Abstract
BACKGROUND
Although surgery for endometriosis can improve pain and fertility, the risk of disease recurrence is high. There is little consensus regarding the benefit of medical therapy in preventing recurrence of endometriosis following surgery.
OBJECTIVE AND RATIONALE
We performed a review of prospective observational studies and randomised controlled trials (RCTs) to evaluate the risk of endometriosis recurrence in patients undergoing post-operative hormonal suppression, compared to placebo/expectant management.
SEARCH METHODS
The following databases were searched from inception to March 2020 for RCTs and prospective observational cohort studies: MEDLINE, Embase, Cochrane CENTRAL and Web of Science. We included English language full-text articles of pre-menopausal women undergoing conservative surgery (conserving at least one ovary) and initiating hormonal suppression within 6 weeks post-operatively with either combined hormonal contraceptives (CHC), progestins, androgens, levonorgesterel-releasing intra-uterine system (LNG-IUS) or GnRH agonist or antagonist. We excluded from the final analysis studies with <12 months of follow-up, interventions of diagnostic laparoscopy, experimental/non-hormonal treatments or combined hormonal therapy. Risk of bias was assessed using the Cochrane Risk of Bias Tool for RCTs and the Newcastle-Ottawa Scale (NOS) for observational studies.
OUTCOMES
We included 17 studies (13 RCTs and 4 cohort studies), with 2137 patients (1189 receiving post-operative suppression and 948 controls), which evaluated various agents: CHC (6 studies, n = 869), progestin (3 studies, n = 183), LNG-IUS (2 studies, n = 94) and GnRH agonist (9 studies, n = 1237). The primary outcome was post-operative endometriosis recurrence, determined by imaging or recurrence of symptoms, at least 12 months post-operatively. The secondary outcome was change in endometriosis-related pain. Mean follow up of included studies ranged from 12 to 36 months, and outcomes were assessed at a median of 18 months. There was a significantly decreased risk of endometriosis recurrence in patients receiving post-operative hormonal suppression compared to expectant management/placebo (relative risk (RR) 0.41, 95% CI: 0.26 to 0.65), 14 studies, 1766 patients, I2 = 68%, random effects model). Subgroup analysis on patients treated with CHC and LNG-IUS as well as sensitivity analyses limited to RCTs and high-quality studies showed a consistent decreased risk of endometriosis recurrence. Additionally, the patients receiving post-operative hormonal suppression had significantly lower pain scores compared to controls (SMD −0.49, 95% CI: −0.91 to −0.07, 7 studies, 652 patients, I2 = 68%).
WIDER IMPLICATIONS
Hormonal suppression should be considered for patients not seeking pregnancy immediately after endometriosis surgery in order to reduce disease recurrence and pain. Various hormonal agents have been shown to be effective, and the exact treatment choice should be individualised according to each woman’s needs.
Keywords: endometriosis, recurrence, suppression, post-operative, laparoscopy, surgery
Introduction
Endometriosis is a common condition affecting women of reproductive age, often presenting with pelvic pain and infertility. In addition to significantly impacting a woman’s quality of life, the treatment of endometriosis carries a considerable economic burden (Levy et al., 2011). Surgery can help relieve symptoms, restore anatomy and improve fertility outcomes (Lyons et al., 2006; Jacobson et al., 2010). However, endometriosis recurrence rates following surgery are high and patients remain at risk of requiring re-intervention. Re-operation rates following endometriosis surgery in which the ovaries are preserved have been reported as between 27% and 58% (Abbott et al., 2003; Weir et al., 2005; Shakiba et al., 2008).
Post-operative hormonal suppression has been proposed as a means of reducing the need for re-intervention following conservative (ovarian sparing) surgery for endometriosis (Somigliana et al., 2017; Murji et al., 2020). A Cochrane review published in 2004, updated in 2011, synthesised findings of randomised controlled trials (RCTs) comparing medical therapies to prevent post-operative recurrence of endometriosis and pain symptoms (Furness et al., 2004). This review found no evidence of benefit of post-operative hormonal suppression, compared to surgery alone; however, the majority of included studies examined outcomes at only 3 months following surgery. Despite the rigorous methodological quality well-known to Cochrane reviews, the publication is dated and a number of newer therapeutics have been introduced for the medical management of endometriosis. Furthermore, other systematic reviews and meta-analyses, particularly on oral contraceptive pills (OCPs) have demonstrated a benefit to post-operative hormonal suppression (Muzii et al., 2016; Grandi et al., 2019). Our current understanding of endometriosis would suggest that a short course of suppressive therapy following surgery to treat residual endometriosis may not confer long-term benefits. Patients need to be on hormone suppression for long periods of time to prevent disease recurrence, as relapse occurs when patients come off medication.
Our objective was to perform a systematic review and meta-analysis of prospective observational studies and RCTs of women undergoing conservative endometriosis surgery to evaluate the risk of disease recurrence in patients receiving post-operative hormonal suppression compared to placebo/expectant management.
Methods
Search strategy
A comprehensive search strategy of electronic databases was used to locate relevant published studies from inception up to 18 March 2020 and included MEDLINE, Embase, Cochrane CENTRAL and Web of Science. Search terms were used to identify patients with endometriosis and receiving medical management. We opted for these broad inclusive search concepts with subsequent limitation for studies of patients undergoing surgery placed during the title/abstract screen. For the search strategy, see Supplementary Data File S1.
Study selection
RCTs and prospective observational cohort studies of pre-menopausal women undergoing conservative endometriosis surgery (conserving at least one ovary) were included. Eligible studies were published manuscripts that required both an intervention and control arm post-operatively, in which patients received either post-operative medical suppression of endometriosis for a minimum duration of 6 months or placebo/expectant management. Medical suppression was required to be commenced within 6 weeks following surgery. Acceptable medical therapies included combined hormonal contraception (CHC), progestins, androgens, levonorgesterel-releasing intra-uterine system (LNG-IUS) and GnRH agonist or antagonist. We excluded studies with patients undergoing diagnostic laparoscopy only, ovarian cyst aspiration and sclerosis, experimental treatment with novel agents (such as interferon alpha-2b) or medical therapy remote from surgery (beyond 6 weeks post-operatively), as well as patients receiving a combination of medical treatments post-operatively (eg. GnRH agonist and LNG-IUS). Conference proceeding abstracts and unpublished studies were excluded. Study selection was limited to full-text English publications.
The database search was performed by an information specialist, and the subsequent title and abstract screen, full-text review and risk of bias analysis were performed by two independent reviewers (A.Z. and E.D.). Conflicts were resolved by consensus after consulting a third reviewer (A.M.). Title and abstract screening, full-text review and risk of bias analysis were all performed with the use of the online platform Covidence (www.covidence.org). The study protocol was registered online through PROSPERO (CRD42018111394) and was completed following PRISMA guidelines for systematic reviews (Moher et al., 2009).
Outcome measures
The primary outcome was post-operative endometriosis recurrence, defined radiologically or clinically. Radiographic evidence of endometriosis recurrence included presence of new endometrioma, endometriosis plaques and nodules either on ultrasound (US) or MRI. The clinical definition of recurrence consisted of new onset or increasing pelvic pain symptoms, palpation of new endometriosis nodules on physical examination, initiation of additional therapy for endometriosis treatment and findings from second-look surgery, when available. The secondary outcome was changed in endometriosis-related pain. As pain is inconsistently reported across studies (i.e. pelvic pain, dyspareunia, dysmenorrhoea, non-cyclic pelvic pain), we chose change in dysmenorrhoea as our primary pain endpoint when more than one pain parameter was evaluated. When dysmenorrhoea was not specifically reported, pelvic pain was substituted as the outcome of interest. Due to heterogeneous pain scoring across studies, change in pain from pre-operative baseline to follow-up was standardised using standard mean difference (SMD) to allow for comparisons. Outcomes of interest were analysed collectively for all medical interventions, and subgroup analyses were then performed for each specific medical therapy. Outcomes were assessed at 12 months, and when data were available at both 12 months and beyond, outcome data at 12 months were preferentially extracted. Data from validated quality of life measurement tools such as the Medical Outcomes Survey Short Form 36 (SF-36) were extracted and descriptively analysed.
Data extraction
Two reviewers (A.Z. and E.D.) independently completed the data extraction using custom pre-tested worksheets. For studies with more than one intervention arm, the data were extracted for each specific intervention. When studies had multiple intervention arms, data for the control group were used once in the analysis.
Risk of bias
The risk of bias for each study was evaluated independently by two reviewers (A.Z. and E.D.) and discrepancies were resolved by a third reviewer (A.M.). For RCTs, the risk of bias was determined using the Cochrane Risk of Bias Tool. The Newcastle-Ottawa Scale (NOS) was used to score observational studies. Scores obtained on the NOS were then used to assign study quality as good, fair or poor based on the Agency of Healthcare Research and Quality (AHRQ) standards.
Statistical analysis
Post-operative recurrence of endometriosis was reported as relative risk (RR) with corresponding 95% confidence interval (CI). As pain was reported on different scales across studies, effects on pain scores were reported as an SMD. When the mean or standard deviation were not reported, the median was used as an estimate of the mean and the standard deviation was calculated from the range or inter-quartile range (Hozo et al., 2005). To calculate the number needed to treat (NNT) from the pooled RR, we took into account the pooled background risk of endometriosis recurrence in the control group in the most recently published studies and calculated NNT with 95% CI for various background risks. Statistical heterogeneity was assessed using the I2 statistic and presented by calculating a prediction interval. Statistical heterogeneity was further explored by calculating the prediction interval with each study omitted in turn (IntHout et al., 2016). We performed post-hoc meta-regression to evaluate if study follow-up duration affected heterogeneity. Planned subgroup analysis by medical intervention (CHC, progestins, androgens, LNG-IUS, GnRH agonist, GnRH antagonist versus placebo/no intervention) was performed. Post-hoc subgroup analysis based on endometriosis phenotype was performed to assess effectiveness of medical suppression based on disease behaviour. To test the robustness of our primary outcome, we performed pre-defined sensitivity analyses between fixed and random effects modelling and by limiting to only RCTs and high-quality studies. Publication bias was assessed using a funnel plot with trim-and-fill (Duval and Tweedie, 2000). Data analysis was performed using the meta package in R and Review Manager version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration) (Schwarzer, 2007; R-Core-Team, 2019).
Results
Study selection and characteristics
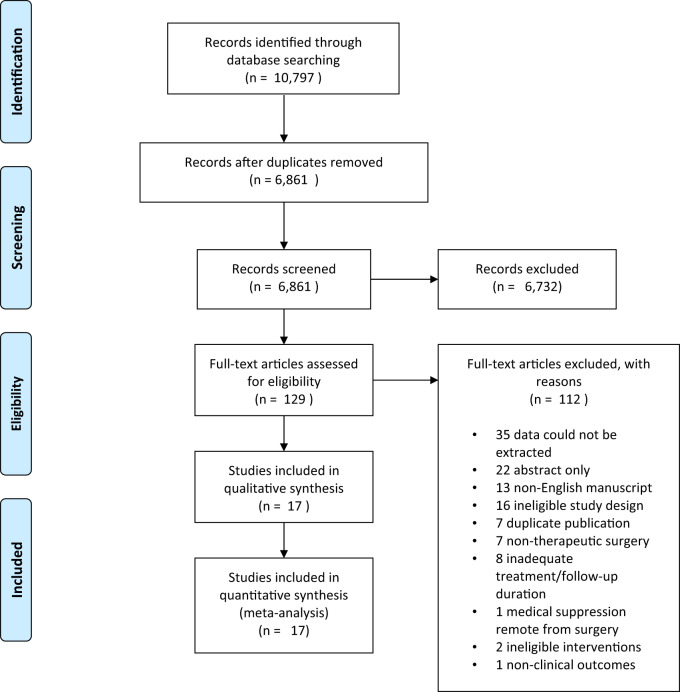
The electronic database search produced 10 797 articles, including 3936 duplicate results. Title and abstract screening led to a total of 129 articles for full-text review; of these 112 articles were excluded, as detailed in the PRISMA flowchart in Fig. 1. Ultimately, 17 studies were eligible for inclusion, comprising 13 RCTs and 4 observational cohort studies. Studies originated from six countries, including Italy (10), China (3), Thailand (1), Russia (1), the USA (1) and Japan (1).
Figure 1.
PRISMA flow chart for study identification and inclusion/exclusion.
In total, 2137 patients were included in the meta-analysis: 1189 received post-operative hormonal suppression and 948 were managed expectantly or were given placebo. Six studies (n = 869 patients) (Muzii et al., 2000; Sesti et al., 2007; Vercellini et al., 2008; Sesti et al., 2009; Seracchioli et al., 2010; Cucinella et al., 2013) evaluated CHC which was administered continuously in all studies, except one (Muzii et al., 2000). Three studies (n = 183 patients) evaluated various progestins including gestrinone and dienogest (Yang et al., 2006; Takaesu et al., 2016; Dobrokhotova et al., 2017) and two studies evaluated LNG-IUS (n = 94 patients) (Vercellini et al., 2003b; Tanmahasamut et al., 2012). Three studies had, in addition to a control group, two active intervention arms for which data could be independently extracted and analysed (Sesti et al., 2007, 2009; Takaesu et al., 2016). Nine studies evaluated various formulations of GnRH agonist including leuprolide, triptorelin, nafarelin and goserelin (n = 1237) (Campo et al., 2014; Hornstein et al., 1997; Vercellini et al., 1999; Sesti et al., 2007, 2009; Angioni et al., 2015; Takaesu et al., 2016; Huang et al., 2018; Yang et al., 2019). Table I describes the individual studies, their characteristics and outcomes.
Table I.
Study characteristics.
| Study | Design | Phenotype | Surgical procedure | Intervention | Control | Mean follow-up (months) | Primary outcome (recurrence) | Secondary outcome (pain) |
|---|---|---|---|---|---|---|---|---|
| Angioni et al. (2015) | RCT | DIE | EE | Triptorelin 3.75 mg IM monthly × 6 months (n = 80) | Expectant (n = 79) | 12 | N/A | SF-36 *Cumulative Pain Scores and QoL |
| Campo et al. (2014) | Prospective Cohort | OE | Cystectomy | GnRH-a NOS monthly × 6 months (n = 46) | Expectant (n = 102) | 30 | TVUS endometrioma measuring ≥ 2cm | N/A |
| Cucinella et al. (2013) | Prospective Cohort | OE | Cystectomy | Cyclic OCP daily (monophasic n = 87, multiphasic n = 43) | Expectant (n = 38) | 24 | TVUS endometrioma measuring ≥ 2 cm | N/A |
| Dobrokhotova et al. (2017) | Prospective Cohort | NOS | EE and Cystectomy | Dienogest 2 mg PO daily × 6 months (n = 12) | Expectant (n = 9) | 12 | N/A | Dysmenorrhea by 10-point VAS |
| Hornstein et al. (1997) | RCT | NOS | ‘Reductive surgery’ | Nafarelin 200 mcg IN BID × 6 months (n = 49) | Placebo IN BID × 6 months (n = 44) | 24 | Time to initiation of alternative therapy | Cumulative Pain Score: 15-point B+B scale |
| Huang et al. (2018) | RCT | NOS | EE and Cystectomy | GnRH-a NOS IM q 28d × 4-6 months (n = 50) | Expectant (n = 50) | 12 | Recurrence of pain or pelvic mass on TVUS | Pain Relief Rates by 11 point NRS |
| Muzii et al. (2000) | RCT | OE | Cystectomy | Cyclic Monophasic OCP × 6 months (n = 33) | Expectant (n = 35) | 22 | TVUS endometrioma, with confirmatory laparoscopy | Incidence of non-specific pain graded ≥4 on a 10-point visual analog scale. |
| Seracchioli et al. (2010) | RCT | OE | Cystectomy | Monophasic OCP A: Continuous × 24 months (n = 73) B: Cyclic × 24 months (n = 75) | Expectant (n = 69 ) | 24 | TVUS endometrioma measuring ≥ 1.5 cm | N/A |
| Sesti et al. (2009) | RCT | OE | Cystectomy | A:Triptorelin/ Leuprorelin 3.75mg IM q 28d × 6 mo (n = 58) B: Continuous Monophasic OCP × 6 mo (n = 60) C: Dietary Therapy (vitamins etc.) × 6 mo (n = 62) | Placebo (n = 60) | 18 | TVUS endometrioma measuring ≥ 2.0cm, subsequent confirmatory laparoscopy | N/A |
| Sesti et al. (2007) | RCT | rAFS Stage 3/4 | EE | A:Triptorelin/ Leuprolide 3.75 mg IM monthly × 6 months (n = 39) B: Continuous Monophasic OPC × 6 mo (n = 38) C: Dietary Therapy (vitamins etc.) × 6 mo (n = 35) | Placebo (n = 110) | 12 | N/A | Dysmenorrhea by 10-point VAS |
| Takaesu et al. (2016) | RCT | NOS | EE and Cystectomy | A: Dienogest 2mg PO daily × 6 months (n = 54) B: Goserelin 1.8mg SC monthly × 6 months (n = 51) | Expectant (n = 79) | 24 | MRI endometriosis lesions | Dysmenorrhea by 10-point VAS |
| Tanmahasamut et al. (2012) | RCT | NOS | EE and Cystectomy | Levonorgestrel IUD (n = 28) | Expectant (n = 26) | 12 | N/A | Dysmenorrhea by 10-point VAS |
| Vercellini et al. (1999) | RCT | AFS score ≥ 4 | ‘Conservative Surgery’ | Goserelin 3.6 mg SC monthly × 6 months (n = 107) | Expectant (n = 103) | 24 | Total pain recurrence defined by B+B score ≥ 5 after surgery | N/A |
| Vercellini et al. (2008) | Prospective Cohort | OE | Cystectomy | Cyclic monophasic OCP (n = 102) | Expectant (n = 46) | 28 | TVUS endometrioma measuring ≥ 2 cm | N/A |
| Vercellini et al. (2003b) | RCT | AFS 1-4 | EE | Levonorgestrel IUD (n = 20) | Expectant (n = 20) | 12 | N/A | Dysmenorrhea by 10-point VAS |
| Yang et al. (2006) | RCT | ASRM Stage 3/4 | ‘Conservative Surgery’ | Gestrinone 2.5mg twice weekly × 6 mo (n = 19) Traditional Chinese Medicine (n = 20) | Expectant (n = 13) | 12 - 36 | TVUS endometrioma, progressive pelvic pain | N/A |
| Yang et al. (2019) | RCT | NOS | EE and Cystectomy | Triptorelin 3.75 mg IM q 28 d × 6 mo (n = 65) | Expectant (n = 65) | 21 | NOS | N/A |
AFS, American Fertility Society; ASRM, American Society of Reproductive Medicine; B + B, Biberoglu and Behrman; DIE, deeply infiltrating endometriosis; EE, endometriosis excision; EE2, ethinyl oestradiol; IN, intra-nasal; IUD, intra-uterine device; mo, months; N/A, not applicable; NOS, not otherwise specified; NRS, numeric rating score; OCP, oral contraceptive pill; OE, ovarian endometrioma; QoL, Quality of life; rAFS, revised AFS; RCT, randomised controlled trial; SIE, superficially infiltrating endometriosis; TVUS, transvaginal ultrasound; VAS, visual analogue scale.
Primary outcome
Among the 17 studies, 14 reported on endometriosis recurrence rates. Recurrence was most commonly defined as reappearance of an ovarian endometrioma on imaging (US or MRI). Four studies described recurrence of endometriosis as return of pain symptoms (Vercellini et al., 1999, 2003; Tanmahasamut et al., 2012; Huang et al., 2018), and one study used initiation of an alternative therapy due to symptomatology as a marker for disease recurrence (Hornstein et al., 1997). The mean follow up of included studies ranged from 12 to 36 months, and outcomes were assessed at 12 months in 9 studies and only beyond 12 months in 8 studies (median 18 months).
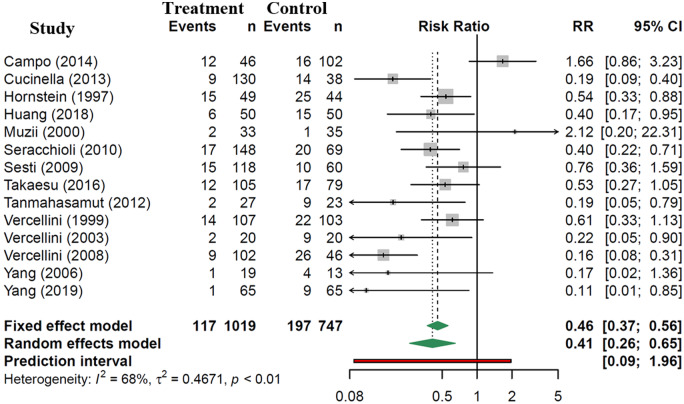
Patients receiving post-operative hormonal suppression were significantly less likely to experience recurrence of endometriosis, with a RR of 0.41 (95% CI: 0.26 to 0.65, 14 studies, 1766 patients, I2 = 68%, random effects model, Fig. 2). Subgroup analyses, presented in Fig. 3 and Supplementary Data File S2, show consistent decreased risk of disease recurrence, compared to controls for CHC (RR 0.36, 95% CI: 0.15 to 0.87, 6 studies, 854 patients, I2 = 67% random effects model) and LNG-IUS (RR 0.21, 95% CI: 0.07 to 0.57, 2 studies, 90 patients, I2 = 0% random effects model). Subgroup analysis for progestin (RR 0.17, 95% CI: 0.02 to 1.36, 1 study, 32 patients) and GnRH agonist studies (RR 0.62, 95% CI: 0.35 to 1.13, 7 studies, 929 patients, I2 = 52%, random effects model) showed a trend towards a decrease in endometriosis recurrence; however, statistical significance was not reached.
Figure 2.
Risk of radiologic or clinical endometriosis recurrence with post-operative hormonal suppression compared to expectant management.
Figure 3.
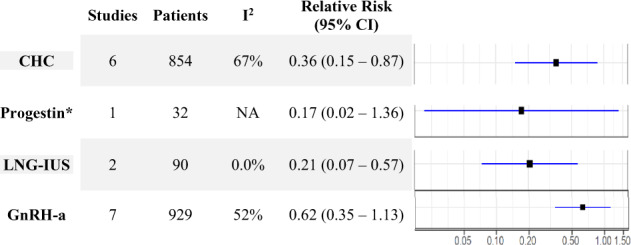
Relative risk of endometriosis recurrence by hormonal intervention (random effects). CHC, combined hormonal contraceptive; CI, confidence interval; LNG-IUS, levonorgesterel intra-uterine system. *Single study—fixed effect model.
In the four most recent studies, the background risk of disease recurrence in the control group ranged from 15% to 25% (95% CI). The NNTs with hormonal suppression to prevent one endometriosis recurrence at least 6 months post-operatively were 16 (95% CI 11–24, based on 15% background risk), 12 (95% CI 8–18, based on 20% background risk) or 10 (95% CI 6–15, based on 25% background risk).
Secondary outcome
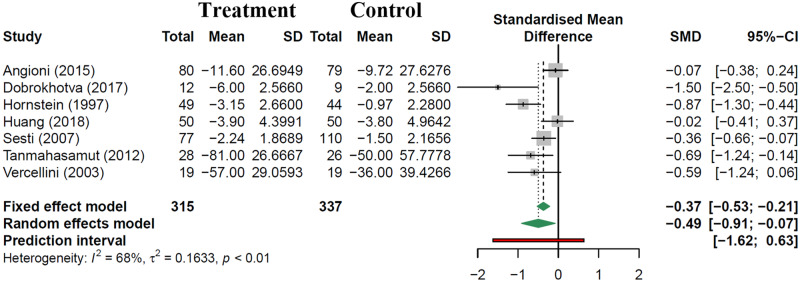
Seven studies reported change in pain symptoms which were amenable to data extraction and analysis. Evaluation methods included use of a 10 point visual analogue scale (VAS) for dysmenorrhoea (4 studies), dysmenorrhoea pain relief rates by 11 point numeric rating scale (1 study), cumulative pain scores from Short Form 36 quality of life tool (1 study) and cumulative pain scores on Biberoglu and Berhman (B + B) scale (1 study). Outcomes were evaluated at 12 months in six studies, and beyond 12 months in one study. Patients receiving post-operative hormonal suppression had significantly lower pain scores compared to controls: SMD of −0.49 (95% CI: −0.91 to −0.07, 7 studies, 652 patients, I2 = 68%, random effects model, Fig. 4). Due to the paucity of studies reporting this outcome, subgroup analysis by intervention type was not performed. Only three studies commented on quality-of-life metrics, all of which used SF-36, one using GnRH agonist therapy, another with multiple intervention arms (GnRH-a therapy and continuous monophasic OCP), and the last with the LNG-IUS (Sesti et al., 2007; Tanmahasamut et al., 2012; Angioni et al., 2015). All three studies reported significant improvement of individual and composite quality-of-life parameters such as physical form, general health and vitality compared to control groups.
Figure 4.
Change in pain scores with post-operative hormonal suppression compared to expectant management.
Subgroup and sensitivity analyses
When we limited the primary outcome to only RCTs, the risk of post-operative endometriosis recurrence was consistent (RR 0.44, 95% CI: 0.30 to 0.66, 11 studies, 1302 patients, I2 = 3%, random effects model) (Supplementary Data File S3). Sensitivity analyses including six high-quality studies suggested even greater reduction in recurrence risk (RR 0.36, 95% CI: 0.18 to 0.72, 6 studies, 843 patients, I2 = 64%, random effects model) (Supplementary Data File S3). Subgroup analysis for risk of endometriosis recurrence based on endometriosis phenotype were as follows: endometrioma RR 0.49 (95% CI: 0.16 to 1.46, 6 studies, 927 patients, I2 = 85% random effects model), deep-infiltrating endometriosis RR 0.55 (95% CI: 0.31 to 1.00, 2 studies, 242 patients, I2 = 25% fixed effects model) and for endometriosis not otherwise specified RR 0.38 (95% CI: 0.22 to 0.66, 6 studies, 597 patients, I2 = 0% random effects model) (Supplementary Data File S3).
For the primary outcome of disease recurrence, there was a large amount of heterogeneity in the treatment effect (I2 = 68%) with a 95% CI Prediction Interval RR 0.09 to 1.96. However, the probability of a future RCT finding no benefit or an increased risk of disease recurrence with post-operative suppression (RR ≥1) was 7%. The probability of a future high-quality study, similar to the six in our meta-analysis, finding RR ≥1 was 4%. In an analysis where each study was sequentially omitted, we found that the Campo et al. (2014) study contributed the greatest heterogeneity and with this study omitted, I2 = 50% with a narrower 95% CI prediction interval of RR 0.10 to 1.36 (Supplementary Data File S3). In this scenario, the probability of the RR ≥1 was 5%. When examining the 13 studies with a follow-up time variable, post-hoc meta-regression reduced the I2 from 69.5% in the meta-analysis to 67.9%, and the estimated proportion of variance explained by follow-up duration was 9.5%. The P-value for the effect of time was 0.272, so differences in the follow-up duration of the studies explain little of the between-study heterogeneity, and not more than we could easily explain by assuming that there was no effect of follow-up duration (Supplementary Data File S3).
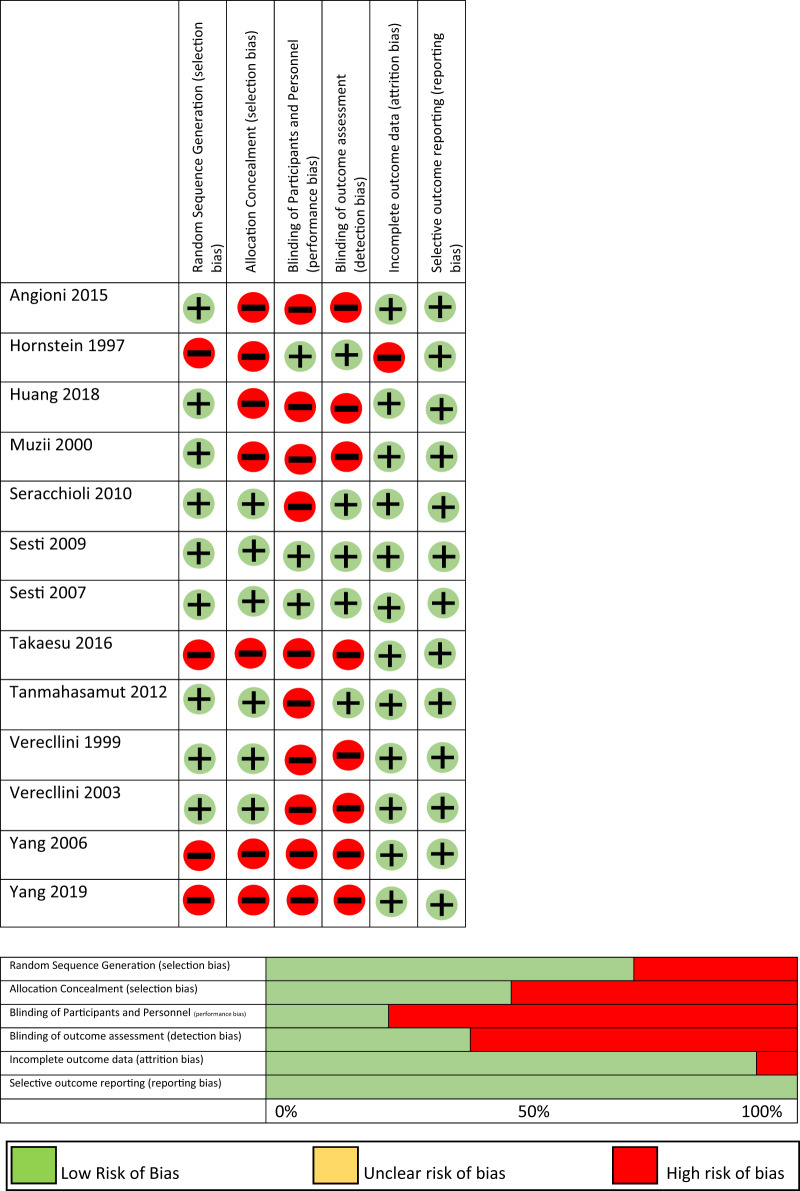
Risk of bias
Risk of bias assessments is shown in Fig. 5 for RCTs and in Table II for observational studies. Among RCTs, the highest risk of bias was for random sequence generation, allocation concealment and blinding of participants and personnel. Of the four observational cohort studies, one was graded as good, two as fair, and one as poor. Selection bias was most common where it was unclear whether patients receiving intervention and control were drawn from a comparable population. We assessed for publication bias using a funnel plot (Supplementary Data File S4) where the effect size of each study was plotted by the inverse of its standard error. We did not find evidence of significant publication bias. After filling in the datasets of two possible studies found to be missing, the summary statistic for the primary outcome was similar.
Figure 5.
Risk of bias for RCTs.
Table II.
Risk of bias for observational studies.
| Selection | Comparability | Outcome | Assessment | |
|---|---|---|---|---|
| Campo et al. (2014) | HIGH (2 pts) | HIGH (1 pt) | LOW (3 pts) | Poor |
| Cucinella et al. (2013) | HIGH (2 pts) | LOW (2 pts) | LOW (3 pts) | Fair |
| Dobrokhotova et al. (2017) | HIGH (2 pts) | LOW (2 pts) | LOW (3 pts) | Fair |
| Vercellini et al. (2008) | LOW (4 pts) | LOW (2 pts) | LOW (3 pts) | Good |
Discussion
In this systematic review and meta-analysis, we identified 17 studies evaluating 2137 women with four types of hormonal suppression following endometriosis surgery: CHC, progestin therapy, LNG-IUS and GnRH agonist. Pooled results of all treatment options, as well as subgroup analyses, consistently demonstrated that post-operative hormonal suppression was effective in reducing endometriosis recurrence and improving patient-reported pain. Based on these data, we found that as few as 8 and as many as 24 women would need to be started on hormonal suppression following surgery in order to prevent one endometriosis recurrence at a median of 18 months.
Strengths of this study include the robust design and thorough nature of the review, with a literature search developed by an information specialist. Exclusion of retrospective studies as well as case series elevates the quality of included studies and the results of the meta-analysis. Furthermore, consolidating literature on a variety of medical options from studies from various countries provides a comprehensive summary of the effect of these treatment options on post-operative outcomes.
Our findings must be interpreted in the context of the study design. There was substantial heterogeneity in the treatment effect. This was likely due to differences between studies such as study design, different interventions, varying endometriosis phenotypes and differences in outcome measurement. Furthermore, there was substantial heterogeneity within individual studies where, for example, CHC was prescribed to half the patients cyclically and the other half continuously; however, the results were pooled (Seracchioli et al., 2010). Other examples include two studies not distinguishing between the types of GnRH agonist (Sesti et al., 2007, 2009) or another study where patients with incomplete and complete surgical resection were combined (Angioni et al., 2015). Such discrepancies and inconsistencies, both between and within studies, demonstrate that endometriosis treatment is complex, and there is a multitude of approaches worldwide. It is reassuring that sensitivity analyses limited to RCTs and highest quality studies were consistent with the overall outcome. Furthermore, selection bias in observational studies may have resulted in patients deemed at higher risk of recurrence being preferentially started on post-operative suppression. However, this would cause regression towards the null hypothesis, making the positive results of this meta-analysis all the more striking. Although the treatment effect (captured by the summary statistic and confidence interval) appears to show decreased disease recurrence with medical suppression, the significant heterogeneity of the included studies and associated wide prediction interval suggest that future studies might not necessarily yield similar findings. We have attempted to explain some of the heterogeneity by various statistical analyses (subgroup analysis, prediction interval, sequential removal of studies) and meta-regression, and by evaluating study methodology. However, there must be a variety of unknown factors that may contribute to heterogeneity leading to uncertainty about future results. Nonetheless, based on our results, clinicians can be reassured that the probability of not finding a benefit in a future high-quality study is considered low, at 4%.
Defining the primary and secondary outcomes of this review was challenging due to the paradigm shift of clinical diagnosis and monitoring of endometriosis, rather than relying on laparoscopy (Agarwal et al., 2019). Although a purist definition of endometriosis recurrence calls for second-look laparoscopy, real-world monitoring of disease progression is performed clinically, using medical imaging, recurrence of symptoms or the need to initiate alternative therapy. Given these criteria for determining recurrence, instances of recurrent deep disease or subtle plaques cannot be adequately excluded. This becomes especially more complicated for studies were endometriosis was incompletely excised. Additionally, imaging findings suggestive of recurrence may not always be as clinically meaningful in the context of a patient who remains asymptomatic, without an effect on quality of life. As far as pain reporting, the subjective, complex and layered nature of pain (whether it be dysmenorrhoea, dyspareunia or pelvic pain) makes attempts at measurement and tracking fraught with imperfections and inaccuracies. Despite these challenges, the outcome measures used in the study are clinically meaningful and results were consistently in favour of post-operative suppression. Our study was not designed to assess for differences between medical therapies in the prevention of specific disease phenotype recurrences, such as endometriomas. Although our data do not provide guidance on this issue, a 2013 systematic review and meta-analysis by Vercellini et al. (2013) demonstrated the efficacy of CHC in preventing endometrioma recurrence, with a pooled odds ratio of 0.12 (95% CI: 0.05, 0.29, 4 studies, 423 CHC users, 341 controls) for always-users compared to never-users. In contrast to this, in a 2017 RCT, Chen et al. (2017) showed no difference in endometrioma recurrence after a year of LNG-IUS use compared to expectant management in patients undergoing laparoscopic cystectomy followed by 6 months of GnRH agonist. These nuanced differences are important in tailoring care to patients and their unique clinical context. Prospective, comparative trials are needed to best define the ideal role of the various medications used to treat endometriosis and establish clear guidance.
Following surgical treatment, endometriosis has a relatively high rate of spontaneous recurrence, with an ∼5-year cumulative recurrence rate of 40–50% (Wheeler and Malinak, 1983; Guo, 2009). Risk factors for recurrence remain unclear, with some studies suggesting increased rates in younger patients, and others suggesting the opposite (Busacca et al., 2006; Kikuchi et al., 2006; Tandoi et al., 2011). However, there is general consensus that complete excision of endometriotic lesions results in lower rates of recurrence for both deeply infiltrating endometriosis as well as ovarian disease (Vercellini et al., 2003a; Alborzi et al., 2004; Vignali et al., 2005). The lower rate of recurrence noted in patients having undergone complete excision would suggest that, at least in part, recurrence may be reactivation or progression of residual disease, rather than newly appearing endometriosis, underscoring the importance of surgical technique and, consequently, a potential source of bias in studies on post-operative outcomes. Nonetheless, whether recurrence truly represents new lesions or simply a flare up of persistent disease, post-operative medical therapy has a role to play in suppressing the activity of this endometriosis. The complex pathogenesis of endometriosis suggests that a combination of genetic alterations, ectopic endometrial cells (whether through retrograde menstruation, induced metaplasia, haematogenous/lymphatic spread or other), ovulation and altered immune clearance work synchronously to produce the condition we know as endometriosis (Burney and Giudice, 2012; Vercellini et al., 2014). Assuming that surgery has relieved the bulk, if not all, of the ectopic tissue, the main strategy in preventing recurrence with post-operative suppression hinges on minimising ovulation, the activity of endometrial cells and their risk of re-implanting in the peritoneal cavity, as no treatments or interventions exist to alter the genetics or immune aspects of this disease. In this way, hormonal suppression of endometriosis is a valuable tool in the post-operative setting. Given the high risk of recurrence, and the positive results of this meta-analysis, patients not seeking to conceive immediately after surgery may benefit from post-operative hormonal suppression to reduce the risk of disease recurrence. Managing endometriosis in this way could translate to fewer repeat surgeries and decreased morbidity of multiple procedures. Clinicians have an armamentarium of effective post-operative options. This can facilitate dialogue between physicians and patients to choose the treatment that is most in line with the patient’s goals (e.g. tolerability, contraception, comorbidities and affordability).
Although the benefits of post-operative hormonal suppression have been established, many clinical questions remain unanswered. Comparative trials evaluating the various hormonal therapies are necessary to guide clinicians and patients in choosing appropriate post-operative management for various clinical situations based on symptoms and endometriosis phenotype. It is essential that future studies have standardised outcome reporting to allow for comparison, combination and synthesis of data for clinical decision-making (Becker et al., 2014).
Conclusion
When hormonal suppression (CHC, progestin, LNG-IUS, GnRH agonist) is initiated within 6 weeks of endometriosis surgery, there is a significant reduction in endometriosis recurrence and pain scores at up to 1 year post-operatively. Medical suppression should be considered and discussed with patients not seeking pregnancy immediately after surgery. As various hormonal agents have been shown to be effective, the choice of treatment should be individualised according to each woman’s needs.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Authors’ roles
A.Z.: study design, execution, analysis, manuscript drafting, critical discussion. E.D.: execution, analysis, manuscript drafting. S.M.: literature search, data acquisition. G.T.: data analysis, critical discussion. O.B.: study design, manuscript drafting, critical discussion. A.M.: study design, execution, analysis, manuscript drafting, critical discussion.
Funding
Mount Sinai Hospital (Toronto, Canada) Department of Obstetrics & Gynaecology Generalist Practice Plan and the Comprehensive Research Experience for Medical Students (CREMS) (University of Toronto, Toronto, Canada) provided financial support for the study but had no involvement in the study design, collection/analysis/interpretation of data, writing of the report or the decision to submit the article for publication.
Conflict of interest
A.Z.: honoraria from Hologic, outside the submitted work. E.D., S.M. and G.T.: none to disclose. O.B.: personal fees from Bayer, Abbvie, Hologic and Allergan, grants from Bayer and Allergan, outside the submitted work. A.M.: speaker bureau/advisory board for Abbvie, Allergan, Bayer, Hologic and Pfizer, outside the submitted work.
Supplementary Material
References
- Abbott J, Hawe J, Clayton R, Garry R.. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2–5 year follow‐up. Human Reprod 2003;18:1922–1927. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, Singh SS, Taylor HS.. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol 2019;220:354.e1–354.e12. [DOI] [PubMed] [Google Scholar]
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S.. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril 2004;82:1633–1637. [DOI] [PubMed] [Google Scholar]
- Angioni S, Pontis A, Dessole M, Surico D, De Cicco Nardone C, Melis I.. Pain control and quality of life after laparoscopic en-block resection of deep infiltrating endometriosis (DIE) vs. incomplete surgical treatment with or without GnRHa administration after surgery. Arch Gynecol Obstet 2015;291:363–370. [DOI] [PubMed] [Google Scholar]
- Becker CM, Laufer MR, Stratton P, Hummelshoj L, Missmer SA, Zondervan KT, Adamson GD, Adamson G, Allaire C, Anchan R.. World endometriosis research foundation endometriosis phenome and biobanking harmonisation project: I. Surgical phenotype data collection in endometriosis research. Fertil Steril 2014;102:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Giudice LC.. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busacca M, Chiaffarino F, Candiani M, Vignali M, Bertulessi C, Oggioni G, Parazzini F.. Determinants of long-term clinically detected recurrence rates of deep, ovarian, and pelvic endometriosis. Am J Obstet Gynecol 2006;195:426–432. [DOI] [PubMed] [Google Scholar]
- Campo S,, Campo V,, Gambadauro P. Is a positive family history of endometriosis a risk factor for endometrioma recurrence after laparoscopic surgery? Reprod Sci 2014;21:526–531. [DOI] [PubMed] [Google Scholar]
- Chen Y-J, Hsu T-F, Huang B-S, Tsai H-W, Chang Y-H, Wang P-H.. Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: a randomized controlled study. Am J Obstet Gynecol 2017;216:582.e1–582.e9. [DOI] [PubMed] [Google Scholar]
- Cucinella G, Granese R, Calagna G, Svelato A, Saitta S, Tonni G, De Franciscis P, Colacurci N, Perino A.. Oral contraceptives in the prevention of endometrioma recurrence: does the different progestins used make a difference? Arch Gynecol Obstet 2013;288:821–827. [DOI] [PubMed] [Google Scholar]
- Dobrokhotova JE, Ilyina IJ, Grishin II,, Ibragimova DM, Kalimatova DM, Narimanova MR, Bondarenko KR, Ilchenko VJ.. Evaluation of dienogest treatment efficacy in patients with endometriosis. J Endometr Pelvic Pain Disord 2017;9:44–49. [Google Scholar]
- Duval S, Tweedie R.. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- Furness S, Yap C, Farquhar C, Cheong YC. Pre and post‐operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev2004. CD003678-CD003678. [DOI] [PMC free article] [PubMed]
- Grandi G, Barra F, Ferrero S, Sileo FG, Bertucci E, Napolitano A, Facchinetti F.. Hormonal contraception in women with endometriosis: a systematic review. Eur J Contracept Reprod Health Care 2019;24:61–70. [DOI] [PubMed] [Google Scholar]
- Guo S-W. Recurrence of endometriosis and its control. Hum Reprod Update 2009;15:441–461. [DOI] [PubMed] [Google Scholar]
- Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL.. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril 1997;68:860–864. [DOI] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I.. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wu M, Liu Z, Shi H, Han Y, Song X.. Clinical efficacy and safety of gonadotropin-releasing hormone agonist combined with laparoscopic surgery in the treatment of endometriosis. Int J Clin Exp Med 2018;11:4132–4137. [Google Scholar]
- IntHout J, Ioannidis JP, Rovers MM, Goeman JJ.. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6:e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson TZ, Duffy JM, Barlow DH, Farquhar C, Koninckx PR, Olive D. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev2010. CD001398-CD001398. [DOI] [PubMed]
- Kikuchi I, Takeuchi H, Kitade M, Shimanuki H, Kumakiri J, Kinoshita K.. Recurrence rate of endometriomas following a laparoscopic cystectomy. Acta Obstet Gynecol Scand 2006;85:1120–1124. [DOI] [PubMed] [Google Scholar]
- Levy AR, Osenenko KM, Lozano-Ortega G, Sambrook R, Jeddi M, Bélisle S, Reid RL.. Economic burden of surgically confirmed endometriosis in Canada. J Obstet Gynaecol Canada 2011;33:830–837. [DOI] [PubMed] [Google Scholar]
- Lyons SD, Chew SS, Thomson AJ, Lenart M, Camaris C, Vancaillie TG, Abbott JA.. Clinical and quality-of-life outcomes after fertility-sparing laparoscopic surgery with bowel resection for severe endometriosis. J Minim Invasive Gynecol 2006;13:436–441. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murji A, Biberoğlu K, Leng J, Mueller MD, Römer T, Vignali M, Yarmolinskaya M.. Use of dienogest in endometriosis: a narrative literature review and expert commentary. Curr Med Res Opin 2020: 1–13. [DOI] [PubMed] [Google Scholar]
- Muzii L, Di Tucci C, Achilli C, Di Donato V, Musella A, Palaia I, Panici PB.. Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: a systematic review and metaanalysis. Am J Obstet Gynecol 2016;214:203–211. [DOI] [PubMed] [Google Scholar]
- Muzii L, Marana R, Caruana P, Catalano GF, Margutti F, Panici PB.. Postoperative administration of monophasic combined oral contraceptives after laparoscopic treatment of ovarian endometriomas: a prospective, randomized trial. Am J Obstet Gynecol 2000;183:588–592. [DOI] [PubMed] [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://wwwR-projectorg/2019 (9 December 2019, date last accessed).
- Schwarzer G. meta: An R package for meta-analysis. R News 2007;7:40–45. [Google Scholar]
- Seracchioli R, Mabrouk M, Frasca C, Manuzzi L, Montanari G, Keramyda A, Venturoli S.. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010;93:52–56. [DOI] [PubMed] [Google Scholar]
- Sesti F, Capozzolo T, Pietropolli A, Marziali M, Bollea MR, Piccione E.. Recurrence rate of endometrioma after laparoscopic cystectomy: a comparative randomized trial between post-operative hormonal suppression treatment or dietary therapy vs. placebo. Eur J Obstet Gynecol Reprod Biol 2009;147:72–77. [DOI] [PubMed] [Google Scholar]
- Sesti F, Pietropolli A, Capozzolo T, Broccoli P, Pierangeli S, Bollea MR, Piccione E.. Hormonal suppression treatment or dietary therapy versus placebo in the control of painful symptoms after conservative surgery for endometriosis stage III-IV. A randomized comparative trial. Fertil Steril 2007;88:1541–1547. [DOI] [PubMed] [Google Scholar]
- Shakiba K, Bena JF, McGill KM, Minger J, Falcone T.. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol 2008;111:1285–1292. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Busnelli A, Benaglia L, Viganò P, Leonardi M, Paffoni A, Vercellini P.. Postoperative hormonal therapy after surgical excision of deep endometriosis. Eur J Obstet Gynecol Reprod Biol 2017;209:77–80. [DOI] [PubMed] [Google Scholar]
- Takaesu Y, Nishi H, Kojima J, Sasaki T, Nagamitsu Y, Kato R, Isaka K.. Dienogest compared with gonadotropin‐releasing hormone agonist after conservative surgery for endometriosis. J Obstet Gynaecol Res 2016;42:1152–1158. [DOI] [PubMed] [Google Scholar]
- Tandoi I, Somigliana E, Riparini J, Ronzoni S, Candiani M.. High rate of endometriosis recurrence in young women. J Pediatr Adolesc Gynecol 2011;24:376–379. [DOI] [PubMed] [Google Scholar]
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P.. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-related pain: a randomized controlled trial. Obstet Gynecol 2012;119:519–526. [DOI] [PubMed] [Google Scholar]
- Vercellini P, De Matteis S, Somigliana E, Buggio L, Frattaruolo MP, Fedele L.. Long‐term adjuvant therapy for the prevention of postoperative endometrioma recurrence: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand 2013;92:8–16. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Chapron C, De Giorgi O, Consonni D, Frontino G, Crosignani PG.. Coagulation or excision of ovarian endometriomas? Am J Obstet Gynecol 2003. a;188:606–610. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Crosignani PG, Fadini R, Radici E, Belloni C, Sismondi P.. A gonadotrophin‐releasing hormone agonist compared with expectant management after conservative surgery for symptomatic endometriosis. Br J Obstet Gynaecol 1999;106:672–677. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG.. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril 2003. b;80:305–309. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Somigliana E, Daguati R, Vigano P, Meroni F, Crosignani PG.. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol 2008;198:504.e1–504.e5. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Viganò P, Somigliana E, Fedele L.. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261. [DOI] [PubMed] [Google Scholar]
- Vignali M, Bianchi S, Candiani M, Spadaccini G, Oggioni G, Busacca M.. Surgical treatment of deep endometriosis and risk of recurrence. J Minim Invasive Gynecol 2005;12:508–513. [DOI] [PubMed] [Google Scholar]
- Weir E, Mustard C, Cohen M, Kung R.. Endometriosis: what is the risk of hospital admission, readmission, and major surgical intervention? Journal of minimally invasive gynecology 2005;12:486–493. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Malinak LR.. Recurrent endometriosis: incidence, management, and prognosis. Am J Obstet Gynecol 1983;146:247–253. [DOI] [PubMed] [Google Scholar]
- Yang DX, Ma WG, Qu F, Ma BZ. Comparative study on the efficacy of Yiweining and Gestrinone for post-operational treatment of stage III endometriosis. Chin J Integr Med 2006 Sep;12:218–220. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhu W, Chen S, Zhang G, Chen M, Zhuang Y.. Laparoscopic surgery combined with GnRH agonist in endometriosis. J Coll Physicians Surg Pak 2019;29:313–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.