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2019; [DOI] [PubMed] [Google Scholar]
- [335].Koops HS, Vaglini M, Suciu S, Kroon BB, Thompson JF, Gohl J, Eggermont AM, Di Filippo F, Krementz ET, Ruiter D, and Lejeune FJ, Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol. 1998;16:2906–12. [DOI] [PubMed] [Google Scholar]
- [336].Eggermont AM, de Wilt JH, and ten Hagen TL, Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003;4:429–37. [DOI] [PubMed] [Google Scholar]
- [337].Bull JM, Scott GL, Strebel FR, Nagle VL, Oliver D, Redwine M, Rowe RW, Ahn CW, and Koch SM, Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-alpha: a description of a phase I-II protocol. Int J Hyperthermia. 2008;24:649–62. [DOI] [PubMed] [Google Scholar]
- [338].Hildebrandt B, Hegewisch-Becker S, Kerner T, Nierhaus A, Bakhshandeh-Bath A, Janni W, Zumschlinge R, Sommer H, Riess H, and Wust P, Current status of radiant whole-body hyperthermia at temperatures > 41.5 degrees C and practical guidelines for the treatment of adults. The German ‘Interdisciplinary Working Group on Hyperthermia’. International Journal of Hyperthermia. 2005;21:169–183. [DOI] [PubMed] [Google Scholar]
- [339].Janssen CW, Lowry CA, Mehl MR, Allen JJ, Kelly KL, Gartner DE, Medrano A, Begay TK, Rentscher K, White JJ, Fridman A, Roberts LJ, Robbins ML, Hanusch KU, Cole SP, and Raison CL, Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2016;73:789–95. [DOI] [PubMed] [Google Scholar]
- [340].Song CW, Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44:4721s–4730s. [PubMed] [Google Scholar]
- [341].Franckena M, Fatehi D, de Bruijne M, Canters RA, van Norden Y, Mens JW, Van Rhoon GC, and Van der Zee J, Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur.J.Cancer 2009;45:1969–1978. [DOI] [PubMed] [Google Scholar]
- [342].Linthorst M, Baaijens M, Wiggenraad R, Creutzberg C, Ghidey W, van Rhoon GC, and van der Zee J, Local control rate after the combination of re-irradiation and hyperthermia for irresectable recurrent breast cancer: Results in 248 patients. Radiother Oncol. 2015;117:217–22. [DOI] [PubMed] [Google Scholar]
- [343].Bakker A, Kolff MW, Holman R, van Leeuwen CM, Korshuize-van Straten L, de Kroon-Oldenhof R, Rasch CRN, van Tienhoven G, and Crezee H, Thermal Skin Damage During Reirradiation and Hyperthermia Is Time-Temperature Dependent. Int J Radiat Oncol Biol Phys. 2017;98:392–399. [DOI] [PubMed] [Google Scholar]
- [344].Saccomandi P, Schena E, and Silvestri S, Techniques for temperature monitoring during laser-induced thermotherapy: An overview. International Journal of Hyperthermia. 2013;29:609–619. [DOI] [PubMed] [Google Scholar]
- [345].Lewis MA, Staruch RM, and Chopra R, Thermometry and ablation monitoring with ultrasound. International Journal of Hyperthermia. 2015;31:163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].De Leeuw AAC, Crezee J, and Lagendijk JJW, Temperature and SAR measurements in deep-body hyperthermia with thermocouple thermometry. Int.J.Hyperthermia 1993;9:685–697. [DOI] [PubMed] [Google Scholar]
- [347].Hynynen K and Edwards DK, Temperature measurements during ultrasound hyperthermia. Med Phys. 1989;16:618–26. [DOI] [PubMed] [Google Scholar]
- [348].Morris H, Rivens I, Shaw A, and ter Haar G, Investigation of the viscous heating artefact arising from the use of thermocouples in a focused ultrasound field. Physics in Medicine and Biology. 2008;53:4759–4776. [DOI] [PubMed] [Google Scholar]
- [349].Winter L, Oberacker E, Paul K, Ji Y, Oezerdem C, Ghadjar P, Thieme A, Budach V, Wust P, and Niendorf T, Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int J Hyperthermia. 2016;32:63–75. [DOI] [PubMed] [Google Scholar]
- [350].Fani F, Schena E, Saccomandi P, and Silvestri S, CT-based thermometry: an overview. Int J Hyperthermia. 2014;30:219–27. [DOI] [PubMed] [Google Scholar]
- [351].Miyakawa M and Bolomey JC, Non-Invasive Thermometry of the Human Body: CRC Press, 1995. [Google Scholar]
- [352].Bharat S, Techavipoo U, Kiss MZ, Liu W, and Varghese T, Monitoring stiffness changes in lesions after radiofrequency ablation at different temperatures and durations of ablation. Ultrasound in Medicine and Biology. 2005;31:415–422. [DOI] [PubMed] [Google Scholar]
- [353].Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, and Suzuki Y, A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34:814–23. [DOI] [PubMed] [Google Scholar]
- [354].Kothapalli S VVN, Altman MB, Zhu L, Partanen A, Cheng G, Gach HM, Straube W, Zoberi I, Hallahan DE, and Chen H, Evaluation and selection of anatomic sites for magnetic resonance imaging-guided mild hyperthermia therapy: a healthy volunteer study. Int J Hyperthermia. 2018;34:1381–1389. [DOI] [PubMed] [Google Scholar]
- [355].Li Z, Vogel M, Maccarini PF, Stakhursky V, Soher BJ, Craciunescu OI, Das S, Arabe OA, Joines WT, and Stauffer PR, Improved hyperthermia treatment control using SAR/temperature simulation and PRFS magnetic resonance thermal imaging. Int J Hyperthermia. 2011;27:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].Salomir R, Palussiere J, Vimeux FC, de Zwart JA, Quesson B, Gauchet M, Lelong P, Pergrale J, Grenier N, and Moonen CT, Local hyperthermia with MR-guided focused ultrasound: spiral trajectory of the focal point optimized for temperature uniformity in the target region. J Magn Reson Imaging. 2000;12:571–83. [DOI] [PubMed] [Google Scholar]
- [357].Kim YS, Advances in MR image-guided high-intensity focused ultrasound therapy. Int J Hyperthermia. 2015;31:225–32. [DOI] [PubMed] [Google Scholar]
- [358].Siedek F, Yeo SY, Heijman E, Grinstein O, Bratke G, Heneweer C, Puesken M, Persigehl T, Maintz D, and Grull H, Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MR-HIFU): Technical Background and Overview of Current Clinical Applications (Part 1). Rofo. 2019;191:522–530. [DOI] [PubMed] [Google Scholar]
- [359].Siedek F, Yeo SY, Heijman E, Grinstein O, Bratke G, Heneweer C, Puesken M, Persigehl T, Maintz D, and Grull H, Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MR-HIFU): Overview of Emerging Applications (Part 2). Rofo. 2019;191:531–539. [DOI] [PubMed] [Google Scholar]
- [360].Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, and Jolesz FA, MR-guided focused ultrasound surgery. J Comput Assist Tomogr. 1992;16:956–65. [DOI] [PubMed] [Google Scholar]
- [361].Sanghvi NT, Chen WH, Carlson R, Weis C, Seip R, Uchida T, and Marberger M, Clinical validation of real-time tissue change monitoring during prostate tissue ablation with high intensity focused ultrasound. J Ther Ultrasound. 2017;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Pichardo S, Gelet A, Curiel L, Chesnais S, and Chapelon JY, New integrated imaging high intensity focused ultrasound probe for transrectal prostate cancer treatment. Ultrasound Med Biol. 2008;34:1105–16. [DOI] [PubMed] [Google Scholar]
- [363].Shahmirzadi D, Hou GY, Chen J, and Konofagou EE, Ex Vivo characterization of canine liver tissue viscoelasticity after high-intensity focused ultrasound ablation. Ultrasound Med Biol. 2014;40:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Kok HP, Wust P, Stauffer PR, Bardati F, van Rhoon GC, and Crezee J, Current state of the art of regional hyperthermia treatment planning: a review. Radiat Oncol. 2015;10:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].De Greef M, Kok HP, Correia D, Bel A, and Crezee J, Optimization in hyperthermia treatment planning: the impact of tissue perfusion uncertainty. Med.Phys 2010;37:4540–4550. [DOI] [PubMed] [Google Scholar]
- [366].De Greef M, Kok HP, Correia D, Borsboom PP, Bel A, and Crezee J, Uncertainty in hyperthermia treatment planning: the need for robust system design. Phys.Med.Biol 2011;56:3233–3250. [DOI] [PubMed] [Google Scholar]
- [367].van de Kamer JB, Van Wieringen N, De Leeuw AAC, and Lagendijk JJW, The significance of accurate dielectric tissue data for hyperthermia treatment planning. Int.J.Hyperthermia 2001;17:123–142. [DOI] [PubMed] [Google Scholar]
- [368].Edd JF and Davalos RV, Mathematical modeling of irreversible electroporation for treatment planning. Technol Cancer Res Treat. 2007;6:275–86. [DOI] [PubMed] [Google Scholar]
- [369].Neal RE, Garcia PA, Robertson JL, and Davalos RV, Experimental Characterization and Numerical Modeling of Tissue Electrical Conductivity during Pulsed Electric Fields for Irreversible Electroporation Treatment Planning. Ieee Transactions on Biomedical Engineering. 2012;59:1076–1085. [DOI] [PubMed] [Google Scholar]
- [370].Porcheron J, Talabard JN, Breton C, Szafnicki K, Luxembourger O, Dufraisse G, D M, Clavreul G, Mosnier JF, Perpoint B, and Balique JG, Intraperitoneal chemohyperthermia for peritoneal carcinomatosis: Original modeling, clinical tolerance and results study about 30 patients. Hepato-Gastroenterology. 2000;47:1411–1418. [PubMed] [Google Scholar]
- [371].Ladhari T and Szafnicki K, Modelling of some aspects of a biomedical process: application to the treatment of digestive cancer (HIPEC). 3 Biotech. 2018;8:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Stigliano RV, Shubitidze F, Petryk AA, Tate JA, and Hoopes PJ, Magnetic nanoparticle hyperthermia: Predictive model for temperature distribution. Proc SPIE Int Soc Opt Eng. 2013;8584:858410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Berjano EJ, Theoretical modeling for radiofrequency ablation: state-of-the-art and challenges for the future. Biomed Eng Online. 2006;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Schumann C, Rieder C, Haase S, Teichert K, Suss P, Isfort P, Bruners P, and Preusser T, Interactive multi-criteria planning for radiofrequency ablation. Int J Comput Assist Radiol Surg. 2015;10:879–89. [DOI] [PubMed] [Google Scholar]
- [375].Reinhardt M, Brandmaier P, Seider D, Kolesnik M, Jenniskens S, Sequeiros RB, Eibisberger M, Voglreiter P, Flanagan R, Mariappan P, Busse H, Moche M, and Clinic ISG, A prospective development study of software-guided radio-frequency ablation of primary and secondary liver tumors: Clinical intervention modelling, planning and proof for ablation cancer treatment (ClinicIMPPACT). Contemp Clin Trials Commun. 2017;8:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Lopresto V, Pinto R, Farina L, and Cavagnaro M, Treatment planning in Microwave Thermal Ablation: clinical gaps and recent research advances. Int J Hyperthermia. 2016;1–39. [DOI] [PubMed] [Google Scholar]
- [377].Yero DD, Gonzalez FG, Van Troyen D, and Vandenbosch GAE, Dielectric Properties of Ex Vivo Porcine Liver Tissue Characterized at Frequencies Between 5 and 500 kHz When Heated at Different Rates. Ieee Transactions on Biomedical Engineering. 2018;65:2560–2568. [DOI] [PubMed] [Google Scholar]
- [378].Sebek J, Albin N, Bortel R, Natarajan B, and Prakash P, Sensitivity of microwave ablation models to tissue biophysical properties: A first step toward probabilistic modeling and treatment planning. Med Phys. 2016;43:2649. [DOI] [PubMed] [Google Scholar]
- [379].Chen D, Xia R, Chen X, Shafirstein G, Corry PM, Griffin RJ, Penagaricano JA, Tulunay-Ugur OE, and Moros EG, SonoKnife: feasibility of a line-focused ultrasound device for thermal ablation therapy. Med Phys. 2011;38:4372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Nikolov SI and Jensen JA, Application of different spatial sampling patterns for sparse array transducer design. Ultrasonics. 2000;37:667–71. [DOI] [PubMed] [Google Scholar]
- [381].de Bruijne M, Wielheesen DH, Van der Zee J, Chavannes N, and Van Rhoon GC, Benefits of superficial hyperthermia treatment planning: five case studies. Int.J.Hyperthermia 2007;23:417–429. [DOI] [PubMed] [Google Scholar]
- [382].Trujillo-Romero CJ, Paulides MM, Drizdal T, and van Rhoon GC, Impact of silicone and metal port-a-cath implants on superficial hyperthermia treatment quality. Int J Hyperthermia. 2015;31:15–22. [DOI] [PubMed] [Google Scholar]
- [383].Franckena M, Canters R, Termorshuizen F, Van der Zee J, and Van Rhoon GC, Clinical implementation of hyperthermia treatment planning guided steering: A cross over trial to assess its current contribution to treatment quality. Int.J.Hyperthermia 2010;26:145–157. [DOI] [PubMed] [Google Scholar]
- [384].Kok HP, Ciampa S, De Kroon-Oldenhof R, Steggerda-Carvalho EJ, Van Stam G, Zum Vörde Sive Vörding PJ, Stalpers LJA, Geijsen ED, Bardati F, Bel A, and Crezee J, Toward on-line adaptive hyperthermia treatment planning: correlation between measured and simulated specific absorption rate changes caused by phase steering in patients. Int.J.Radiat.Oncol.Biol.Phys 2014;90:438–445. [DOI] [PubMed] [Google Scholar]
- [385].Kok HP, Schooneveldt G, Bakker A, De Kroon-Oldenhof R, Korshuize - van Straten L, De Jong CE, Steggerda-Carvalho E, Geijsen ED, Stalpers LJA, and Crezee J, Predictive value of simulated SAR and temperature for changes in measured temperature after phase-amplitude steering during locoregional hyperthermia treatments. Int J Hyperthermia. 2018;35:330–339. [DOI] [PubMed] [Google Scholar]
- [386].Kok HP, Korshuize-van Straten L, Bakker A, de Kroon-Oldenhof R, Westerveld GH, Versteijne E, Stalpers LJA, and Crezee J, Feasibility of on-line temperature-based hyperthermia treatment planning to improve tumour temperatures during locoregional hyperthermia. Int J Hyperthermia. 2018;34:1082–1091. [DOI] [PubMed] [Google Scholar]
- [387].Rijnen Z, Bakker JF, Canters RA, Togni P, Verduijn GM, Levendag PC, Van Rhoon GC, and Paulides MM, Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int.J.Hyperthermia 2013;29:181–193. [DOI] [PubMed] [Google Scholar]
- [388].Kok HP, Crezee J, Franken NAP, Stalpers LJA, Barendsen GW, and Bel A, Quantifying the combined effect of radiation therapy and hyperthermia in terms of equivalent dose distributions. Int.J.Radiat.Oncol.Biol.Phys 2014;88:739–45. [DOI] [PubMed] [Google Scholar]
- [389].Crezee J, van Leeuwen CM, Oei AL, van Heerden LE, Bel A, Stalpers LJ, Ghadjar P, Franken NA, and Kok HP, Biological modelling of the radiation dose escalation effect of regional hyperthermia in cervical cancer. Radiat Oncol. 2016;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Kok HP, Van Dijk I, Crama KF, Franken NAP, Rasch CRN, Merks JHM, Crezee J, Balgobind BV, and Bel A, Reirradiation plus hyperthermia for recurrent pediatric sarcoma; a simulation study to investigate feasibility. Int J Oncol. 2019;54:209–218. [DOI] [PubMed] [Google Scholar]
- [391].Crezee H, van Leeuwen CM, Oei AL, Stalpers LJ, Bel A, Franken NA, and Kok HP, Thermoradiotherapy planning: Integration in routine clinical practice. Int J Hyperthermia. 2016;32:41–49. [DOI] [PubMed] [Google Scholar]
- [392].Beck M, Ghadjar P, Weihrauch M, Burock S, Budach V, Nadobny J, Sehouli J, and Wust P, Regional hyperthermia of the abdomen, a pilot study towards the treatment of peritoneal carcinomatosis. Radiat Oncol. 2015;10:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Schooneveldt G, Dobsicek Trefna H, Persson M, de Reijke TM, Blomgren K, Kok HP, and Crezee H, Hyperthermia Treatment Planning Including Convective Flow in Cerebrospinal Fluid for Brain Tumour Hyperthermia Treatment Using a Novel Dedicated Paediatric Brain Applicator. Cancers (Basel). 2019;11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Coluccia D, Fandino J, Schwyzer L, O’Gorman R, Remonda L, Anon J, Martin E, and Werner B, First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound. 2014;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Kok HP, Beck M, Loke DR, Helderman R, van Tienhoven G, Ghadjar P, Wust P, and Crezee H, Locoregional peritoneal hyperthermia to enhance the effectiveness of chemotherapy in patients with peritoneal carcinomatosis: a simulation study comparing different locoregional heating systems. Int J Hyperthermia. 2020;37:76–88. [DOI] [PubMed] [Google Scholar]
- [396].Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, Crary-Burney M, Ariaspulido H, Veliz FA, Steinmetz NF, and Fiering SN, Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation. Mol Pharm. 2018;15:3717–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Chao Y, Chen G, Liang C, Xu J, Dong Z, Han X, Wang C, and Liu Z, Iron Nanoparticles for Low-Power Local Magnetic Hyperthermia in Combination with Immune Checkpoint Blockade for Systemic Antitumor Therapy. Nano Lett. 2019;19:4287–4296. [DOI] [PubMed] [Google Scholar]
- [398].Oei AL, Korangath P, Mulka K, Helenius M, Coulter JB, Stewart J, Velarde E, Crezee J, Simons B, Stalpers LJA, Kok HP, Gabrielson K, Franken NAP, and Ivkov R, Enhancing the abscopal effect of radiation and immune checkpoint inhibitor therapies with magnetic nanoparticle hyperthermia in a model of metastatic breast cancer. Int J Hyperthermia. 2019;36:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Duval KEA, Vernice NA, Wagner RJ, Fiering SN, Petryk JD, Lowry GJ, Tau SS, Yin J, Houde GR, Chaudhry AS, and Hoopes PJ, Immunogenetic effects of low dose (CEM43 30) magnetic nanoparticle hyperthermia and radiation in melanoma cells. Int J Hyperthermia. 2019;36:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Ohno S, Tomoda M, Tomisaki S, Kitamura K, Mori M, Maehara Y, and Sugimachi K, Improved surgical results after combining preoperative hyperthermia with chemotherapy and radiotherapy for patients with carcinoma of the rectum. Dis Colon Rectum. 1997;40:401–6. [DOI] [PubMed] [Google Scholar]
- [401].Takahashi M, Fujimoto S, Kobayashi K, Mutou T, Kure M, Masaoka H, Shimanskaya RB, Takai M, Endoh F, and Ohkubo H, Clinical outcome of intraoperative pelvic hyperthermochemotherapy for patients with Dukes’ C rectal cancer. Int J Hyperthermia. 1994;10:749–54. [DOI] [PubMed] [Google Scholar]
- [402].Huilgol NG, Gupta S, and Dixit R, Chemoradiation with hyperthermia in the treatment of head and neck cancer. Int J Hyperthermia. 2010;26:21–5. [DOI] [PubMed] [Google Scholar]
- [403].Ohguri T, Imada H, Yahara K, Narisada H, Morioka T, Nakano K, and Korogi Y, Concurrent chemoradiotherapy with gemcitabine plus regional hyperthermia for locally advanced pancreatic carcinoma: initial experience. Radiat Med. 2008;26:587–96. [DOI] [PubMed] [Google Scholar]
- [404].Saeki H, Kawaguchi H, Kitamura K, Ohno S, and Sugimachi K, Recent advances in preoperative hyperthermochemoradiotherapy for patients with esophageal cancer. J Surg Oncol. 1998;69:224–9. [DOI] [PubMed] [Google Scholar]
- [405].Sugimachi K, Kuwano H, Ide H, Toge T, Saku M, and Oshiumi Y, Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma: a prospective randomized trial. Int.J.Hyperthermia 1994;10:485–493. [DOI] [PubMed] [Google Scholar]
- [406].Maluta S, Schaffer M, Pioli F, Dall’oglio S, Pasetto S, Schaffer PM, Weber B, and Giri MG, Regional hyperthermia combined with chemoradiotherapy in primary or recurrent locally advanced pancreatic cancer : an open-label comparative cohort trial. Strahlenther Onkol. 2011;187:619–25. [DOI] [PubMed] [Google Scholar]
- [407].Geijsen ED, De Reijke TM, Koning CCE, Zum Vörde Sive Vörding P, De la Rosette J, Rasch CRN, Van Os RM, and Crezee J, Combining Mitomycin C and Regional 70 MHz Hyperthermia in Patients with Nonmuscle Invasive Bladder Cancer: A Pilot Study. J Urol. 2015;194:1202–1208. [DOI] [PubMed] [Google Scholar]
- [408].Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, and Vujaskovic Z, A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2014;30:171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [409].Westermann A, Mella O, van der ZJ, Jones EL, Van DS-B, Koper P, Uitterhoeve AL, de WR, Van d V, Burger C, Schem BC, Van Der WC, Dahl O, Prosnitz LR, and Van TH, Long-term survival data of triple modality treatment of stage IIB-III-IVA cervical cancer with the combination of radiotherapy, chemotherapy and hyperthermia - an update. Int.J.Hyperthermia 2012;28:549–553. [DOI] [PubMed] [Google Scholar]
- [410].Hulshof MC, Van Haaren PM, van Lanschot JJ, Richel DJ, Fockens P, Oldenborg S, Geijsen ED, Van Berge Henegouwen MI, and Crezee J, Preoperative chemoradiation combined with regional hyperthermia for patients with resectable esophageal cancer. Int.J.Hyperthermia 2009;25:79–85. [DOI] [PubMed] [Google Scholar]
- [411].Cho C, Wust P, Hildebrandt B, Issels RD, Sehouli J, Kerner T, Deja M, Budach V, and Gellermann J, Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: evaluation of two annular-phased-array applicators. Int J Hyperthermia. 2008;24:399–408. [DOI] [PubMed] [Google Scholar]
- [412].Petrovich Z, Langholz B, Kapp DS, Emami B, Oleson JR, Luxton G, and Astrahan M, Deep regional hyperthermia of the liver. A clinical study of 49 patients. Am J Clin Oncol. 1989;12:378–83. [DOI] [PubMed] [Google Scholar]
- [413].Engin K, Tupchong L, Waterman FM, Nerlinger RT, Hoh LL, McFarlane JD, and Leeper DB, Thermoradiotherapy with combined interstitial and external hyperthermia in advanced tumours in the head and neck with depth > or = 3 cm. Int J Hyperthermia. 1993;9:645–54. [DOI] [PubMed] [Google Scholar]
- [414].Johannsen M, Thiesen B, Wust P, and Jordan A, Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia. 2010;26:790–5. [DOI] [PubMed] [Google Scholar]
- [415].Ren RL, Chou CK, Vora N, Luk K, Vora L, Ma L, Ahn C, Staud CL, Li B, McDougall JA, Chan KW, Xiong XB, and Li DJ, A pilot study of intracavitary hyperthermia combined with radiation in the treatment of oesophageal carcinoma. Int J Hyperthermia. 1998;14:245–54. [DOI] [PubMed] [Google Scholar]
- [416].You QS, Wang RZ, Suen GQ, Yan FC, Gao YJ, Cui SR, Zhao JH, Zhao TZ, and Ding L, Combination preoperative radiation and endocavitary hyperthermia for rectal cancer: long-term results of 44 patients. Int J Hyperthermia. 1993;9:19–24. [DOI] [PubMed] [Google Scholar]
- [417].Abe M, Hiraoka M, Takahashi M, Egawa S, Matsuda C, Onoyama Y, Morita K, Kakehi M, and Sugahara T, Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer. 1986;58:1589–95. [DOI] [PubMed] [Google Scholar]
- [418].Matsuda T, Tanaka Y, Takeshita N, Ishiwata J, and Awane Y, [Clinical significance of the combined use of radiation therapy and hyperthermia]. Gan To Kagaku Ryoho. 1987;14:1508–14. [PubMed] [Google Scholar]
- [419].Arcangeli G, Benassi M, Cividalli A, Lovisolo GA, and Mauro F, Radiotherapy and hyperthermia. Analysis of clinical results and identification of prognostic variables. Cancer. 1987;60:950–6. [DOI] [PubMed] [Google Scholar]
- [420].Valdagni R and Amichetti M, Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28:163–9. [DOI] [PubMed] [Google Scholar]
- [421].Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, and Sawada S, A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia. 2001;17:97–105. [DOI] [PubMed] [Google Scholar]
- [422].Yamada S, Takai Y, Nemoto K, Ogawa Y, Kakuto Y, Hoshi A, Sakamoto K, Kimura Y, and Kobari M, Intraoperative radiation therapy combined with hyperthermia against pancreatic carcinoma. Tohoku J Exp Med. 1992;166:395–401. [DOI] [PubMed] [Google Scholar]
- [423].Yamaguchi S, Ohguri T, Imada H, Yahara K, Moon SD, Higure A, Yamaguchi K, Yoshikawa I, Harada M, and Korogi Y, Multimodal approaches including three-dimensional conformal re-irradiation for recurrent or persistent esophageal cancer: preliminary results. J Radiat Res. 2011;52:812–20. [DOI] [PubMed] [Google Scholar]
- [424].Maluta S, Dall’oglio S, Romano M, Marciai N, Pioli F, Giri MG, Benecchi PL, Comunale L, and Porcaro AB, Conformal radiotherapy plus local hyperthermia in patients affected by locally advanced high risk prostate cancer: preliminary results of a prospective phase II study. Int.J.Hyperthermia 2007;23:451–456. [DOI] [PubMed] [Google Scholar]
- [425].Tilly W, Gellermann J, Graf R, Hildebrandt B, Weissbach L, Budach V, Felix R, and Wust P, Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther.Onkol 2005;181:35–41. [DOI] [PubMed] [Google Scholar]
- [426].Schlottmann F and Patti MG, Current Concepts in Treatment of Barrett’s Esophagus With and Without Dysplasia. Journal of Gastrointestinal Surgery. 2017;21:1354–1360. [DOI] [PubMed] [Google Scholar]
- [427].Merten R, Ott O, Haderlein M, Bertz S, Hartmann A, Wullich B, Keck B, Kuhn R, Rodel CM, Weiss C, Gall C, Uter W, and Fietkau R, Long-Term Experience of Chemoradiotherapy Combined with Deep Regional Hyperthermia for Organ Preservation in High-Risk Bladder Cancer (Ta, Tis, T1, T2). Oncologist. 2019;24:e1341–e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [428].Shin BJ, Chick JF, and Stavropoulos SW, Contemporary Status of Percutaneous Ablation for the Small Renal Mass. Curr Urol Rep. 2016;17:23. [DOI] [PubMed] [Google Scholar]


