Abstract
Purpose:
The aim of this investigation is to study patient reported outcomes of patients with microbial keratitis (MK) using the 9-item National Eye Institute Visual Function Questionnaire (NEI VFQ-9).
Methods:
Using the Sight Outcomes Research Collaborative (SOURCE) ophthalmology electronic health record repository, patients with MK and control patients who completed the NEI VFQ-9 within 7 days of their appointment were identified. The questionnaire is scored as a mean of the 9 items on a scale from 0 to 100, with higher scores indicating better functioning. Composite and individual item scores were compared between groups using analysis of variance (ANOVA).
Results:
916 questionnaires were completed from patients with acute MK (n=83), non-acute MK (n=30), MK with a corneal transplant (n=22), from controls seen in a satellite comprehensive ophthalmology clinic (n=528), and controls seen at a sub-specialty ophthalmology clinic (n=253). The mean NEI VFQ-9 composite score per group was 66.2 (standard deviation, SD = 26.8), 78.1 (SD = 17.1), 60.3 (SD=22.4), 88.0 (SD = 10.2), and 83.5 (SD = 13.0), respectively (p<0.0001). Both acute MK patients and MK patients requiring transplant reported significantly worse function than non-acute MK, comprehensive, and specialty patients. Non-acute MK patients reported significantly worse function than comprehensive control patients (all Tukey-adjusted p<0.05).
Discussion:
Patients who had or eventually require corneal transplant for management of their MK report worse visual function than patients with non-acute MK. This may be important in helping physicians counsel their patients.
Keywords: Microbial keratitis, Patient Reported Outcomes, Corneal Transplant
Microbial keratitis (MK) is a corneal infectious disease with varied pathologies, presentations, and severities.1 The prevalence of MK and the inciting pathogen are influenced by a combination of climate, hygiene, ocular, and systemic risk factors.1–6 In 2010, MK resulted in 930,000 clinic visits and 58,000 emergency department visits in the United States (US).7 In 2012, an estimated one out of five patients who had been hospitalized in the US for MK required a corneal transplant8 and a 2018 estimate of the global disease burden of MK exceeded two million cases annually.9
Patient reported outcomes (PROs) are “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”10 PROs can be obtained electronically or from a paper survey.10,11 Of course, objective clinical measures (e.g., visual acuity, infiltrate size) are critical for health assessment, but PROs are increasingly being used as a measure of symptoms and function in clinical trials, such as monitoring surgical and medical retina disease outcomes.12,13
The National Eye Institute-Visual Function Questionnaire (NEI VFQ-25) is a tool designed to measure patients’ perception of visual function and quality of life.14,15 The 25-item NEI VFQ has been used in a randomized control trial of fungal MK to investigate outcomes after different antifungal treatments.16 In a prospective study, the Chinese version was used to look at vision-related quality of life in infectious keratitis and its correlation to visual acuity and duration of disease.17
Kodjebacheva et al. published a validated shortened version of this survey, the NEI VFQ-9.18 This 9-item version covers the following functional domains: general vision, near vision, distance vision, driving, peripheral vision, role limitation, and mental health.18 The purpose of this investigation was to evaluate PROs from the NEI VFQ-9 in a cohort of patients with MK compared to a control sample.
Methods
Starting in September 2017, all ophthalmology patients seen at the University of Michigan, Kellogg Eye Center were assigned the NEI VFQ-9 at least annually. The 9 items ask about visual function on a Likert scale, where each response is scored from 0 (worse visual function) to 100 (better visual function). The overall composite score is calculated as the mean of the item scores. Questionnaires are self-administered and completed electronically before an appointment through the patient portal or during the appointment using a tablet.
Using ICD-9 and ICD-10 codes (Appendix 1), patient encounters with an associated diagnosis of MK were identified in the Sight Outcomes Research Collaborative (SOURCE) ophthalmology electronic health record (EHR) repository from September 2017 to May 2019. The encounters were included if an NEI VFQ-9 had been completed within 7 days of that appointment. For patients with >1 encounter with an NEI VFQ-9 and a diagnosis of MK, the first encounter was selected. MK patients were categorized as follows: (1) acute MK infection (NEI VFQ-9 was completed within 30 days of initial MK diagnosis), (2) non-acute MK infection (NEI VFQ-9 was completed >30 days from initial MK diagnosis), and (3) MK patient who also underwent a corneal transplant (penetrating keratoplasty, PKP, or deep anterior lamellar keratoplasty, DALK; Appendix 1). A control group of patients who completed the NEI VFQ-9 within 7 days of an appointment were identified from a satellite comprehensive ophthalmology clinic. For control patients with >1 visit with an NEI VFQ-9, the first visit was selected. Control patients were subdivided based on whether they saw a comprehensive ophthalmologist or a specialist (cornea or glaucoma). Any patient in the control sample with a history of MK was excluded to maintain independent groups.
Statistical Methods:
Patient demographics and NEI VFQ-9 scores were summarized with descriptive statistics (mean, standard deviation [SD], frequency, percentage) separately for the MK and control patient cohorts. Stacked bar charts and boxplots were used to display the distribution of NEI VFQ-9 responses at the item level and the overall composite score, respectively. Group differences were tested with Chi-square tests, Fisher’s exact tests, Kruskal-Wallis tests, and analysis of variance (ANOVA). Significant Kruskal-Wallis or ANOVA results were followed by post-hoc pairwise comparisons with Tukey adjustment. Univariate and multivariable linear regression models were used to assess factors associated with NEI VFQ-9 composite scores. Variables investigated included age, sex, race, and insurance type. SAS version 9.4 was used for all statistical analysis (SAS institute; Cary, NC).
Results
A total of 12,250 ophthalmology patients completed at least 1 NEI VFQ-9 over the 21-month period, of which 99% (12,165 of 12,220) had completed one survey within 7 days of an appointment (Figure 1). Patients include those with acute MK (n=84), non-acute MK (n=30), MK with cornea transplant (n=21), comprehensive patients (n=528), and comprehensive patients who saw a specialist (n=253). Patients with an NEI VFQ-9 but not selected for inclusion in this study (n=11,249) were subjects with a diagnosis other than MK and who were seen in a specialty ophthalmology clinic. Because questionnaires were not necessarily filled out at initial MK diagnosis, rather simply a visit with an MK diagnosis, the timing of the questionnaire could be before or after a transplant (range: 0.6 years [223 days] before to 4.3 years after a graft, in the 21 MK patient with transplant). No significant differences between the study groups were found with respect to gender (p=0.48) or ethnicity (p=0.51; Table 1). However, patients seen in the comprehensive clinic by a specialist were significantly older (mean=63.3, SD=15.5) than all other patient groups (acute MK: mean=55.0, SD=18.6; non-acute MK: mean=53.4, SD=15.6; MK with cornea transplant: mean=51.6, SD=15.8; comprehensive patients: mean=52.9, SD=18.9; p<0.0001). A smaller percentage of patients with MK were Black or other minority races (11.0% acute MK, 10.0% non-acute MK, 14.3% MK with corneal transplant) compared to control patients (25.6% comprehensive, 18.9% specialist; p=0.0055). MK patients who had corneal transplant showed a smaller percentage having commercial insurance compared to non-acute MK patients, comprehensive control patients, and specialty satellite clinic patients (52.4% versus 86.7%, 76.8%, and 69.4%, respectively; p=0.0015).
Figure 1.
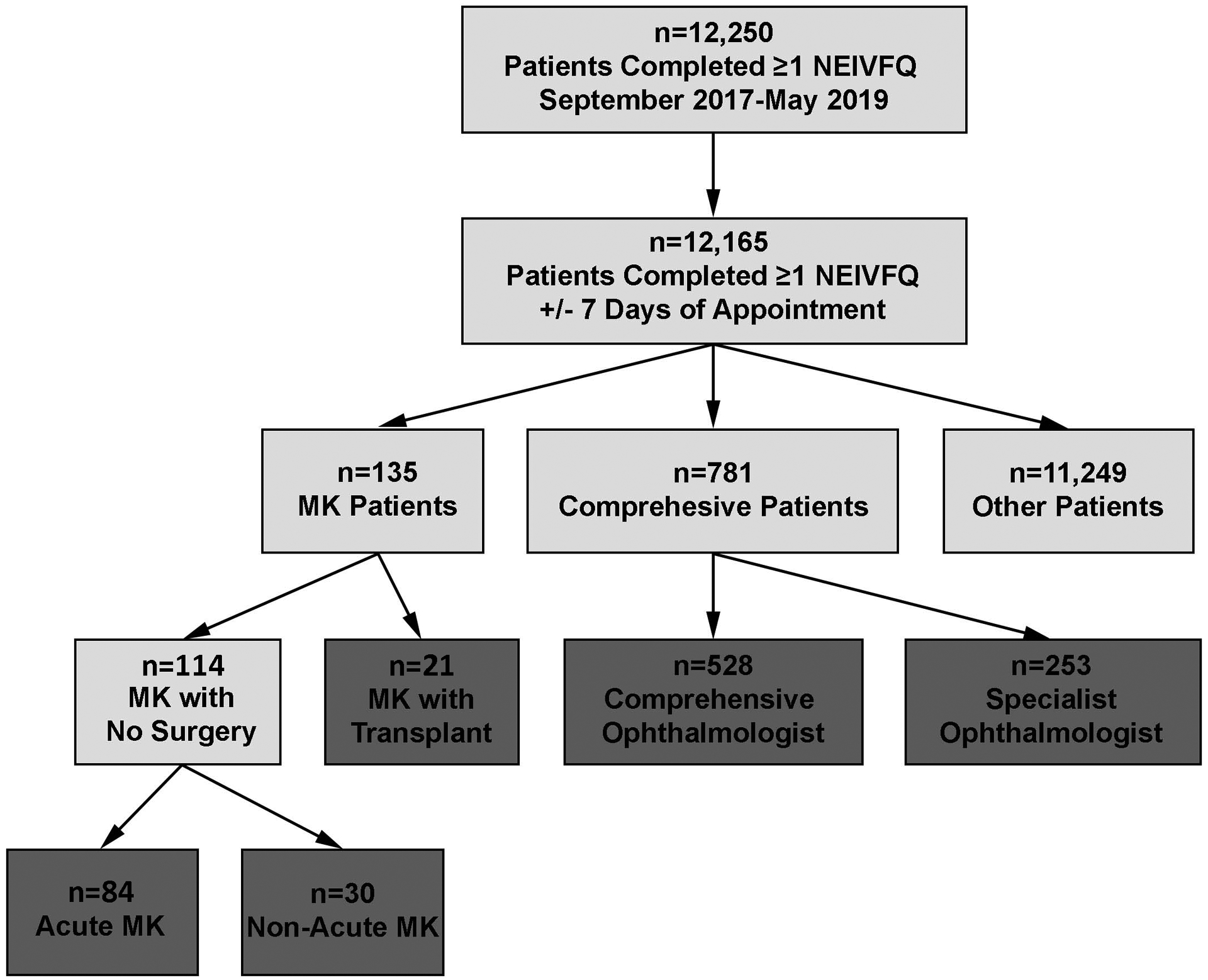
Flow chart for selection of patient cohorts
MK, Microbial Keratitis; NEI VFQ, National Eye Institute Visual Function Questionnaire
Table 1.
Descriptive statistics of patient demographics stratified by patient cohorts
| Acute MK (n=84) | Non-Acute MK (n=30) | MK with Transplant (n=21) | Comprehensive/No Specialist (n=528) | Comprehensive/Specialist (n=253) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Categorical Variable | # | % | # | % | # | % | # | % | # | % | P-value* |
| Gender | |||||||||||
| Female | 53 | 63.1 | 20 | 66.7 | 10 | 47.6 | 308 | 58.3 | 140 | 55.3 | 0.4765 |
| Male | 31 | 36.9 | 10 | 33.3 | 11 | 52.4 | 220 | 41.7 | 113 | 44.7 | |
| Race | |||||||||||
| White | 73 | 89.0 | 27 | 90.0 | 18 | 85.7 | 384 | 74.4 | 198 | 81.2 | 0.0055 |
| Black or Other | 9 | 11.0 | 3 | 10.0 | 3 | 14.3 | 132 | 25.6 | 46 | 18.8 | |
| Ethnicity | |||||||||||
| Hispanic | 2 | 2.4 | 2 | 7.1 | 0 | 0.0 | 14 | 2.8 | 5 | 2.1 | 0.5142 |
| Non-Hispanic | 82 | 97.6 | 26 | 92.9 | 20 | 100.0 | 482 | 97.2 | 237 | 97.9 | |
| Insurance | |||||||||||
| CAID | 3 | 3.9 | 0 | 0.0 | 1 | 4.8 | 3 | 0.6 | 0 | 0.0 | 0.0015 |
| CARE | 17 | 22.1 | 4 | 13.3 | 9 | 42.9 | 116 | 22.6 | 77 | 30.6 | |
| COMM | 57 | 74.0 | 26 | 86.7 | 11 | 52.4 | 394 | 76.8 | 175 | 69.4 | |
| Continuous Variable | Mean (SD) | Min, Max | Mean (SD) | Min, Max | Mean (SD) | Min, Max | Mean (SD) | Min, Max | Mean (SD) | Min, Max | p-value** |
| Age (years) | 55.0 (18.6) | 18.3, 93.7 | 53.4 (15.6) | 22.7, 88.9 | 51.6 (15.8) | 22.2, 78.5 | 52.9 (18.9) | 18.1, 90.8 | 63.3 (15.5) | 18.4, 95.8 | <0.0001*** |
MK, Microbial Keratitis; CAID, Medicaid; CARE, Medicare; COMM, Commercial; SD, Standard Deviation; Min, Minimum; Max, Maximum;
Chi-square test or Fisher’s Exact test (when cell counts <5),
ANOVA;
post-hoc pairwise comparisons with Tukey adjustment show average age of patients seeing a specialist at the comprehensive clinic are significantly older than all other groups (all p<0.05)
The distribution of NEI VFQ-9 item and composite scores are displayed in Figures 2 and 3. A smaller percentage of MK patients who also had corneal transplants endorsed better visual function responses on all 9 items of the NEI VFQ-9 compared to all other MK patient groups and controls. For example, when asked how much difficulty they had noticing objects off to the side while walking along, only 4.8% of MK patients who had corneal transplants endorsed “no difficulty at all”. In comparison, 45.1% of acute MK patients, 72.4% of non-acute MK patients, 86.8% of general comprehensive patients, and 75.3% of specialty satellite clinic patients endorsed “no difficulty at all.” Alternatively, a larger percentage of the general comprehensive patients reported better visual functioning responses on all 9 items of the NEI VFQ, compared to the other patient cohorts. In addition, a larger percentage of non-acute MK patients reported better visual functioning responses on 7 of 9 items of the NEI VFQ-9 than acute MK patients.
Figure 2.
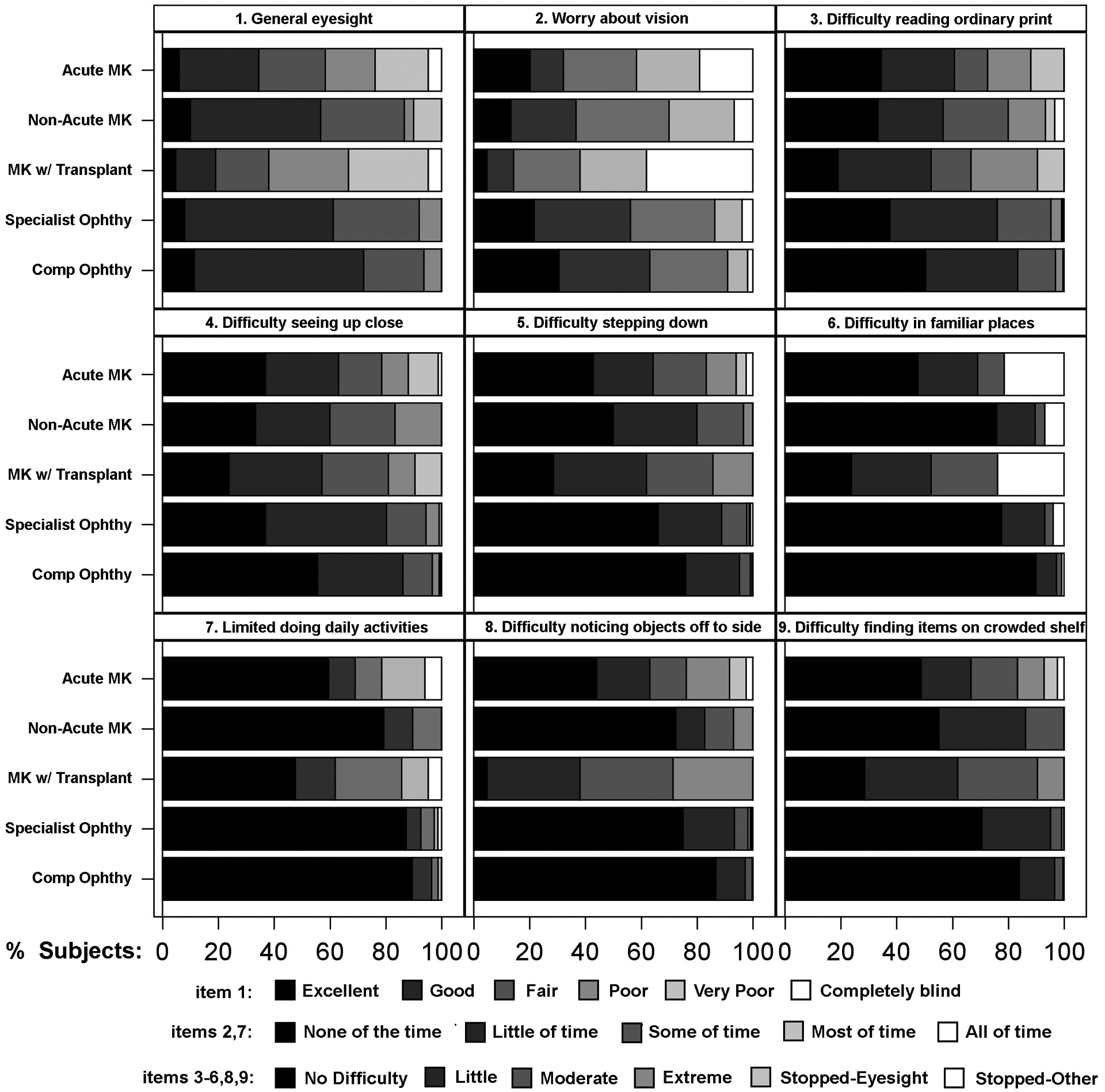
Stacked bar charts showing the distribution of responses to each item of the 9-item National Eye Institute Visual Function Questionnaire (NEI VFQ-9), stratified by patient cohort
Figure 3.
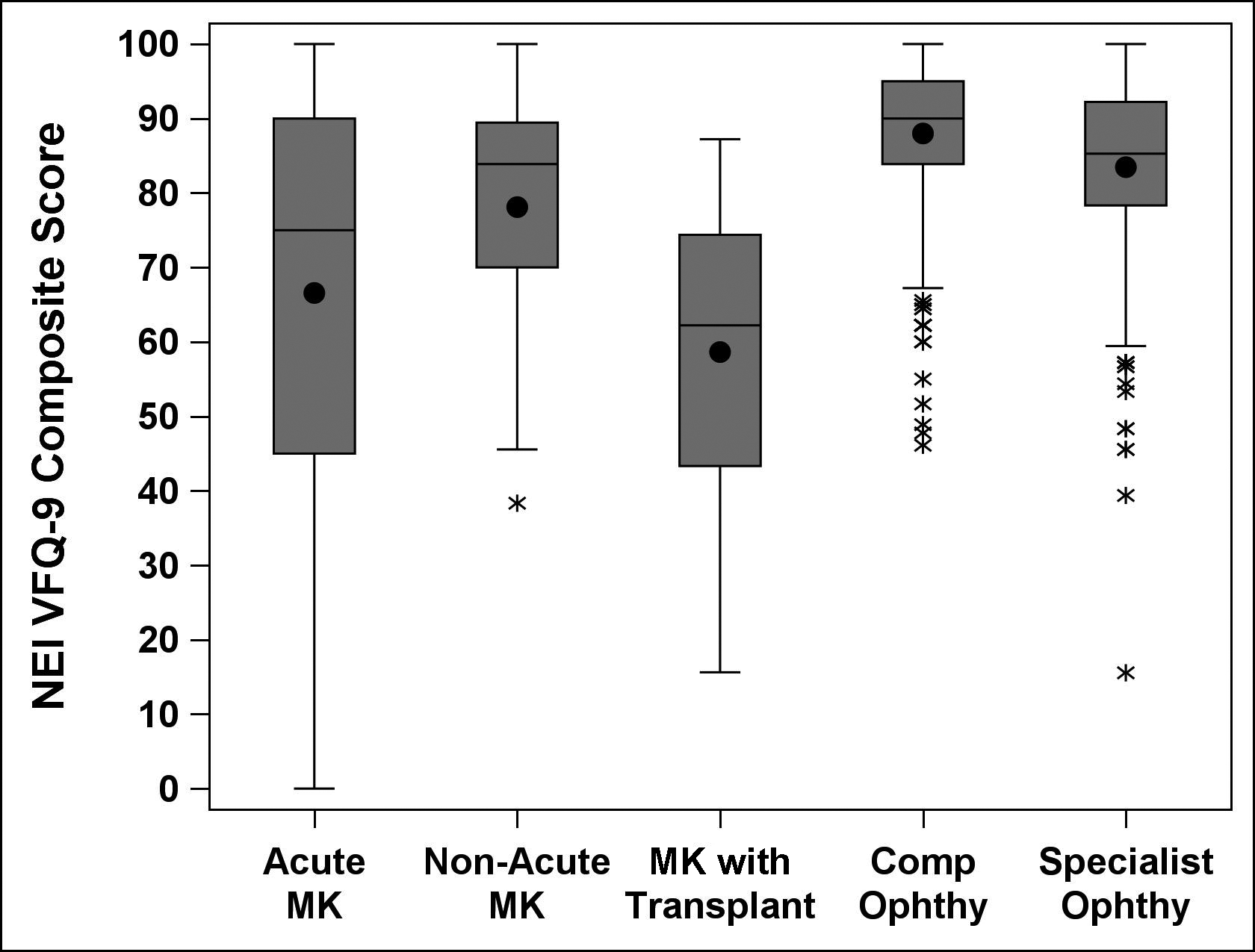
Side-by-side boxplots displaying the distribution of NE IVFQ-9 composite scores by patient cohorts. Boxplots display means (black dot inside box), medians (horizontal line within box), interquartile range (IQR, bottom of box to top of box), outliers (asterisks, observations outside 1.5 times the IQR). NEI VFQ, National Eye Institute Visual Function Questionnaire; MK, Microbial Keratitis; Comp; Comprehensive; Ophthy, Ophthalmology
Table 2 presents summary scores for the NEI VFQ-9 by patient cohort. The mean NEI VFQ-9 composite score was 66.6 (SD=26.8) for acute MK patients, 78.1 (SD=17.1) for non-acute MK patients, 58.6 (SD=21.6) for MK patients with corneal transplant, 88.0 (SD=10.2) for general comprehensive patients, and 83.5 (SD=13.0) for specialty satellite clinic patients (ANOVA p<0.0001). Post-hoc pairwise comparisons showed acute MK patients reported significantly worse visual function than non-acute MK patients, comprehensive patients and specialty clinic patients (all Tukey-adjusted p<0.05) but reported similar visual function to MK patients who also had corneal transplants. MK patients requiring corneal transplant reported significantly worse visual function than non-acute MK patients, and comprehensive and specialty clinic patients (all Tukey-adjusted p<0.05). Non-acute MK patients reported significantly worse visual function than both types of control patients (all Tukey-adjusted p<0.05). Individual items of the NEI VFQ-9 showed significantly worse visual function in acute MK patients compared to both control samples (comprehensive ophthalmology and specialist ophthalmology patients; all Tukey-adjusted p<0.05).
Table 2.
Descriptive statistics of 9-item National Eye Institute Visual Function Questionnaire responses and composite score, stratified by patient cohorts
| Acute MK (n=84) | Non-Acute MK (n=30) | MK with Transplant (n=21) | Comprehensive Ophthy (n=528) | Specialist Ophthy (n=253) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NEIVFQ | Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | P-value* |
| General eyesight | 54.0 (27.0) | 60.0 | 68.7 (21.5) | 80.0 | 44.8 (26.0) | 40.0 | 75.4 (14.6) | 80.0 | 72.2 (15.0) | 80.0 | <0.0001a,c,d,e,h,i |
| Worry about vision | 47.9 (34.8) | 50.0 | 53.3 (28.4) | 50.0 | 29.8 (30.2) | 25.0 | 70.7 (25.1) | 75.0 | 65.1 (26.3) | 75.0 | <0.0001b,c,d,e,f,h,i |
| Difficulty reading ordinary print | 64.0 (35.2) | 75.0 | 68.1 (29.8) | 75.0 | 57.1 (32.7) | 75.0 | 82.8 (20.5) | 100.0 | 77.4 (21.8) | 75.0 | <0.0001c,d,f,h,i,j |
| Difficulty seeing up close | 67.5 (33.8) | 75.0 | 69.2 (27.6) | 75.0 | 63.1 (31.2) | 75.0 | 84.8 (20.2) | 100.0 | 77.7 (21.9) | 75.0 | <0.0001c,d,f,h,i,j |
| Difficulty stepping down | 72.9 (29.7) | 75.0 | 81.7 (21.7) | 87.5 | 69.0 (26.1) | 75.0 | 92.5 (14.8) | 100.0 | 88.6 (18.3) | 100.0 | <0.0001c,d,f,h,i |
| Difficulty in familiar places | 87.1 (17.7) | 100.0 | 94.4 (12.7) | 100.0 | 75.0 (20.4) | 75.0 | 97.2 (9.3) | 100.0 | 94.4 (12.2) | 100.0 | <0.0001a,b,c,d,e,h,i,j |
| Limited doing daily activities | 75.3 (34.2) | 100.0 | 92.2 (16.5) | 100.0 | 72.6 (31.5) | 75.0 | 96.2 (12.5) | 100.0 | 94.0 (18.0) | 100.0 | <0.0001a,c,d,e,h,i |
| Difficulty noticing objects off to side | 70.4 (33.1) | 75.0 | 87.1 (23.7) | 100.0 | 53.6 (22.8) | 50.0 | 96.0 (11.2) | 100.0 | 91.9 (16.3) | 100.0 | <0.0001a,b,c,d,e,f,h,i,j |
| Difficulty finding items on crowded shelf | 74.7 (30.8) | 87.5 | 85.3 (18.3) | 100.0 | 70.2 (24.5) | 75.0 | 95.2 (11.9) | 100.0 | 91.2 (15.0) | 100.0 | <0.0001a,c,d,e,f,h,i,j |
| Composite | 66.6 (26.8) | 75.0 | 78.1 (17.1) | 83.9 | 58.6 (21.6) | 62.2 | 88.0 (10.2) | 90.0 | 83.5 (13.0) | 85.3 | <0.0001a,c,d,e,f,h,i,j |
MK, Microbial Keratitis; SD, Standard Deviation; Ophthy, Ophthalmology
ANOVA (Kruskal-Wallis tests also showed all p<0.0001); Post-hoc pairwise comparisons show significant differences after Tukey adjustment for:
Acute MK vs. non-acute MK,
Acute MK vs. MK with corneal transplant,
Acute MK vs. Comprehensive/No Specialist,
Acute MK vs. Comprehensive/Specialist,
Non-acute MK vs. MK with corneal transplant,
Non-acute MK vs. Comprehensive/No Specialist,
Non-acute MK vs. Comprehensive/Specialist,
MK with corneal transplant vs. Comprehensive/No Specialist,
MK with corneal transplant vs. Comprehensive/Specialist,
Comprehensive/No Specialist vs. Comprehensive/Specialist
Univariable and multivariable linear regression models of the composite NEI VFQ-9 score are presented in Table 3. Univariate models found older age (p<0.0001), Medicare insurance (versus commercial insurance, p<0.0001), and female sex (p=0.02) were all associated with reporting worse visual function. Patients of Other race reported significantly better visual function (p=0.02) compare to those who were White. Patients with acute MK, non-acute MK, MK with corneal transplant, and those who saw a specialist in the satellite clinic, all reported significantly worse visual functioning compared to comprehensive ophthalmology patients, by 21.4, 9.9, 29.3, and 4.5 points, respectively (all p<0.05). After adjusting for age, insurance type, and sex, similar findings in reported visual function were observed between patient groups (multivariable model, Table 3; all p<0.05). Specifically, patients with acute MK, non-acute MK, MK with corneal transplant, and those who saw a specialist in the satellite clinic, all reported significantly worse visual functioning compared to comprehensive ophthalmology patients, by 21.6, 9.8, 28.6, and 2.8 points, respectively (all p<0.05, after adjustment for age, sex, and insurance type).
Table 3.
Univariate and Multivariable linear regression models for 9-item National Eye Institute Visual Function Questionnaire composite score
| Univariate Models | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Variable | Estimate | 95% CI | P-value | Estimate | 95% CI | P-value |
| Age (per 10 years) | −1.8 | −2.4, −1.3 | <0.0001 | −1.5 | −2.1, −0.1 | <0.0001 |
| Race (vs White) | ||||||
| Black | 0.1 | −3.7, 4.0 | 0.9461 | Non-significant after adjustment for type of insurance | ||
| Other | 3.8 | 0.5, 7.0 | 0.0230 | |||
| Insurance (vs Commercial) | ||||||
| Medicare | −6.5 | −9.0, −4.0 | <0.0001 | −2.9 | −5.4, −0.5 | 0.0188 |
| Sex (vs Male) | ||||||
| Female | −2.5 | −4.7, −0.3 | 0.0236 | −2.9 | −4.8, −1.0 | 0.0024 |
| Group (vs Comprehensive Ophthy) | ||||||
| Active MK | −21.4 | −24.7, −18.1 | <0.0001 | −21.6 | −24.9, −18.3 | <0.0001 |
| Inactive MK | −9.9 | −15.2, −4.6 | 0.0003 | −9.8 | −14.8, −4.7 | 0.0002 |
| MK with Transplant | −29.3 | −35.5, −23.2 | <0.0001 | −28.6 | −34.7, −22.6 | <0.0001 |
| Specialty Ophthy | −4.5 | −6.7, −2.3 | <0.0001 | −2.8 | −5.0, −0.6 | 0.0134 |
CI, Confidence Interval; MK, Microbial Keratitis; Ophthy, Ophthalmology
Discussion:
MK is an acute vision-threatening infection of the cornea. As such, the impact on patients’ function and quality of life must be considered. Published literature regarding PROs in patients who experience MK is limited. This study demonstrates the self-reported visual function loss associated with MK for patients followed at an academic center.
Patients in this cohort with acute MK reported significantly worse visual function as compared to 3 of the remaining 4 patient groups studied. This included control groups of patients with and without specialist care, and patients with non-acute MK—which likely includes both chronic and inactive infections. Previous studies have found a relationship between disease duration of MK and NEI VFQ scores.17 This study looked at the interval between diagnosis of MK and NEI VFQ score rather than disease duration. Patients with acute MK had significantly worse NEI-VFQ scores, perhaps because they had experienced a recent decrease in visual function and higher severity of symptoms as compared to those with non-acute MK in which patients may have recovered visual function or have had time to adapt their lives to their new level of visual function. This decrease in PRO score is not necessarily reflective of the expected disease duration rather that these patients are at the early stage of their disease course.
Another important finding is that patients with a history of both corneal transplant and MK have significantly lower self-reported visual function than patients with non-acute MK. This is consistent with a prior study that found that generally, corneal transplant recipients had decreased vision-related quality of life.19 Our study likely captures both the impaired vision-related quality of life caused by both MK and corneal transplant. This is likely confounded by the fact that the corneal transplant patients likely had more severe disease at baseline (necessitating the corneal transplant) or may have ongoing issues with their transplant; however, it highlights nonetheless that patients with histories of MK and a corneal transplant have significantly worse quality of life.
These results show that MK has significant effects on vision-related quality of life across the course of disease. Both acute and non-acute MK patients showed significantly reduced NEI VFQ-9 composite scores compared to general comprehensive patients. While a prior study also examined NEI VFQ-25 scores in MK patients, these patients were enrolled in a clinical trial in which the NEI VFQ was captured at prespecified time points.16 Our study captures NEI VFQ-9 responses at a spectrum of time points in the clinical course showing that patients have a diminished quality of life across many stages of MK. This reduced quality of life is likely in large part due to the pain and vision loss related to the MK. There is also a significant treatment burden associated with MK due to the high quantity of drops prescribed and cost of treatment.20 MK patients may also have psychological burden from their disease. A lower PRO score has been found to be associated with worse psychological attitudes of patients towards their own health,21 as well as depression independent of vision, mental status, and general health.22
MK has similar effects on visual function and perceived quality of life as chronic ophthalmologic conditions. Our study found that patients report an average NEI VFQ-9 composite score of 66 for acute MK, and 78 for non-acute MK. Patients with dry eye reported an average NEI VFQ-25 scores of 88, displaying better functioning than those with MK.23 Other ophthalmic diseases have similar impairment in visual function to MK such as diabetic macular edema (composite score of 78),24 chronic cataract (73),25 and retinitis pigmentosa (63).26 Comparing NEI VFQ-25 scores to NEI VFQ-9 scores is not perfect but reasonable due to the same scale and mean scoring, but the comparison should be interpreted with caution.
This study has limitations. First, the NEI VFQ-9 was given automatically through the electronic health record without targeting a specific population. MK severity undoubtedly has an effect on patient’s visual function. The lack of a published definition of MK severity meant we had to use surgical indications and time since diagnosis as a method to stratify patients on their disease severity. Lastly, the non-acute MK group in this study includes a wide variety of MK patients, ranging from inactive disease to chronic MK. The heterogeneity of this group with respect to disease severity and stages of healing limits the conclusions we can make about this group specifically, but still allows for valid comparisons between groups.
MK has a direct impact on patients’ perceived quality of life. Although MK is an acute eye condition, it can have short-term and long-term effects on patients’ quality of life, visual function, and independence. Capturing quality of life metrics allows clinicians to individualize care by understanding a patient’s health from their perspective. This could help providers tailor management and treatments to respond to patient’s reported functional limitations.
Acknowledgments:
Personnel effort (LMN) for this research at the University of Michigan was supported by Ms. Susan Lane.
Financial Support: This work was supported by the National Institutes of Health R01EY031033 (MAW) (Rockville, MD). The funding organization had no role in study design or conduct, data collection, management, analysis, interpretation of the data, decision to publish, or preparation of the manuscript. MAW had full access to the data and takes responsibility for the integrity and accuracy of the data analysis.
Acronyms:
- MK
Microbial Keratitis
- EHR
Electronic Health Record
- PROs
Patient Reported Outcomes
- NEI VFQ
National Eye Institute Visual Function Questionnaire
- PKP
Penetrating Keratoplasty
- DALK
Deep Anterior Lamellar Keratoplasty
Appendix 1
Diagnosis codes used to identify microbial keratitis in the electronic health record:
ICD-9: 370.00, 370.03, 370.03, 370.04, 370.04, 370.04, 370.04, 370.05, 370.05, 370.05
ICD-10: H16.009, H16.009, H16.002, H16.001, H16.002, H16.001, 370.00, H16.003, H16.001, H16.002, H16.009, H16.009, H16.009, 370.03, H16.012, H16.031, H16.032, H16.039, H16.039, H16.069, H16.061, H16.069, H16.012, H16.032
Current procedural terminology (CPT) codes used to identify penetrating keratoplasty (PKP) or deep anterior lamellar keratoplasty (DALK) in the electronic health record:
PKP: 65730, 65750, 65755
DALK: 65710
Footnotes
Conflict of Interest: The authors have no proprietary or commercial interest in any of the materials discussed in this article.
Meeting Presentation: This material has been accepted for presentation at the World Cornea Congress in Boston, Massachusetts in May 2020.
References
- 1.Musch DC, Sugar A, Meyer RF. Demographic and Predisposing Factors in Corneal Ulceration. Arch Ophthalmol. 1983;101(10):1545–1548. [DOI] [PubMed] [Google Scholar]
- 2.Werli-Alvarenga A, Ercole FF, Botoni FA, et al. Corneal injuries: incidence and risk factors in the Intensive Care Unit. Rev Lat Am Enfermagem. 2015;19(5):1088–1095. [DOI] [PubMed] [Google Scholar]
- 3.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22–27. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: The Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93(10):1319–1324. [DOI] [PubMed] [Google Scholar]
- 5.Saeed A, D’Arcy F, Stack J, et al. Risk factors, microbiological findings, and clinical outcomes in cases of microbial keratitis admitted to a tertiary referral center in Ireland. Cornea. 2009;28(3):285–292. [DOI] [PubMed] [Google Scholar]
- 6.Ni N, Nam EM, Hammersmith KM, et al. Seasonal, Geographic, and Antimicrobial Resistance Patterns in Microbial Keratitis. Cornea. 2015;34(3):296–302. [DOI] [PubMed] [Google Scholar]
- 7.Collier SA, Gronostaj MP, Macgurn AK, et al. Estimated Burden of Keratittis -- United States, 2010. Morbitidty Mortal Wkly Rep. 2014;63(45):1027–1030. [PMC free article] [PubMed] [Google Scholar]
- 8.Lee R, Manche EE. Trends and Associations in Hospitalizations Due to Corneal Ulcers in the United States, 2002–2012. Ophthalmic Epidemiol. 2016;23(4):257–263. [DOI] [PubMed] [Google Scholar]
- 9.Ung L, Bispo PJM, Shanbhag SS, et al. ScienceDirect The persistent dilemma of microbial keratitis : Global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2018;64(3):255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Services H Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual Life Outcomes. 2006;4:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA H Guidance for Industry Patient-reported Outcome measures: Use in Medical Product Development to Support Labeling Claims. Clin Fed Regist. 2009; (December):1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lois N, Burr J, Norrie J, et al. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: A pragmatic randomized controlled trial. Investig Ophthalmol Vis Sci. 2011;52(3):1586–1592. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–1267. [DOI] [PubMed] [Google Scholar]
- 14.Mangione CM, Phillips RS, Seddon JM, et al. Development of the “Activities of Daily Vision Scale”: A Measure of Visial Functional Status. Med Care. 2017;30(12):1111–1126. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg E, Legro MW, Diener-west M, et al. The VF-14. An index of functional impairment in patients with cataract. Arch Ophthalmol. 1994;112(5):630–638. [DOI] [PubMed] [Google Scholar]
- 16.Rose-Nussbaumer J, Prajna NV, Krishnan KT, et al. Vision-related quality-of-life outcomes in the Mycotic Ulcer Treatment Trial I: A randomized clinical trial. JAMA Ophthalmol. 2015;133(6):642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Hong J, Wei A, et al. Vision-Related Quality of Life in Patients with Infectious Keratitis. Optom Vis Sci. 2014;91(3):278–283. [DOI] [PubMed] [Google Scholar]
- 18.Kodjebacheva G, Coleman AL, Ensrud KE, et al. Reliability and validity of abbreviated surveys derived from teh NAtional Eye Institute Visual Function Questionnaire: The Study of Osteoporotic Fractures. Am J Ophthalmol. 2010;149(2):330–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak ST, Wong ACM. Vision-related quality of life in corneal graft recipients. Eye. 2012;26(9):1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballouz D, Maganti N, Tuohy M, et al. Medication Burden for Patients With Bacterial Keratitis. 2019;38(8):933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carta A, Braccio L, Belpoliti M, et al. Self-assessment of the quality of vision: Assosciation of questionnaire score with objective clinical tests. Curr Eye Res. 1998;17(5):506–511. [DOI] [PubMed] [Google Scholar]
- 22.Owsley C, McGwin G. Depression and the 25-item National Eye Institute Visual Function Questionnaire in older adults. Ophthalmology. 2004;111(12):2259–2264. [DOI] [PubMed] [Google Scholar]
- 23.Nichols KK, Mitchell GL, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea. 2002;21(6):578–583. [DOI] [PubMed] [Google Scholar]
- 24.Granström T, Forsman H, Leksell J, et al. Visual functioning and health-related quality of life in diabetic patients about to undergo anti-vascular endothelial growth factor treatment for sight-threatening macular edema. J Diabetes Complications. 2015;29(8):1183–1190. [DOI] [PubMed] [Google Scholar]
- 25.Ni W, Li X, Hou Z, et al. Impact of cataract surgery on vision-related life performances: The usefulness of Real-Life Vision Test for cataract surgery outcomes evaluation. Eye. 2015;29(12):1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siqueira RC, Messias A, Messias K, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial). Stem Cell Res Ther. 2015;6(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


