Abstract
Functional dependence is an important determinant of longevity and quality of life. The purpose of the current study was to determine the prevalence and correlates of functional dependence among patients with end-stage renal disease (ESRD) receiving maintenance dialysis. We enrolled 148 participants with ESRD from five clinics. Functional status, as measured by basic and instrumental activities of daily living (ADL, IADL), was ascertained by validated questionnaires. Functional dependence was defined as needing assistance in at least one of seven IADLs or at least one of four ADLs. Demographic characteristics, chronic health conditions, anthropometric measurements, and laboratories were assessed by a combination of self-report and chart review. Cognitive function was assessed with a neurocognitive battery, and depressive symptoms were assessed by questionnaire. Mean age of the sample was 56.2 ± 14.6 years. Eighty-seven participants (58.8%) demonstrated dependence in ADLs or IADLs, 70 (47.2%) exhibited IADL dependence alone, and 17 (11.5%) exhibited combined IADL and ADL dependence. In a multivariable-adjusted model, stroke, cognitive impairment, and higher systolic blood pressure were independent correlates of functional dependence. We found no significant association between demographic characteristics, chronic health conditions, depressive symptoms or laboratory measurements, and functional dependence. Impairment in executive function was more strongly associated with functional dependence than memory impairment. Functional dependence is common among ESRD patients and independently associated with stroke, systolic blood pressure, and executive function impairment.
Keywords: Functional dependence, ESRD, dialysis, cognitive impairment
INTRODUCTION
Functional dependence is defined as difficulty carrying out activities essential to independent living,1 and is often measured with the basic and/or instrumental activities of daily living (ADL and IADL, respectively). ADLs are self-care tasks such as bathing, dressing, and ambulation, whereas IADLs involve household management tasks such as shopping, housecleaning, and telephone use.2 Functional dependence is predictive of survival, nonadherence to drug treatment,3,4 and poorer quality of life.5,6 Several professional organizations recommend that end-stage renal disease (ESRD) providers assess functional status in order to manage the disease continuum and intervene if deficits in function occur.7,8
Like functional dependence, cognitive impairment is common among patients with ESRD, affecting 10–30% of middle-aged patients and 30–55% of patients over 75 years of age.9 Cognitive impairment is associated with functional dependence in non-ESRD populations, including community-dwelling adults over age 65.10–12 The association between cognitive impairment and functional dependence has not been carefully investigated among patients with ESRD, but is important to determine because it may have implications for disability detection and prevention.
The objective of the present study was to determine the correlates of functional dependence in patients with ESRD. Our hypothesis was that functional dependence would be positively correlated with age and cognitive impairment.
METHODS
Participants
From March 2009 through October 2010, we recruited participants from 5 outpatient dialysis clinics near Stanford University. Eligible participants were at least 21 years of age and receiving maintenance dialysis for at least 90 days. Participants were excluded if they were not fluent in English or Spanish, if they had an active psychiatric disorder (including dementia), or if they had significant visual or hearing impairment. A total of 720 individuals were screened, and 477 were considered eligible for the study. We contacted 348 eligible individuals based on geographic proximity to our center, and 150 consented to participate. Two participants were found to be ineligible and were subsequently withdrawn, resulting in a study sample of 148 participants. Eligible individuals who declined participation were older (63 ± 16 vs. 56 ± 15 years), less likely to be white (25 vs. 43%), and received dialysis for a longer period of time compared with study participants (66 ± 41 vs. 40 ± 37 months). The Institutional Review Boards at Stanford University and Santa Clara Valley Medical Center, and the Research and Development Committee of Veterans Affairs Palo Alto Health Care System approved the study and all participants provided informed consent.
Measurements
Participants completed a study questionnaire that assessed demographic, clinical, and psychosocial characteristics, as well as functional dependence. We evaluated functional dependence by assessing the degree of assistance required in Lawton’s 7 IADLs—telephone use, shopping for groceries, using transportation, meal preparation, housework, taking medications, and handling finances, and in Katz’s 4 basic ADLs—bathing, dressing, ambulating, and transferring from a chair.13,14 Each item on the 7-item IADL scale is rated from 0 to 2 and each item on the 4-item ADL scale ranges is rated from 0 to 3, with higher scores indicating higher functional status. IADL dependence was defined as needing assistance in any of the 7 IADLs, and ADL dependence was defined as needing assistance in any of the 4 ADLs.
We assessed cognitive function with a neuropsychological battery assessing domains of global cognition, attention, psychomotor speed, executive function, verbal fluency, and verbal memory. Testing was conducted in a quiet room prior to a midweek dialysis session and administered by trained research staff. Global cognition was assessed with the Modified Mini-Mental State Examination. Attention was assessed with the Trail Making Test Part A (Trails A) and executive function with Part B (Trails B) as well as the Digit Symbol Substitution Test. Memory was assessed with the Rey Auditory Verbal Learning Test immediate and delayed recall components, psychomotor speed in dominant and nondominant hands was assessed with the Grooved Pegboard Test, and verbal fluency was assessed among English-speaking participants only with the Controlled Oral Word Association Test. Cognitive impairment was defined as a score at least 2 standard deviation below normative values for age and education (where available) in 2 or more cognitive domains.15,16 If a test was not attempted, then the participant was classified as not impaired on this test. Ten subjects did not attempt the tests of executive function, 21 did not attempt the tests of motor function, 7 did not attempt the test of attention, and 10 did not attempt the test of language fluency; there were no subjects missing memory assessments. Educational attainment was classified as less than high school graduate, high school graduate, or some college education. Diabetes was defined as self-report or chart history of diabetes or use of medications for diabetes. Hypertension was defined as self-report of hypertension or use of medications for hypertension. Coronary artery disease was defined as self-report or chart history of a myocardial infarction, coronary angioplasty, or coronary stent placement. Peripheral arterial disease was defined as self-report or chart history of lower extremity angioplasty or stent placement. Heart failure was defined by self-report or chart history. Depressive symptoms were defined as a score of 6 or more on the Geriatric Depression Scale short form.17 Laboratory results within 1 month of enrollment were abstracted from the dialysis chart. Blood pressure and weight were measured prior to the start of dialysis.
Statistical analysis
We compared characteristics of participants with no IADL or ADL dependence to participants with IADL and ADL dependence using analysis of variance or the Kruskal-Wallis test for continuous variables and the chi-square test for categorical variables. Because there were few subjects with ADL dependence, we grouped subjects with IADL and ADL dependence together for all subsequent analyses of functional dependence. We then used logistic regression to determine the association, expressed as an odds ratio (OR) and 95% confidence interval (95% CI), between demographic and clinical characteristics with functional dependence. We used backward selection to fit the multivariable models, forcing age, sex, and race in the models, in addition to all other effects significant at the P < 0.1 level. We first fit a parsimonious model in which demographic characteristics, chronic health conditions, depression, and cognitive impairment were considered for inclusion. Next, we fit a “full” model in which laboratory and anthropometric measurements were considered for inclusion in addition to other participant characteristics (with the exception of hypertension to avoid collinearity with blood pressure measurements). Next, we investigated whether there were differing associations according to the type of cognitive impairment, memory vs. executive function impairment. For these analyses, we used logistic regression, adjusting for the same set of variables described above. Analyses were conducted using SAS v9.2 (http://www.sas.com).
RESULTS
The mean age of participants was 56.2 ± 14.6 years. Of the 148 participants, 96 (65%) were male, 134 (90%) were on hemodialysis (vs. peritoneal dialysis), 84 (57%) were nonwhite, and 68 (46%) had diabetes (Table 1). There were 87 (58.8%) participants with dependence in at least one IADL or ADL activity. Among these participants, 70 (47.2%) exhibited IADL dependence alone and 17 (11.5%) exhibited combined IADL and ADL dependence.
Table 1.
Characteristics of participants receiving chronic dialysis according to functional status
| No ADL or IADL impairment (N = 61) | IADL impairment only (N = 70) | ADL impairment (N = 17) | P value | |
|---|---|---|---|---|
| Age (years) | 54.9 ± 14.5 | 57.0 ± 14.9 | 57.7 ± 14.0 | 0.65 |
| Months since start of dialysis | 37.0 ± 32.1 | 38.7 ± 36.7 | 54.7 ± 53.7 | 0.57 |
| Male sex, N (%) | 44 (72.1%) | 45 (64.3%) | 7 (41.2%) | 0.06 |
| Race, N (%) | ||||
| White | 26 (42.6%) | 33 (47.1%) | 5 (29.4%) | 0.09 |
| Black | 4 (6.6%) | 7 (10.0%) | 5 (29.4%) | |
| Other | 31 (50.8%) | 30 (42.9%) | 7 (41.2%) | |
| Education level, N (%) | ||||
| <High school graduate | 9 (14.8%) | 7 (10.0%) | 2 (11.8%) | 0.86 |
| High school graduate | 18 (29.5%) | 18 (25.7%) | 5 (29.4%) | |
| College | 34 (55.7%) | 45 (64.3%) | 10 (58.8%) | |
| English speaking, N (%) (vs. Spanish) | 56 (91.8%) | 68 (97.1%) | 16 (94.1%) | 0.37 |
| Living arrangement | ||||
| Live with family/friends | 49 (81.7%) | 53 (75.7%) | 14 (82.4%) | 0.77 |
| Live alone | 11 (18.3%) | 15 (21.4%) | 3 (17.6%) | |
| Live in nursing home | 0 (0%) | 2 (2.9%) | 0 (0%) | |
| Hemodialysis, N (%) (vs. peritoneal dialysis) | 51 (83.6%) | 66 (94.3%) | 17 (100%) | 0.06 |
| Diabetes, N (%) | 27 (44.3%) | 33 (47.1%) | 8 (47.1%) | 0.94 |
| Hypertension, N (%) | 58 (95.1%) | 66 (94.3%) | 15 (88.2%) | 0.49 |
| Stroke, N (%) | 2 (3.3%) | 13 (18.6%) | 3 (17.7%) | 0.01 |
| Heart failure, N (%) | 15 (24.6%) | 21 (30.0%) | 6 (35.3%) | 0.63 |
| Coronary artery disease, N (%) | 10 (16.4%) | 21 (30.0%) | 2 (11.8%) | 0.09 |
| Peripheral arterial disease, N (%) | 3 (4.9%) | 2 (2.9%) | 4 (23.5%) | 0.02 |
| Depressive symptoms, N (%) | 20 (32.8%) | 25 (35.7%) | 6 (35.3%) | 0.94 |
| Cognitive impairment, N (%) | 20 (32.8%) | 38 (54.3%) | 12 (70.6%) | 0.01 |
| Executive function impairment, N (%) | 23 (37.7%) | 47 (67.1%) | 14 (82.4%) | 0.01 |
| Memory impairment, N (%) | 7 (11.5%) | 15 (21.4%) | 4 (23.5%) | 0.26 |
| Systolic blood pressure (mmHg) | 138.4 ± 21.4 | 141.3 ± 29.6 | 159.6 ± 27.6 | 0.07 |
| Diastolic blood pressure (mmHg) | 78.2 ± 14.3 | 76.3 ± 15.8 | 77.6 ± 13.2 | 0.77 |
| Predialysis weight (kg) | 84.0 ± 25.1 | 82.2 ± 21.7 | 82.1 ± 29.0 | 0.92 |
| Hemoglobin (g/dL) | 12.0 ± 1.1 | 11.9 ± 0.9 | 12.2 ± 0.9 | 0.38 |
| Albumin (g/dL) | 4.0 ± 0.3 | 3.9 ± 0.8 | 3.9 ± 0.3 | 0.38 |
| Blood urea nitrogen (mg/dL) | 70.3 ± 19.5 | 64.2 ± 18.0 | 68.7 ± 19.5 | 0.32 |
| Sodium (mg/dL) | 137.8 ± 3.0 | 137.6 ± 2.7 | 137.1 ± 2.1 | 0.81 |
P value represents trend across all functional status categories.
ADL = activities of daily living; IADL = instrumental activities of daily living.
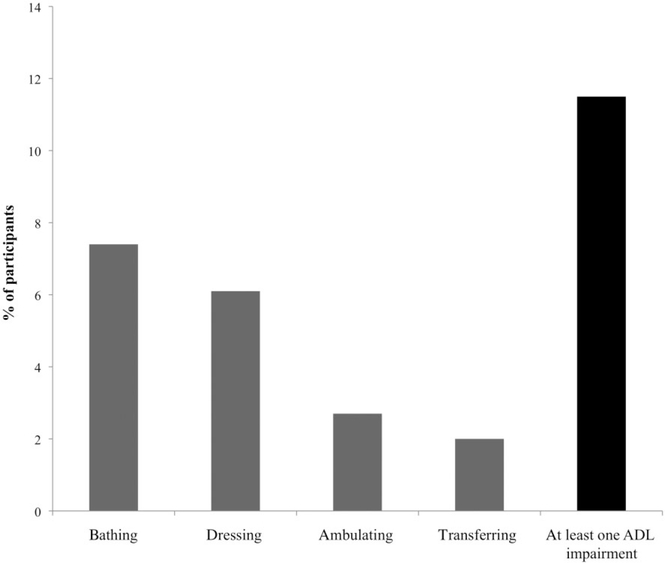
Housework was the most frequent impaired IADL, followed by transportation use, grocery shopping, managing finances, meal preparation, taking medications, and finally, telephone use (Figure 1). Regarding the ADL items, bathing was the most frequent impaired activity, whereas transferring from a chair was the least frequent impaired activity (Figure 2).
Figure 1.
Frequency of impairment in each instrumental activities of daily living (IADL) item.
Figure 2.
Frequency of impairment in each activities of daily living (ADL) item.
In unadjusted analyses, stroke and cognitive impairment were associated with higher odds of functional dependence, whereas higher levels of blood urea nitrogen were associated with lower odds of functional dependence (Table 2). Higher systolic and lower diastolic blood pressure had borderline significant associations with functional dependence. There was no significant association between age, sex, race, education, other chronic health conditions, depressive symptoms, or other laboratory measurements with functional dependence. In a parsimonious multivariable-adjusted model, cognitive impairment remained associated with a significant 2.5-fold increased odds of functional dependence, and stroke remained associated with a significant fivefold increased odds of functional dependence (Table 2). In the full multivariable model, higher systolic blood pressure was also associated with significantly higher odds of functional dependence, whereas diastolic blood pressure and blood urea nitrogen level had borderline nonsignificant associations with functional dependence.
Table 2.
Association of demographic and clinical characteristics with functional dependence
| Characteristic | Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) |
|---|---|---|---|
| Age (per 10 year increase) | 1.12 (0.89, 1.40) | 1.05 (0.81, 1.36) | 0.72 (0.49, 1.06) |
| Male sex (vs. female) | 0.57 (0.28, 1.16) | 0.65 (0.30, 1.41) | 0.57 (0.23, 1.43) |
| Race | |||
| Black vs. white | 2.05 (0.60, 7.07) | 1.47 (0.38, 5.70) | 1.82 (0.37, 8.96) |
| Other vs. white | 0.82 (0.41, 1.63) | 0.72 (0.32, 1.62) | 0.58 (0.23, 1.50) |
| Stroke | 6.65 (1.47, 30.09) | 5.49 (1.14, 26.37) | 5.89 (1.11, 31.10) |
| Cognitive impairment | 2.77 (1.40, 5.48) | 2.55 (1.23, 5.32) | 2.95 (1.24, 7.00) |
| Systolic blood pressure (per 10 mmHg increase) | 1.09 (0.96, 1.24) | – | 1.25 (1.02, 1.54) |
| Diastolic blood pressure (per 10 mmHg increase) | 0.92 (0.74, 1.16) | – | 0.69 (0.47, 1.02) |
| Blood urea nitrogen (per 10 mg/dL increase) | 0.86 (0.71, 1.03) | – | 0.80 (0. 63, 1.00) |
Model 1 is unadjusted. Model 2 is adjusted for age, sex, race, stroke, and cognitive impairment. Model 3 is adjusted for age, sex, race, stroke, cognitive impairment, systolic blood pressure, diastolic blood pressure, and blood urea nitrogen.
CI = confidence interval; OR = odds ratio.
Next, we assessed whether the domain of cognitive impairment had differing associations with functional dependence. There were 84 (56.8%) participants who exhibited executive function impairment and 26 (17.6%) participants who exhibited memory impairment. After adjustment for age, sex, race, stroke, systolic and diastolic blood pressure, and blood urea nitrogen, executive function impairment was associated with a 5.26 (95% CI 2.30, 12.05) higher odds of functional dependence, whereas memory impairment was not significantly associated with functional dependence (OR 1.91, 95% CI 0.68, 5.39).
DISCUSSION
In this ethnically diverse primarily community-dwelling cohort of patients with ESRD, we found that functional dependence was present in more than half of patients. In addition to stroke, functional dependence was strongly correlated with cognitive impairment, especially executive function impairment, and with higher systolic blood pressure.
Among patients with chronic kidney disease not receiving dialysis, self-reported disability is present in 18–24% of adults ≥65 years and 7–11% of younger adults.9 A population-based Taiwanese study of 84,000 patients using the Barthel index to longitudinally characterize functional dependence in incident ESRD patients found, at 13-year follow-up, that adult patients starting hemodialysis experience approximately 3 years with disability.18 In a cross-sectional study, Cook et al. characterized functional dependence in 168 patients ≥65 years receiving dialysis. They found that 52% of subjects were dependent in both IADLs and at least one ADL, while 43% had IADL dependence alone, and only 5% were fully independent.19 In a cross-sectional study of 742 hemodialysis patients, Kutner et al. found that 19% of patients were dependent in at least one ADL.20 Compared with our study, which found that approximately 12% of adults were dependent in both IADLs and at least one ADL, the higher prevalence of functional dependence in the Cook study is explained by the 20 year higher average age of the cohort.
In prior research, frailty, polypharmacy, poor mobility, and low educational attainment were correlated with functional dependence.16,20 This study extends previous findings by demonstrating that cognitive impairment and higher systolic blood pressure, in addition to stroke, are independent correlates of functional dependence in patients with ESRD. Consistent with earlier studies among older adults without ESRD, we found that patients with ESRD and cognitive impairment had a threefold higher prevalence of functional dependence. In contrast to our findings, Cook et al. found no significant association between cognitive function and functional dependence in ESRD patients;19 however, this may be due to the use of a screening test of cognitive function rather than a cognitive battery.
There are several pathways that could link cognitive impairment with functional dependence. Cognitive impairment may reduce the ability of patients to engage in activities that promote independence, such as exercise.21 Conversely, functional dependence could lead to fewer social interactions, thereby contributing to cognitive decline. Cognitive impairment and functional dependence could also have a similar pathophysiologic origin. The syndromes of cognitive impairment and functional dependence overlap with that of frailty and are sometimes conceptualized as consequences of frailty, and at other times, manifestations of the frailty spectrum.22 Although the exact causes of frailty have yet to be elucidated, some have speculated that the central nervous system may play an important role.23 Both cognitive impairment and functional dependence are strongly correlated with stroke. In the same cohort, we have recently shown that stroke-like symptoms in the absence of diagnosed stroke are associated with cognitive impairment and functional dependence,24 supporting the idea that similar structural brain changes may underlie both conditions. Aging and cardiovascular risk factors are thought to preferentially affect frontal subcortical brain regions responsible for executive function.
The relationship between blood pressure and functional dependence is controversial and may be bidirectional. In mid-life, elevated systolic blood pressure may contribute to functional dependence through its effects on cerebral and lower extremity vascular disease.20,25 As patients age and become more functionally dependent, clinicians may apply less stringent blood pressure targets for fear of precipitating syncope or exacerbating cognitive decline.26,27
There are several clinical implications of our findings. The majority of subjects in our study were community-dwelling; thus, the high prevalence of functional dependence implies a large degree of support on family and other informal caregivers, most of whom are unpaid. Second, functional dependence is closely linked with hospitalizations and mortality.28–30 In an increasingly older and sicker ESRD patient population, assessment of functional dependence may inform prognosis, reduce the number of adverse events resulting from functional dependence, and improve health outcomes and quality of life. We found that more than 70% of individuals with cognitive impairment had concomitant functional dependence. Because cognitive testing can be logistically challenging in the dialysis unit and time consuming, targeting cognitive screening to individuals with functional dependence may be an efficient way to detect cognitive impairment in the dialysis population.
What measures can patients and health-care providers take to reduce or reverse dependence? Physical activity and a socially active lifestyle may play an essential role in preserving function and improving survival.31,32 Clinicians should be alert to the possibility of functional decline during major health events, such as dialysis initiation or hospitalizations, and assess capacity to return to independent living.33 A referral to rehabilitation services is appropriate if patients are not meeting rehabilitation goals. Functionally dependent people are also at increased risk for fall-related injury after the onset of disability.34 Thus, fall prevention strategies for the home and dialysis unit are essential among functionally dependent patients.35 Whether the identification and treatment of cognitive impairment slows functional decline has not yet been determined.
There are several strengths of our study, including the ethnically diverse sample and the use of a neurocognitive battery to evaluate cognitive function. There are also several limitations. Our sample was small and drawn from a single geographic area, and thus may not be generalizable to the larger ESRD population. Patients who were eligible but did not participate in the study were older, more likely to be nonwhite, and receiving dialysis for a longer period of time. The effect of this potential bias cannot be determined. We relied on self-report to assess functional dependence, and this method may be subject to bias, although it should be noted that self-reported functional status assessments have been validated in patients without ESRD. Because this is a cross-sectional study, we could not determine whether functional dependence was causally related to cognitive impairment or other correlates. We did not assess functional status over time, so we could not determine whether functional dependence was transient or sustained. Finally, the ORs reported here should not be interpreted as relative risks as ORs overestimate the relative risk when the outcome is common.
CONCLUSIONS
In conclusion, stroke, systolic blood pressure, and cognitive impairment, specifically, executive function impairment, were associated with an increased prevalence of functional dependence among patients with ESRD.
ACKNOWLEDGMENTS
This project is supported by K23AG028952 and R01DK092241 from the National Institutes of Health. Views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs or other affiliated organizations.
REFERENCES
- 1.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004; 59:255–263. [DOI] [PubMed] [Google Scholar]
- 2.McDowell I, Newell C, eds. Measuring Health: A Guide to Rating Scales and Questionnaires. New York, New York: Oxford University Press; 1987. [Google Scholar]
- 3.Hayes TL, Larimer N, Adami A, Kaye JA. Medication adherence in healthy elders: Small cognitive changes make a big difference. J Aging Health. 2009; 21:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hain DJ. Cognitive function and adherence of older adults undergoing hemodialysis. Nephrol Nurs J. 2008; 35:23–29. [PubMed] [Google Scholar]
- 5.Gill TM, Desai MM, Gahbauer EA, et al. Restricted activity among community-living older persons: Incidence, precipitants, and health care utilization. Ann Intern Med. 2001; 135:313–321. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 7.Rettig RA, Sadler JH. Assessing health and quality of life outcomes in dialysis. Am J Kidney Dis. 1997; 30:140–155. [DOI] [PubMed] [Google Scholar]
- 8.Eknoyan G, Levin NW. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis. 2002; 39(2 Suppl1):1–266. [PubMed] [Google Scholar]
- 9.Tamura MK, Yaffe K. Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int. 2010; 79:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moritz DJ, Kasl SV, Berkman LF. Cognitive functioning and the incidence of limitations in activities of daily living in an elderly community sample. Am J Epidemiol. 1995; 141:41–49. [DOI] [PubMed] [Google Scholar]
- 11.Burton CA, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009; 55:570–581. [DOI] [PubMed] [Google Scholar]
- 12.Farias ST, Mungas D, Reed B, Harvey D, Cahn-Weiner D, DeCarli C. MCI is associated with deficits in everyday functioning. Alzheimer Dis Assoc Disord. 2006; 20:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969; 9:179–186. [PubMed] [Google Scholar]
- 14.Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. New Engl J Med. 1983; 309:1218–1224. [DOI] [PubMed] [Google Scholar]
- 15.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006; 67:216–223. [DOI] [PubMed] [Google Scholar]
- 16.Yaffe K, Ackerson L, Kurella Tamura M, et al. Chronic kidney disease and cognitive function in older adults: Findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2010; 58:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesher EL, Berryhill JS. Validation of the geriatric depression scale—short form among inpatients. J Clin Psychol. 1994; 50:256–260. [DOI] [PubMed] [Google Scholar]
- 18.Hung MC, Sung JM, Chang YT, Hwang JS, Wang JD. Estimation of physical functional disabilities and long-term care needs for patients under maintenance hemodialysis. Med Care. 2014; 52:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008; 73:1289–1295. [DOI] [PubMed] [Google Scholar]
- 20.Kutner NG, Zhang R, Allman RM, Bowling CB. Correlates of ADL difficulty in a large hemodialysis cohort. Hemodial Int. 2014; 18:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauvaget C, Yamada M, Fujiwara S, Sasaki H, Mimori Y. Dementia as a predictor of functional disability: A four-year follow-up study. Gerontology. 2002; 48:226–233. [DOI] [PubMed] [Google Scholar]
- 22.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: An emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007; 62:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006; 54:991–1001. [DOI] [PubMed] [Google Scholar]
- 24.Tamura MK, Meyer JB, Saxena AB, Huh JT, Wadley VG, Schiller B. Prevalence and significance of stroke symptoms among patients receiving maintenance dialysis. Neurology. 2012; 79:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller M, Sigurdsson S, Kjartansson O, et al. Joint effect of mid-and-late-life blood pressure on the brain The AGES-Reykjavik Study. Neurology. 2014; 82:2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obisesan TO, Obisesan OA, Martins S, et al. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: The Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2008; 56:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James PA, Oparil S, Carter B, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 28.van Diepen M, Schroijen MA, Dekkers OM, et al. Predicting mortality in patients with diabetes starting dialysis. PLoS ONE. 2014; 9:e89744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fields SD, MacKenzie CR, Charlson ME, Sax FL. Cognitive impairment: Can it predict the course of hospitalized patients? J Am Geriatr Soc. 1986; 34:579–585. [DOI] [PubMed] [Google Scholar]
- 30.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz R, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998; 279:1187–1193. [DOI] [PubMed] [Google Scholar]
- 31.Physical Activity Guidelines Advisory Committee. Report of the Physical Activity Guidelines Advisory Committee, 2008. Washington (DC): US Dept of Health and Human Services; 2008. [Google Scholar]
- 32.Stack AG, Molony DA, Rives T, Tyson J, Murthy BV. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005; 45:690–701. [DOI] [PubMed] [Google Scholar]
- 33.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. New Engl J Med. 2009; 361:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010; 304:1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heung M, Adamowski T, Segal JH, Malani PN. A successful approach to fall prevention in an outpatient hemodialysis center. Clin J Am Soc Nephrol. 2010; 5:1775–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]