Autologous mitochondrial transplantation was recently proposed as a novel therapeutic strategy to restore cardiac function after myocardial infarction.1 For this intervention, functional mitochondria are isolated from skeletal muscle and then injected directly into the ischemic heart or coronary arteries of the same subject.1 The declared treatment goal is to replace mitochondria in cardiac myocytes damaged by ischemia with respiration-competent mitochondria, whose ATP formation supposedly sustains cardiac vitality and contraction. This approach was tested in preclinical models of ischemia/reperfusion injury1 and subsequently applied to pediatric patients with myocardial ischemia in an open-label, single-armed clinical trial ( NCT02851758). We recently argued that the translation of this approach to the clinical arena may be premature, since it appears unlikely that injected mitochondria withstand high Ca2+ concentrations in the extracellular environment.2 In response to our article, McCully and colleagues, who had introduced this approach,1 emphasized that “donor mitochondria are viable in both the isolated perfused heart, where Ca2+ concentration was 1.7 mM and in in vitro cell studies where Ca2+ concentration was 1.8 mM”.3
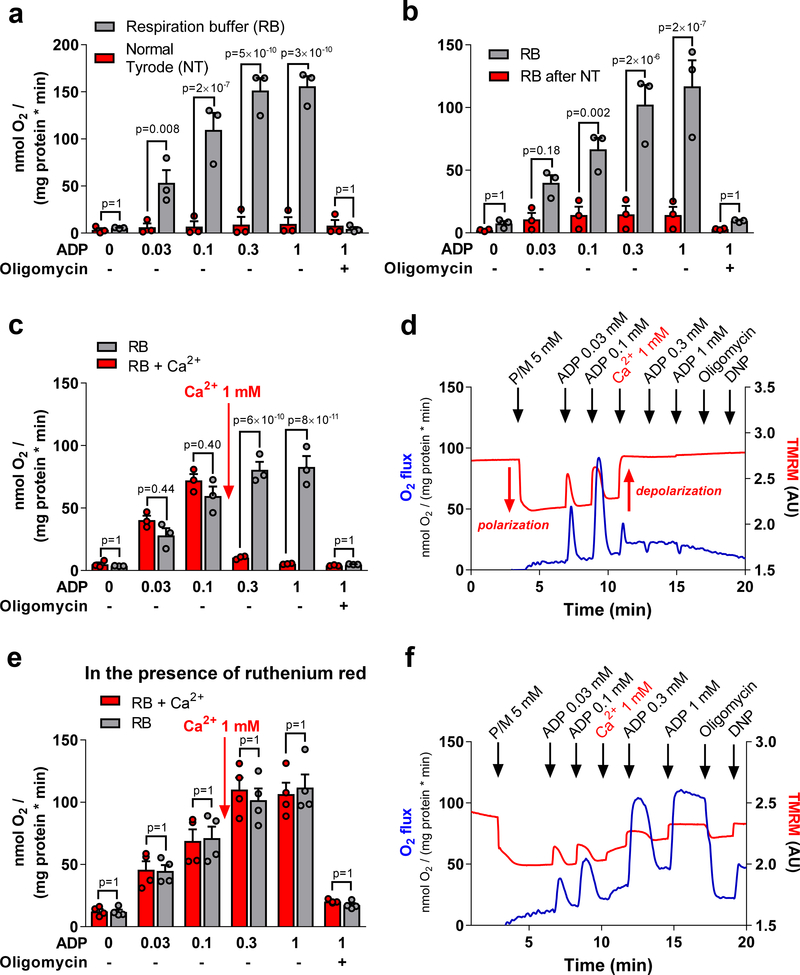
It is widely accepted that upper micromolar Ca2+ concentrations induce mitochondrial Ca2+ overload with fatal activation of the permeability transition pore. However, high extramitochondrial Na+ concentrations in the bloodstream or extracellular fluid may potentially protect mitochondria from Ca2+ overload by inducing Ca2+ efflux via the mitochondrial Na+/Ca2+ exchanger. To address this hypothesis, we isolated skeletal muscle mitochondria (as used in the studies by McCully et al.1) from mice and determined oxygen (O2) consumption and the mitochondrial membrane potential (Δψm; using the potentiometric dye tetramethylrhodamine methyl ester, TMRM). Mitochondria were resuspended in either conventional Ca2+-free respiration buffer (RB) or – to mimic the extracellular environment – in Normal Tyrode’s solution (NT), containing 140 mM Na+, 5 mM K+ and 1 mM Ca2+. Using pyruvate and malate as complex I substrates, respiration was stimulated by increasing concentrations of ADP. In Ca2+-free RB, ADP accelerated O2 consumption, while in Ca2+-containing NT, respiration was abrogated (Figure 1A). This effect was irreversible, since respiration could not be restored by resuspending mitochondria in Ca2+-free RB after 10 min incubation in NT (Figure 1B).
Figure 1:
A, Mitochondria were isolated from murine musculus quadriceps and resuspended either in respiration buffer (RB) containing (in mM) EGTA 0.5, taurine 20, MgCl2 3, sucrose 110, K-lactobionate 60, KH2PO4 10, K-HEPES 20, BSA 1 mg/mL or Normal Tyrodés solution (NT), containing (in mM): NaCl 130, KCl 5, MgCl2 1, CaCl2 1, Na-HEPES 10, glucose 10, sodium pyruvate 2 and ascorbic acid 0.3. Mitochondria were supplied with pyruvate and malate (5 mM, respectively) as complex I substrates, and respiration (measured by an Oroboros O2k system) was induced by sequential additions of increasing concentrations of ADP (“State 3” respiration), before oligomycin (1.2 μM) was added to block the ATP synthase. B, Similar conditions as in A, unless that mitochondria were incubated in NT for 10 min and then centrifuged at 12.000 rpm for 5 min and resuspended in Ca2+-free RB. The control group was treated in the same way except that mitochondria were preincubated with Ca2+-free RB. C, Similar conditions as the RB groups in A and B, unless that in one group (RB + Ca2+), 1 mM Ca2+ was added during state 3 respiration. D, representative example of the group RB + Ca2+ from C, showing (besides respiration) also the fluorescence of TMRM (1 μM). Upon polarization, TMRM fluorescence decreases due to quenching of TMRM fluorescence, while upon depolarization, fluorescence increases due to dequenching of the signal. ADP concentrations are given in mM. Oligomycin (1.2 μM) and DNP (5 μM) were added at the end of the protocol. E and F, similar conditions as in C and D, except that all experiments were performed in the presence of the MCU blocker ruthenium red (25 μM). Animal procedures were approved by the local animal ethics committee and conducted in accordance with institutional guidelines. All cumulative data are the means ±SEM of n=3 experiments, respectively. The Shapiro-Wilk normality test was performed to determine data distribution. Data were analyzed with 2-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test. The p-values presented are corrected for multiple testing. A p-value <0.05 was considered statistically significant.
When acutely applying 1 mM Ca2+ during ADP-stimulated respiration in RB, O2 consumption slightly and transiently accelerated, but shortly thereafter ceased (Figures 1C, D). Immediately after Ca2+ addition, Δψm depolarized within 20 s and did not repolarize when the ATP synthase was inhibited with oligomycin. Moreover, the mitochondrial uncoupler 2,4-dinitrophenol (DNP) did not further depolarize Ca2+-exposed mitochondria (Figure 1D). These data indicate that after Ca2+ addition, Δψm dissipates completely and is uncoupled from the control by ADP via the ATP synthase. To interrogate whether this effect requires Ca2+ uptake via the mitochondrial Ca2+ uniporter (MCU), mitochondria were preincubated with the MCU inhibitor ruthenium red. Under these conditions, Δψm was only partially depolarized by Ca2+ addition, and ADP-induced control of respiration was maintained at levels comparable to that of mitochondria in Ca2+-free RB (Figures 1E and F).
We conclude that skeletal muscle mitochondria are unable to withstand the ionic milieu of blood or the extracellular space, characterized by millimolar Ca2+ and Na+ concentrations, since mitochondria are immediately and irreversibly damaged by depolarization of Δψm as a result of rapid MCU-mediated Ca2+ overload. These results argue against the plausibility of the proposed concept of mitochondrial transplantation, according to which a) extracellular mitochondria provide ATP to ischemic myocardium and b) functional mitochondria eventually become internalized into cardiac myocytes to replace mitochondria damaged by ischemic injury.1, 2 Therefore, although the scientific question behind our experiments may appear trivial, the question how mitochondria can survive a “high Ca2+ shock” during mitochondrial transplantation, especially when applied to patients, should no longer be ignored.
Instead, our results raise the new question whether the contents of permeabilized mitochondria (e.g., peptides, glutathione, adenosine di- or triphosphate, mitochondrial DNA etc.) might account for the beneficial effects reported by McCully and colleagues1, 2 in a paracrine fashion rather than the mitochondria directly contributing to respiration. In this context, it is possible that mitochondria act as delivery vehicles for molecules released into the interstitial space of the myocardium, which could then act as damage-associated molecular patterns4 to shape the immune response to myocardial ischemia in a protective manner, similar to the model recently proposed to explain the benefits of stem cell therapy after myocardial infarction.5 If this is the case, the increased membrane permeability induced by the Ca2+ shock could even be a requirement, rather than a threat, for the efficiency of this novel and intriguing treatment strategy. In conclusion, our results may guide the focus of emerging research in this area towards further clarifying the mechanisms that underlie the cardioprotective effects before this procedure is further applied in humans.
Acknowledgments
SOURCES OF FUNDING
C. M. is supported by the Deutsche Forschungsgemeinschaft (DFG; Ma 2528/7-1, SFB-894, TRR-219) and the Bundesministerium für Bildung und Forschung (BMBF; 01EO1504). B.O’R. is supported by NIH grants R01HL108917 and R01HL137259.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.McCully JD, Cowan DB, Emani SM and Del Nido PJ. Mitochondrial transplantation: From animal models to clinical use in humans. Mitochondrion. 2017;34:127–134. [DOI] [PubMed] [Google Scholar]
- 2.Bertero E, Maack C and O’Rourke B. Mitochondrial transplantation in humans: “magical” cure or cause for concern? The Journal of clinical investigation. 2018;128:5191–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCully JD, Emani SM and Del Nido PJ. Response to Bertero, Maack and O’Rourke. The Journal of clinical investigation. 2018. [Google Scholar]
- 4.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K and Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AK, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, et al. An acute immune response underlies the benefit of cardiac stem-cell therapy. Nature. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]