Abstract
Purpose of Review
Flame retardant (FR) compounds can adversely impact neurodevelopment. This updated literature review summarizes epidemiological studies of FRs and neurotoxicity published since 2015, covering historical (polybrominated biphenyls [PBBs], polychlorinated biphenyls [PCBs]), contemporary (polybrominated diphenyl ethers [PBDEs], hexabromocyclododecane [HBCD], and tetrabromobisphenol A [TBBPA]), and current-use organophosphate FRs (OPFRs) and brominated FRs (2-ethylhexyl 2,3,4,5-tetrabromobezoate [EH-TBB] TBB), bis(2-ethylhexyl) tetrabromophthalate [BEH-TEBP]), focusing on prenatal and postnatal periods of exposure.
Recent Findings
Continuing studies on PCBs still reveal adverse associations on child cognition and behavior. Recent studies indicate PBDEs are neurotoxic, particularly for gestational exposures with decreased cognition and increased externalizing behaviors. Findings were suggestive for PBDEs and other behavioral domains and neuroimaging. OPFR studies provide suggestive evidence of reduced cognition and more behavioral problems.
Summary
Despite a lack of studies of PBBs, TBBPA, EH-TBB, and BEH-TEBP, and only two studies of HBCD, recent literature of PCBs, PBDEs, and OPFRs are suggestive of developmental neurotoxicity, calling for more studies of OPFRs.
Keywords: Flame retardants, developmental neurotoxicity, children, cognition, behavior, epidemiology
1. Introduction
Flammability regulations required that chemical flame retardants (FRs) be embedded in consumer products, including textiles, plastics, furnishings, electronics, building materials, and transportation products, to reduce flame propagation and prevent combustion. Historical FRs, polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs), were initially used due to their resistance to fire. However, most industrialized nations banned their production amid evidence supporting their toxicity, major accidental human poisoning incidents, and their persistence in the environment and in humans [1–4]. Contemporary FRs, including polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD), and tetrabromobisphenol A (TBBPA), replaced PBBs and PCBs. Since the 1970s, PBDEs have been the most commonly used FR until their voluntary phase-out from the United States (US) market in 2004 for mixtures penta-BDE and octa-BDE and in 2013 for deca-BDE. Production ceased once studies confirmed their presence in a wide range of environmental samples and human tissues, their tendency to bioaccumulate, and evidence of their neurotoxicity, thyrotoxicity, estrogenicity, and carcinogenicity [5–8]. TBBPA and HBCD are still in production, though both are highly scrutinized, because of their persistent, bioaccumulative, and toxic properties [9, 10]. Consequently, organophosphate FRs (OPFRs), including tris(1,3-dichloropropyl) phosphate (TDCIPP), triphenyl phosphate (TPHP), and mono-substituted isopropyl triphenyl phosphate (mono-ITP), which were used since the 1970s, have emerged as high production substitutes for PBDEs. Toxicological studies indicate that OPFRs may adversely affect human health, with findings suggesting developmental toxicity, endocrine disruption, and carcinogenicity [11, 12]. Other brominated FRs, including 2-ethylhexyl 2,3,4,5-tetrabromobezoate (EH-TBB) and bis(2-ethylhexyl) tetrabromophthalate (BEH-TEBP) were also used to replace PBDEs as components of Firemaster 550 (along with TPHP and mono-ITP).
Fetuses and children are highly susceptible to neurotoxic insults from exogenous chemicals due to marked structural and functional brain development during gestation and childhood [13]. Toxicological studies have found evidence that FRs disrupt thyroid hormone homeostasis, interfere with ɣ-aminobutyric acid (GABA) signaling, affect neuronal viability via apoptosis and oxidative stress, modify intracellular calcium signaling, alter gene and protein expression in cellular targets, and affect neuronal differentiation [14, 15, 11, 16–26]. Human exposure to FRs is nearly universal as evidenced by their detection in maternal serum, cord/child serum, urine, and breastmilk [27–41].
Several of the halogenated FRs are stringently regulated, but they remain a public health concern due to their persistence in the environment and in humans. Chlorinated and brominated FRs have long half-lives. For instance, CB-153 and CB-180 have half-lives of 7–9 years [42], PBB has an estimated half-life of 10.8 years [43], congeners within the penta-BDE mixture have half-lives between 2–4 years, and BDE-153 has a half-life of 14–16 years [44, 45]. Further, humans continue to be exposed to PBDEs despite the phase-out, because of exposures to reservoirs that remain in usage; reservoir sources, such as recycled items containing PBDEs, contribute to environmental levels as compounds are released as dust particles and higher-brominated PBDEs metabolize into lower-brominated congeners [46]. Despite the phase-out, the body burden of BDE-47 and BDE-99 plateaued between 2011–2014 and BDE-28 has increased after an initial period of decline among pregnant women in California [46].
2. Epidemiological studies on FRs and neurodevelopment published prior to 2015
Chronic exposure to FRs in the general population and evidence of neurotoxicity from animal studies raise concerns of neurodevelopmental impacts in humans. Numerous epidemiological studies have reported adverse associations between PCBs and PBDE exposures and neurodevelopment in childhood [47–54], although the findings are not entirely consistent for various neurodevelopmental domains.
Inverse associations have been reported between in utero PCB concentrations and verbal and memory scores at 4 years and full scale intelligence quotient (FSIQ) scores at 11 years among children living in the Great Lakes region in Michigan [55, 56]. These findings were similarly observed in the Oswego cohort, with a reduction of 3 FSIQ points (p=0.02) for each 1 ng/g (wet weight) increase in placental concentrations of PCBs [49]. Poorer cognitive development in children have similarly been reported in cohorts in Japan and Slovakia [57, 58]. Decrements in IQ scores were also noted among children ages 4 and 5 years who were prenatally exposed to PCBs as a result of a cooking-oil contamination in Taiwan as compared to children born before the mass poisoning [51]. In contrast, birth cohorts from the Netherlands and upstate New York did not observe a persistent inverse association between prenatal PCBs and cognition in subsequent analyses of children at an older age [59, 60]. Further, null associations were reported in the North Carolina birth cohort and in the Collaborative Perinatal Project [61, 62]. Prenatal PCBs may also impact neurobehavior in children as positive associations were noted with impulsivity, impairments in information processing and executive function, Attention Deficit Hyperactivity Disorder (ADHD) behaviors, and feelings of unhappiness and anxiety in children [63–67, 54, 68]. In other studies, however, no relationship was observed between prenatal PCBs and various neurobehavioral domains, including response inhibition, autistic behaviors, ADHD-like behaviors, and emotional disorders [52, 60, 69–74].
Prenatal PBDEs were first reported to be significantly associated with decreased FSIQ in children at 48 months in the New York City cohort [75]. Concordant findings were later reported in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) Study, specifically a 10-fold increase in ∑PBDEs (BDE-47, −99, −100, and −153) was associated with a decrease of 4.7-points (95% CI −9.4, 0.1) in FSIQ at age 7 years [76]. Eskenazi et al. [77] additionally reported inverse associations between child serum concentrations of ∑PBDEs at 7 years and FSIQ (β=−5.6, 95% CI −10.8, −0.3). Statistically significant inverse associations were also observed in the Health Outcomes and Measures of the Environment (HOME) Study, with a reduction of 4.5-points (95% CI −8.8, −0.1) in FSIQ at 5 years [78]. In contrast, the Menorca birth cohort did not find a relationship between prenatal or postnatal PBDEs and total cognitive function scores at 4 years [79]. However, BDE-47 concentrations were low in the Menorca cohort, with a median of 0.12 ng/g lipid in child serum at 4 years [79]. Further, the Pregnancy, Infection, and Nutrition (PIN) Babies Study in North Carolina reported positive associations between PBDE concentrations measured in breastmilk and cognitive skills at 36 months [80]. Though Adgent et al. [80] conclude that these positive associations may have been mitigated by the benefits of breastfeeding. Increased behavioral problems in children have also been observed with higher concentrations of PBDEs, particularly with externalizing and attention problems. In the CHAMACOS Study, prenatal and postnatal PBDE exposures were associated with attention problems in school-aged children [76]. In addition, concurrent concentrations in child serum were positively associated with more teacher-reported hyperactivity and attention problems at 7 years [76]. Increased hyperactivity problems in children at 5 years was also reported in the HOME Study with prenatal BDE-47 concentrations [78]. A cross-sectional study of children ages 9–11 years found positive associations between PBDEs and conduct problems, hostility, and aggression [81]. In contrast, null associations were reported by the Menorca birth cohort between prenatal and postnatal PBDEs and externalizing problems [79].
New studies are emerging on these and current-use FRs, different neurobehavioral outcomes, and in diverse study populations. This updated literature review focuses on recent findings published from 2015 onward on prenatal and postnatal exposures to historical (PBBs, PCBs), contemporary (PBDEs, TBBPA, HBCD), and current-use FRs (OPFRs, EH-TBB, BEH-TEBP) and several neurodevelopmental domains, including cognition (intelligence quotient), behavior (externalizing, internalizing, attention, social), and neuroimaging, in children up to 18 years of age.
3. Methods
We devised and executed a literature search strategy for PubMed on 11 December 2019. Search strings were developed that would address our population of interest (children), exposures of interest (FRs), and outcomes of interest (cognition, behavior, and neuroimaging). A combination of medical subject headings and free text words were used, with exclusion on publication dates that occurred prior to 2015. The specific search string utilized in PubMed was: (“2015”[Date - Publication]: “2020”[Date - Publication]) AND (“Flame retardants” OR PBDEs OR PCBs OR PBBs OR TBBPA OR HBCD OR OPFRs OR EH-TBB OR BEH-TEBP OR BFRs) AND (IQ OR “Cognitive function” OR Behavior OR Neuroimaging OR “Brain imaging”) AND Children. The search strategy was also limited to studies conducted in humans. Finally, we scanned references of the included studies to screen for any additional studies that were not retrieved by the initial literature search.
Results from PubMed were exported into an Excel file and screened for relevancy based on the title and abstract by two reviewers. Discrepancies between reviewers were marked and resolved by discussion. There was no limitation on the number of exposures examined within each study as some investigated the associations between several toxicants and neurodevelopment. Likewise, there was no limitation on the number of neurodevelopmental outcomes examined so long as one of the assessments aligned with the three domains selected for the present review. Studies that were not original research (e.g., review articles) or not written in English were excluded.
Data extraction from full-text documents were independently completed, with the following information recorded for each bibliographic citation: study type, publication year, geographical location, overall sample size, FR compound assessed, timing of FR quantification (prenatal [weeks], postnatal [days, weeks, months, years]) and corresponding biological (maternal or child serum, cord serum, breastmilk, urine) or environmental matrix (dust), neurobehavioral domains, age of assessment, and overall study findings.
4. Summary of Studies
The PubMed search retrieved 100 studies and hand searching bibliographies yielded two additional studies (Figure 1). A total of 44 articles were considered relevant after title and abstract screening. Of these, we excluded 11 review articles and 1 non-English article. Full text reviews for the remaining studies removed 3 additional articles based on irrelevant exposures and/or outcomes. A total of 29 epidemiological studies were identified (Table 1). Most epidemiological studies used a prospective cohort study design (n=26), though there were a few cross-sectional studies (n=3). Most studies (n=18) examined prenatal exposures, while 5 examined postnatal exposures, and 6 investigated both pre-and postnatal concentrations. FRs evaluated in the studies include: PCBs (n=10), PBDEs (n=18), HBCD (n=2), and OPFRs (n=4). We did not identify any studies on PBBs, TBBPA, EH-TBB, or BEH-TEBP. Neurobehavioral outcomes assessed in the epidemiological studies, ranging from newborn to 15 years, were mainly behavior (n=25), followed by intelligence quotient (IQ) (n=9). Two studies have examined the relationship between FRs and neuroimaging.
Figure 1.
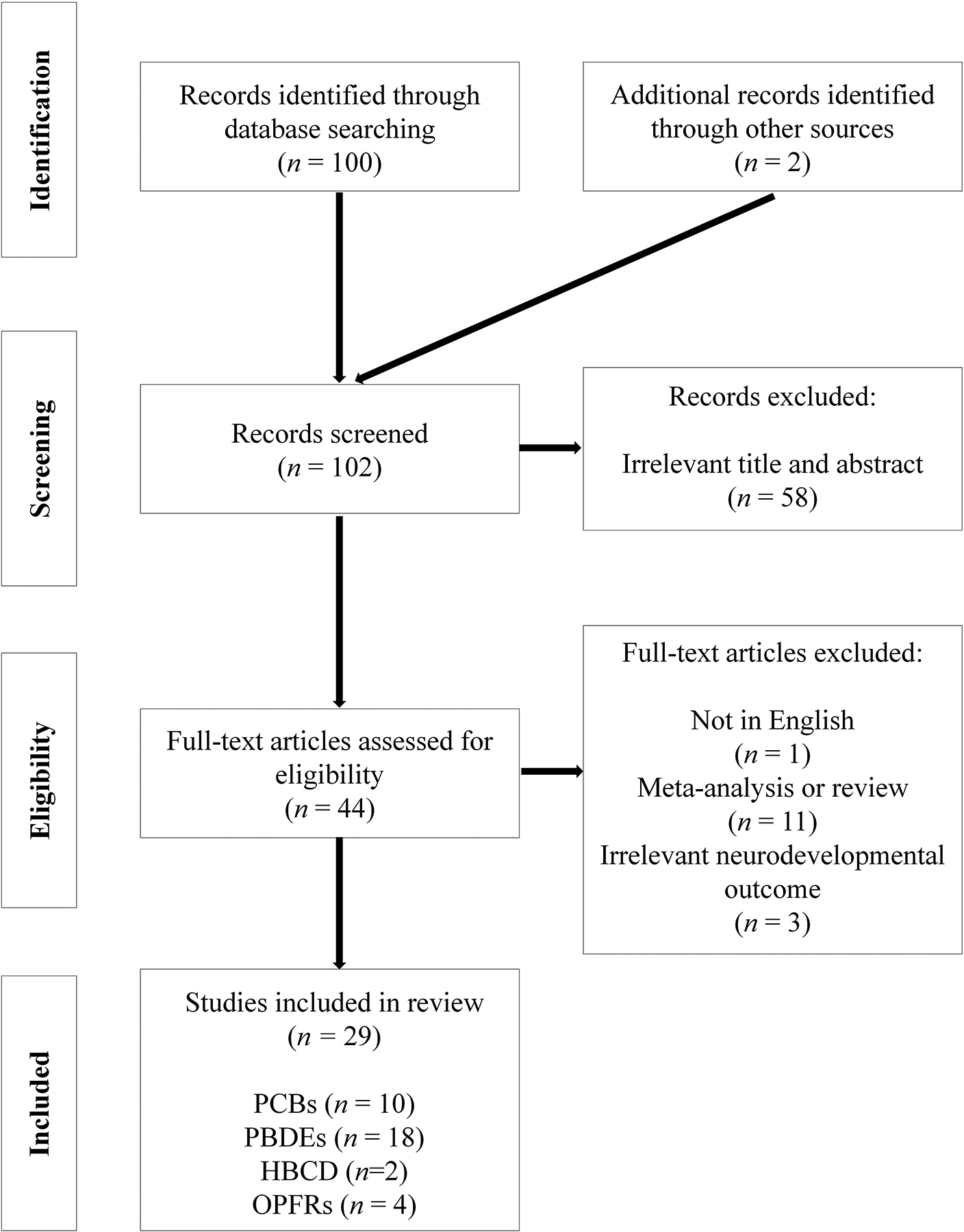
Flow chart showing the process of literature search and study selection
Table 1.
Characteristics of epidemiological studies examining flame retardants and cognition, behavior, and/or neuroimaging in children published from 2015 to 2020
| First Author (Year), Country | Study Design | Sample Size | Flame Retardant Assessment | Neurodevelopment Assessment | Findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective Cohort | Cross-sectional | PCBs | PBDEs | HBCD | Timing | Matrix | Age | IQ | Behavior | Neuroimaging | ||||
| Cowell (2015), United States [101] | ✔ | 210 | ✔ | Delivery | Cord Serum | 3–7 y | ✔ | Positive associations were observed between cord BDE-47 and BDE-153 and attention problems at age 4 years. However, no significant associations were present between cord PBDEs and attention problems at age 6 years. | ||||||
| Ding (2015), China [104] | ✔ | 232 | ✔ | Delivery | Cord Serum | 1 y 2 y |
✔ | A 10-fold increase in cord BDE-47 was associated with a decrease of 1.89-points (95% CI −3.75, −0.03) in the social domain developmental quotient, indicating poorer social skills. | ||||||
| Donauer (2015), United States [96] | ✔ | 349 | ✔ | 16±3 w | Maternal Serum | 5 w | ✔ | Null associations were reported between prenatal PBDEs and neurobehavioral infant profiles at 5 weeks, including hypotonic and social/easygoing, and high arousal/difficult. | ||||||
| Neugebauer (2015), Germany [88] | ✔ | 117 | ✔ | 32 w 2 w after delivery |
Maternal Serum Breast Milk |
8–9 y | ✔ | Positive association between maternal PCBs and omission error, inverse association between maternal and breast milk PCBs and ADHD related behaviors. | ||||||
| Nowack (2015), Germany [91] | ✔ | 116 | ✔ | 32 w | Maternal Serum | 9–10 y | ✔ | Inverse association between maternal PCB concentrations and Social Responsiveness Scale scores, mainly in girls. | ||||||
| Sagiv (2015), United States [102] | ✔ | 622 | ✔ | 26.7±2.6 w Delivery 9 y |
Maternal Serum Cord Serum Child Serum |
9 y 10.5 y 12 y |
✔ | Prenatal ∑PBDEs (BDE-47, −99, −100, and −153) were associated with poorer attention at ages 9–12 years, while no associations were observed between ∑PBDE concentrations in child serum and measures of attention in school-age children. | ||||||
| Sovcikova (2015), Slovakia [89] | ✔ | 307 | ✔ | 8–9 y | Child Serum | 8–9 y | ✔ | Positive association between serum PCB concentrations and parameters μ and τ of the ex-Gaussian distribution of simple reaction time. | ||||||
| Verner (2015), United States [86] | ✔ | 441 | ✔ | Delivery 0–12m | Cord Serum Estimates for postnatal |
8 y | ✔ | Positive associations between cord serum PCB-153 and ADHD-related behaviors in quantile regression at 75th percentile. Association for postnatal PCB-153 was attenuated. | ||||||
| Verner (2015), Canada [87] | ✔ | 98 | ✔ | Delivery | Cord Plasma | 5 y | ✔ | Null association between cord plasma PCB-153 and inattention, but estimated PCB-153 levels at 2 months were associated with increased duration of inattention. | ||||||
| Wang (2015), China [105] | ✔ | 98 | ✔ | 4–5 y | House Dust | 4–5 y | ✔ | BDE-209 and total-BDEs (BDE-99, BDE-153, and BDE-209) were positively associated with depressive behavior problems and lower social development quotients. | ||||||
| Caspersen (2016), Norway [83] | ✔ | 1024 | ✔ | 22 w | Food Frequency Questionnaire | 3.5 y | ✔ | ✔ | Estimated PCB intake during pregnancy was related to lower language skills in girls but not boys. Null association with ADHD, IQ, or executive function. | |||||
| Forns (2016), Norway [99] | ✔ | 548 | ✔ | Median: 32 d | Breastmilk | 12 m | ✔ | In a mixture model of 6 persistent organic pollutants that were selected by elastic net and Bayesian model averaging, null associations were noted between BDE-28 and BDE-47 and behavioral problems at 12 months. | ||||||
| Braun (2017), United States [92] | ✔ | 346 | ✔ | 16±3 w | Maternal Serum | 1–8 y | ✔ | ✔ | Prenatal BDE-47 was associated lower MDI scores at ages 1–3 years and decreased FSIQ at ages 5 and 8 years. Positive associations were also reported between prenatal BDE-47 and externalizing behaviors. | |||||
| Castorina (2017), United States [108] | ✔ | 310 | ✔ | 26 w | Maternal Urine | 7 y | ✔ | ✔ | Inverse associations between DPHP and Full Scale IQ and Working Memory. Positive association between ip-PPP and hyperactivity scores. |
|||||
| Lipscomb (2017), United States [100] | ✔ | 72 | ✔ | ✔ | 3–5 y | Silicone Passive Sampler | 3–5 y | ✔ | ∑PBDEs (BDE-28, −33, −47, −49, −99, −153, and −154) were not associated with externalizing problems in preschool children, while there was a positive relationship with ∑OPFRs (TDCIPP, TPHP, TCPP, and TCEP). | |||||
| Rosenquist (2017), Greenland and Ukraine [85] | ✔ | ✔ | 23–25 w First 12 months after delivery |
Maternal Serum Pharmacokinetic model for postnatal PCBs |
5 and 9 y | ✔ | Pooled adjusted ORs between prenatal and postnatal PCB-153 and abnormal behavior scores for Greenland and Ukraine cohorts did not reveal a statistically significant relationship. | |||||||
| Vuong (2017), United States [103] | ✔ | 214 | ✔ | 16±3 w 1,2,3,5, and 8 y |
Maternal Serum Child Serum |
8 y | ✔ | ✔ | No statistically significant associations were reported between prenatal or postnatal PBDE concentrations and measures of inattention or impulsivity in children at age 8 years. | |||||
| Vuong (2017), United States [93] | ✔ | 208 | ✔ | 1,2,3,5, and 8 y | Child Serum | 8 y | ✔ | ✔ | Postnatal PBDEs were associated with decreased FSIQ and increased hyperactivity and aggressive behaviors at age 8 years. | |||||
| Zhang (2017), United States [94] | ✔ | 239 | ✔ | 16±3 w | Maternal Serum | 8 y | ✔ | ✔ | A 10-fold increase in prenatal ∑PBDEs was associated with a decrement of 5.3-points (95% CI −10.6, −0.02) in FSIQ. Prenatal BDE-153 were associated with increased externalizing problems in children at age 8 years. |
|||||
| Berghuis (2018), Denmark [82] | ✔ | 101 | ✔ | ✔ | ✔ | 35 w | Maternal Serum | 13–15 y | ✔ | ✔ | Prenatal HBCD was associated with decreased total intelligence in children 13–15 years. While PBDEs were not associated with cognition, BDE-153 was observed to decrease sustained auditory attention. | |||
| Kim (2018), South Korea [95] | ✔ | 59 41 |
✔ | Not specified | Maternal Serum Breastmilk |
13–24 m | ✔ | ✔ | Maternal serum BDE-47 was associated with more externalizing behaviors among infants. No associations were noted between PBDEs and MDI. | |||||
| Oulhote (2018), United States [97] | ✔ | 333 | ✔ | 6–13 w | Maternal Serum | 6.9±0.9 m | ✔ | Increased concentrations of prenatal PBDEs were associated with more negative vocalizations in infants. Delayed physical reactivity was also noted with higher PBDEs, but only among boys. | ||||||
| Bernardo (2019), Canada [90] | ✔ | 546 | ✔ | First Trimester | Maternal Plasma | 3–4 y | ✔ | Highest PCB-153 quartile was associated with increased risk of having Social Responsiveness Scale score > 60. | ||||||
| de Water (2019), United States [107] | ✔ | 34 | ✔ | 12.2±2.8 w | Maternal Serum | 5 y | ✔ | Prenatal PBDEs were associated with increased global efficiency in areas of the brain that are involved with visual attention at age 5 years. Increased global efficiency in this area of the brain is associated with more executive function problems. | ||||||
| Doherty (2019), United States [109] | ✔ | 227 149 |
✔ | 27 w | Maternal Urine | 2–3 y | ✔ | Inverse associations between maternal ip-PPP concentrations and MSEL Cognitive Composite Scores and MB-CDI Vocabulary scores. | ||||||
| Doherty (2019), United States [110] | ✔ | 199 | ✔ | 27 w | Maternal Urine | 3 y | ✔ | Positive associations between maternal urinary BDCIPP concentrations and Behavioral Symptoms Index and Externalizing Problems. Inverse association between maternal urinary ip-PPP concentrations and Internalizing Problems. | ||||||
| Ji (2019), China [98] | ✔ | 199 307 |
✔ | Delivery | Cord Serum | 2 y 4 y |
✔ | Cord PBDEs were not associated with externalizing problems, but there was an increase in aggressive behaviors at age 2 years. Cord PBDEs were positively associated with emotional reactivity and internalizing behaviors, but only among girls. | ||||||
| Ruel (2019), Netherlands [84] | ✔ | 181 | ✔ | ✔ | ✔ | 35 w | Maternal Serum | 18 m 30 m |
✔ | Prenatal PCB-153 was associated with a delayed MDI score at 18 months. No associations were observed between PBDEs or HBCD and MDI. | ||||
| Margolis (2020), United States [106] | ✔ | 33 | ✔ | 12.2±2.8 w | Maternal Serum | 5 y | ✔ | Inverse associations were reported between prenatal PBDEs and global efficiency of the reading network. Increased global efficiency of the reading network was associated with improved word reading, suggesting prenatal PBDEs may be involved in reading development. | ||||||
Abbreviations: BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; d, days; DPHP, diphenyl phosphate; ip-PPP, isopropylphenyl phenyl phosphate; m, months; MB-CDI, MacArthur-Bates Communicative Development Inventories; MDI, mental development index; MSEL, Mullen Scales of Early Learning; TCEP, tris(2-chloroethyl) phosphate; TCPP, tris(1-chloro-2-propyl), TDCIPP, tris(1,3-dichloro-2-propyl) phosphate; TPHP, tri-phenylphosphate; w, weeks; y, years
5. Historical flame retardants (PCBs)
5.1. Cognition
In the Development at Adolescence and Chemical Exposure (DACE) Study, prenatal exposure to PCB-183 was associated with higher risk of subclinical cognitive impairment (IQ≤85), and PCB-105, −138, and −183 concentrations were inversely associated with verbal memory at ages 13–15 years [82]. In the Norwegian Mother and Child Cohort Study (MoBa), the estimated maternal dietary exposure during pregnancy to dioxin-like PCBs or PCB-153 was associated with reduced language skills in girls at age 3.5 years, but not IQ [83]. Using two cohorts in the Netherlands (Risk of Endocrine Contaminants on Human Health [RENCO] Study and the Groningen Infant Comparison of Exposure-Effect Pathways to Improve the Assessment of Human Health Risks of Complex Environmental Mixtures of Organohalogens [GIC] Study), Ruel et al. observed a significant association between prenatal PCB-153 concentrations and having a delayed mental development index (MDI) score at age 18 months [84].
5.2. Behavior
ADHD-related behaviors have been examined in several studies. In the Inuit and European (INUENDO) birth cohort, pooled estimates for mother-child pairs from Greenland and Ukraine indicated no statistically significant relationship between concentrations of prenatal and postnatal PCB-153 and abnormal behavior scores at ages 5–9 years [85]. In contrast, cord serum PCB-153 concentrations were significantly associated with increased ADHD behaviors at age 8 years; postnatal exposures had weaker associations [86]. Another study in Inuit preschoolers identified an association between PCB-153 concentrations at 2 months, but not cord plasma concentrations, and inattention at age 5 years [87]. In the Duisburg Birth Cohort Study, prenatal exposure to PCBs were related to increased omission errors on a computer-based test battery of attention performance (KITAP), but reduced ADHD behaviors among children at ages 8–9 years [88]. The MoBa Study did not find associations of estimated prenatal exposure to dioxin-like PCBs or PCB-153 with ADHD and executive function at age 3.5 years [83]. A cross-sectional study in a PCB polluted region in Slovakia found that serum concentrations were related to longer simple reaction time at ages 8–9 years [89]. Two studies examining prenatal PCB exposure and Social Responsiveness Scale (SRS) scores, indicative of more autistic behaviors, had conflicting findings [90, 91]. Specifically, an inverse association was reported between prenatal PCB concentrations among girls at ages 9–10 years, while a positive association was observed among children at ages 3–4 years [90, 91].
6. Contemporary flame retardants (PBDEs and HBCD)
6.1. Cognition
In the HOME Study, investigators reported adverse associations between prenatal and postnatal concentrations of PBDEs and full scale IQ (FSIQ) in children [92–94]. A 10-fold increase in prenatal ∑PBDEs (BDE-47, −99, −100, and −153) was associated with a 5.3-point decrease (95% Confidence Interval [CI] −10.6, −0.02) in FSIQ at age 8 years [94]. Braun et al. [92] further examined the persistence of prenatal PBDEs’ role in longitudinal patterns of child cognition. Prenatal BDE-47 was associated with lower mental development index (MDI) at ages 1–3 years and FSIQ at ages 5 and 8 years. In addition, child serum concentrations of PBDEs, quantified at ages 1, 2, 3, 5, and 8 years, were associated with lower FSIQ at age 8 years [93]. Decrements in FSIQ were noted with higher BDE-153 concentrations measured at multiple time points during childhood [93]. In contrast, the DACE Study reported null associations between prenatal PBDEs (BDE-47, −99, −100, −153, and −154) and the risk of subclinical cognitive impairment (IQ<85) in adolescents at ages 13–15 years, but BDE-154 and HBCD were associated with lower verbal memory and total intelligence in continuous outcome analysis, respectively [82]. In a prospective cohort in the Netherlands, Ruel et al. reported no associations between PBDEs or HBCD measured in maternal serum at 35 weeks gestation and MDI scores at 18 and 30 months [84]. In the Children’s Health and Environmental Chemicals in Korean (CHECK) Study, maternal serum PBDEs were not associated with MDI at 13–24 months [95]. Findings between PBDE concentrations measured in breastmilk yielded similar null findings with MDI scores at 13–24 months [95].
6.2. Behavior
While prenatal PBDEs were not associated with neurobehavioral infant profiles at 5 weeks in the HOME Study [96], Oulhote et al. [97] reported that infants enrolled in the Maternal-Infant Research on Environmental Chemicals (MIREC) cohort displayed more negative vocalizations at 6.9±0.9 months, including incidences of crying and screaming, with increased concentrations of prenatal PBDEs. Poorer emotional reactivity at ages 2 and 4 years was additionally reported in the Shanghai-Minhang Birth Cohort Study with higher concentrations of cord serum PBDEs [98].
Positive associations were additionally reported between prenatal PBDEs and externalizing problems from early childhood to age 8 years in the HOME Study [92, 94]. A 10-fold increase in BDE-153 was associated with a 3.9-point increase in Externalizing Problems score at age 8 years [94]. Similar findings were reported by Kim et al. [95] in the CHECK Study, with increased maternal serum BDE-47 associated with more externalizing behaviors in children at 13–24 months. Findings regarding postnatal PBDEs and externalizing behaviors are inconsistent. In the South Korean cohort, breastmilk PBDEs were not associated with externalizing problems in infants [95]. In addition, Forns et al. [99] utilized a multi-pollutant model to examine six persistent organic pollutant concentrations measured during infancy and behavioral problems at 12 months and reported no statistically significant relationships between breastmilk concentrations of BDE-28 or BDE-47 and behavioral problems. A cross-sectional study of 72 children between ages 3–5 years also reported null findings between PBDEs, quantified via a silicone passive sampler that was worn for 7 days, and externalizing behaviors [100]. In contrast, the HOME Study found associations between PBDEs and increased externalizing behaviors (hyperactivity and aggressive behaviors) at age 8 years [93].
A handful of studies have examined PBDEs and childhood attention, though conclusions were discordant among cohort studies. Prenatal BDE-47 and BDE-153 were associated with more attention problems at age 4 years, though findings were no longer statistically significant when children were 6 years in the New York City cohort [101]. However, maternal serum BDE-153 was associated with poorer attention in adolescents aged 13–15 years in a Danish cohort [82]. In the CHAMACOS Study, prenatal PBDEs were associated with poorer attention in children ages 9–12 years, while no statistically significant relationship was observed with child serum PBDEs [102]. In the HOME Study, findings were suggestive of a potential relationship between prenatal and concurrent PBDE concentrations and inattention in children at age 8 years [103].
Few epidemiological studies have investigated PBDEs’ relationship with social skills and internalizing behaviors. Cord serum BDE-47 was associated with poorer social skills at 1–2 years in a prospective birth cohort in rural China [104]. A statistically significant relationship was also observed in the Shanghai-Minhang Birth Cohort between cord PBDEs and internalizing behaviors, but this association was only present among girls ages 2 and 4 years [98]. In a cross-sectional study of PBDE household dust levels from urban dwellings in Nanjing, China, BDE-209 and total-BDEs (BDE-99, BDE-153, and BDE-209) were associated with poorer social skills and more depressive behaviors at ages 4–5 years [105].
6.3. Neuroimaging
Two preliminary epidemiological studies utilizing participants from the ongoing birth cohort, Endocrine Disruption in Pregnant Women: Thyroid Disruption and Infant Development Study, investigated prenatal PBDEs and the brain’s intrinsic functional network organization involved with executive function and reading development [106, 107]. Resting state functional magnetic resonance imaging (fMRI) was used to examine whether prenatal PBDEs were associated with the intrinsic functional network at age 5 years in a sample of 33–34 children [106, 107]. Prenatal PBDEs were associated with increased global efficiency in areas of the brain that are involved with visual attention [107]. Children with increased global efficiency in this area of the brain were reported to experience more executive function problems. Margolis et al. [106] reported inverse associations between prenatal PBDEs and global efficiency of the reading network, indicating poorer word reading. While there was no statistically significant association between prenatal PBDEs and reading skills in this study, the findings suggest that prenatal PBDEs plays a role in altering network integration, which may result in downstream reading problems [106].
Neuroimaging studies investigating PBDEs would enhance our understanding of the long-term neurodevelopment effects of PBDEs that may not be evident at earlier ages. These preliminary neuroimaging studies provide findings that indicate PBDEs may be involved in altering the brain’s network architecture and intrinsic connectivity.
7. Current-use flame retardants (OPFRs)
7.1. Cognition
Maternal urinary concentrations of diphenyl phosphate (DPHP), a metabolite of TPHP, were inversely associated with FSIQ (−2.9 points, 95% CI: −6.3, 0.5 for a 10-fold exposure increase) and Working Memory (−3.9 points, 95% CI: −7.3, −0.5 for a 10-fold exposure increase) at age 7 years in the CHAMACOS cohort [108]. In the third phase of the Pregnancy, Infection, and Nutrition (PIN3) Study, maternal urinary concentrations of isopropyl-phenyl phenyl phosphate (ip-PPP), a metabolite of mono-IT), but not metabolites of TDCIPP or TPHP, were inversely associated with Composite, Fine Motor, and Expressive Language scores from the Mullen Scales of Early Learning (MSEL) as well as Vocabulary score from the MacArthur-Bates Communicative Development Inventories (MB-CDI) at ages 2–3 years [109].
7.2. Behavior
In the CHAMACOS cohort, maternal urinary concentrations of ip-PPP were positively associated with Hyperactivity scores in the mother-reported Behavior Assessment System for Children-2 (BASC-2), but not teacher reports. Metabolites of TDCIPP and TPHP were not associated with BASC-2 scores or ADHD Index assessed by Conners’ ADHD/DSM-IV Scales assessed at age 7 years [108]. In the PIN3 Study, maternal urinary concentrations of bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), a metabolite of TDCIPP, were positively associated with Behavioral Symptoms Index and Externalizing Problems scores assessed by BASC-2 at age 3 years. Higher ip-PPP concentrations, however, were associated with lower Internalizing Problems scores [110]. Another cross-sectional study using silicon wrist band to assess OPFR exposure identified less responsible behavior and more externalizing behavior problems associated with exposure at age 3–5 years [100].
8. Recommendations
Overall, findings from epidemiological studies within the past five years demonstrate that PBDEs have potential neurotoxic effects, particularly with exposures occurring during gestational development. Epidemiological studies from the United States provide evidence that supports prenatal and postnatal PBDE concentrations may adversely impact childhood intelligence, with findings suggesting that prenatal PBDEs’ neurotoxicity may persist throughout childhood [92–94]. However, cohort studies from Denmark and South Korea present null results. Discrepancies may be due to differences in the body burden of PBDEs between countries. In the HOME Study, prenatal BDE-47 had a median (IQR) of 19.1 (11–34.5) ng/g lipid compared to 0.9 (0.5–1.3) ng/g lipid in the DACE Study, 1.1 (<LOQ-2.1) ng/g lipid in the CHECK Study, and 0.8 (0.5–1.3) ng/g lipid in the RENCO and GIC Studies [82, 111, 84].
Recent epidemiological findings provide additional evidence supporting the hypothesis that prenatal PBDEs are associated with externalizing behaviors. However, the role of postnatal PBDEs are still unclear. Only the HOME Study reported positive associations with externalizing behaviors. Other studies investigating this relationship measured PBDEs at one time point shortly after birth in breastmilk samples or during childhood via a personal silicone passive sampler. Differences in exposure assessment methods and timing may have contributed to the discrepant findings. With regard to PBDEs and attention problems, the findings were varied. Although there is some evidence to suggest that prenatal PBDEs may be associated with more attention problems in children, there is limited evidence on postnatal exposures. Lastly, despite the limited number of studies, suggestive evidence indicates exposure to PBDEs is associated with elevated internalizing behaviors and impaired social skills. Epidemiological findings regarding current-use OPFRs are limited, but provide suggestive evidence of a relationship with neurodevelopment in children. Greater understanding of FR neurotoxicity can be achieved if future studies focus on current-use FRs as limited research thus far have examined pre- and postnatal concentrations of OPFRs and neurodevelopment. Further, the National Academies of Sciences, Engineering, and Medicine (NASEM) have called for the evaluation of OPFRs, putting forth a scoping plan for toxicity assessment [112]. Secondly, very few studies investigating FR neurotoxicity have utilized advanced statistical multi-pollutant models, taking into account the totality of FR exposures. Historical and contemporary FRs have long biological half-lives. Thus, it would be prudent to examine the total impact of FR exposures on cognition and behavior in children. In addition, limited studies have explored potential sexual dimorphism and even fewer have employed neuroimaging to study FR neurotoxicity.
8.1. Current-use flame retardants
OPFR production has increased to 341,000 tons worldwide, more than tripling from 1992 to 2007 [113]. In the US, OPFR production increased from 14,000 tons annually during the mid-1980s to almost 40,000 tons in 2012 [114]. OPFRs have been detected in household dust, cars, air conditioner filters, baby products, and furniture in several countries, including the US, Kuwait, New Zealand, Pakistan, Saudi Arabia, and Sweden [115–121]. Since OPFRs have a low vapor pressure and are hydrophobic [122, 123], they tend to partition into organic matter, such as indoor dust, which is a major source of human exposure [124, 12]. OPFR diester metabolites are now universally detected in urine samples [27, 125, 126], and children have almost 5 times higher urine levels of BDCIPP compared to their mothers [127]. There is sufficient evidence from toxicological studies to warrant concerns regarding OPFRs’ impact on neurodevelopment [128]. As such, future epidemiological studies should investigate whether OPFRs are associated with cognitive and behavioral development, focusing on both prenatal and postnatal exposures. Delayed action regarding removal of PBDEs from the market (i.e., taking over 40 years) resulted in widespread and persistent human exposures. Epidemiological studies investigating OPFR neurotoxicity are necessary so that the similar scenario is not repeated.
Associations between OPFRs and neurodevelopment may be sexually dimorphic, but few epidemiological studies have assessed whether effect modification by sex is present [108, 110, 109]. Sex may also modify OPFR neurotoxicity as there is evidence that OPFR exposure alters thyroid hormones in a sex-dependent measure. TDCIPP and TPHP exposure in adult zebrafish was reported to significantly decrease plasma triiodothyronine and thyroxine in males, while increases were noted in females [129]. TPHP exposure was additionally observed to increase total thyroxine levels, particularly in women, in a sample of 51 office workers in the Boston, MA area [130]. Further, OPFR bioaccumulation was shown to differ sexually in crucian carp, with female eggs having a higher perfusion rate of tri-n-butyl phosphate (TNBP) compared to male gonad concentrations [131].
8.2. Cumulative assessment of FRs using multi-pollutant models
Currently, no epidemiological study has investigated the full extent of all FR exposures, including historical, contemporary, and current-use FRs, on children’s neurodevelopment. Given that most past-use FRs have long half-lives, each compound may act alone or in conjunction with other neurotoxicants to impact brain development. Several advanced mixture models are available to estimate individual and aggregate exposures, identify important mixture components, determine whether non-monotonic relationships exist, and assess whether interactions are present between chemicals [132]. However, no epidemiological study has used advanced statistical methods to evaluate mixtures of FRs. While some studies examined chemical mixtures, they did not comprehensively examine FR compounds [99, 94, 106, 107]. Therefore, given the abundance of toxicological evidence to support the neurotoxicity of FRs, it is pertinent that future epidemiological studies comprehensively estimate associations of FR compounds taking into account potential additive, synergistic, and antagonistic effects.
8.3. Assessment of neurodevelopmental effects utilizing neuroimaging
Neuroimaging could contribute to our ability to investigate FR neurotoxicity by providing a method to examine brain structure and functionality, thus enhancing causal inference and identifying potential biological pathways altered by different FRs. This application advances our understanding by providing an assessment of developmental trajectories of cognition and behavior in children that cannot be achieved via traditional methods of assessment [133]. The field of pediatric neuroimaging is growing, and epidemiological studies examining FR neurotoxicity have begun to apply these techniques to further understand the potential downstream neurodevelopmental effects that may not be evident with assessments using neurodevelopmental batteries. To date, only two preliminary studies have applied neuroimaging to examine PBDE neurotoxicity; both studies yielded interesting findings despite small sample sizes [106, 107]. Neuroimaging has challenges that contribute to its application, including high costs, limited availability in some countries, practical difficulties, such as claustrophobia and motion, especially in young children, and differences in protocols which limit the ability to pool data. Despite this, there is immense promise in the knowledge and mechanistic insights that can be garnered, specifically regarding brain plasticity and developmental trajectories [134].
9. Conclusions
In summary, continuing studies on historically used FRs still reveal long-term adverse impacts of PCB exposures decades after the ban. Recent published studies on PBDE neurotoxicity confirm prenatal exposures are associated with poorer cognition and more externalizing problems in children. Evidence from epidemiological studies indicate that PBDEs may impact attention, internalizing behaviors, and social skills. PBDEs may also alter the intrinsic functional network organization of the brain, resulting in downstream effects on various neurodevelopmental domains, such as reading and executive function. Limited and inconsistent conclusions from epidemiological studies examining postnatal PBDEs make it difficult to conclude that exposures during childhood are as detrimental as those occurring during gestation. Limited studies on OPFRs have indicated adverse impact on child cognitive function, hyperactivity, and externalizing behaviors, calling for more research on this class of FRs and child neurobehavioral development.
However, there is sufficient evidence to justify that a coordinated global effort be taken to reduce FR exposure in humans, because sensitive life stages for brain development should be protected. Further, the fire safety benefit of incorporating FRs in consumer products is questionable [135]. California revised the 1975 flammability standard, Technical Bulletin (TB 117) with TB117-2013. This update replaces the open flame test with the smolder test, which allows furniture to meet fire safety standards without the need of adding FRs, suggesting that these chemicals may not be needed. The phase-out of PBDEs was an important step in decreasing exposure to these neurotoxicants. However, PBDEs remain an important public health problem even though it has been over a decade after its removal from the market. OPFRs are rapidly following its predecessor by making their presence in environmental and human samples universally known. While epidemiological studies are still investigating OPFR neurotoxicity, delaying mitigating actions for several decades – as was done with PBDEs – is an injustice to children’s health and will likely result in another regrettable substitution.
Human and Animal Rights:
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Funding
National Institute of Environmental Health Sciences (P01 ES11261, R01 ES020349, R01 ES024381, R01 ES025214, R01 ES014575, R01ES028277) and Environmental Protection Agency (P01 R829389)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21(1):1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs). J Toxicol Environ Health A. 2006;69(1–2):21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- 4.Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20(6):440–96. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin Med Res. 2003;1(4):281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czerska M, Zielinski M, Kaminska J, Ligocka D. Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int J Occup Med Environ Health. 2013;26(4):498–510. doi: 10.2478/s13382-013-0138-7. [DOI] [PubMed] [Google Scholar]
- 7.Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79(3):172–83. [PubMed] [Google Scholar]
- 8.EPA. Polybrominated diphenyl ethers (PBDEs) significant new use rules (SNUR). 2013. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/polybrominated-diphenylethers-pbdes-significant-new-use. 2019.
- 9.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46(5):583–624. [DOI] [PubMed] [Google Scholar]
- 10.Kemmlein S, Herzke D, Law RJ. Brominated flame retardants in the European chemicals policy of REACH-Regulation and determination in materials. J Chromatogr A. 2009;1216(3):320–33. doi: 10.1016/j.chroma.2008.05.085. [DOI] [PubMed] [Google Scholar]
- 11.Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA et al. Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol. 2011;256(3):281–9. doi: 10.1016/j.taap.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–53. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 13.Rice D, Barone S Jr, Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghassabian A, Trasande L. Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front Endocrinol (Lausanne). 2018;9:204. doi: 10.3389/fendo.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorman DC, Chiu W, Hales BF, Hauser R, Johnson KJ, Mantus E et al. Polybrominated diphenyl ether (PBDE) neurotoxicity: a systematic review and meta-analysis of animal evidence. J Toxicol Environ Health B Crit Rev. 2018;21(4):269–89. doi: 10.1080/10937404.2018.1514829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill KL, Hamers T, Kamstra JH, Willmore WG, Letcher RJ. Organophosphate triesters and selected metabolites enhance binding of thyroxine to human transthyretin in vitro. Toxicol Lett. 2018;285:87–93. doi: 10.1016/j.toxlet.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Jung D, Jo A, Ji K, Moon HB, Choi K. Long-term exposure to triphenylphosphate alters hormone balance and HPG, HPI, and HPT gene expression in zebrafish (Danio rerio). Environ Toxicol Chem. 2016;35(9):2288–96. doi: 10.1002/etc.3395. [DOI] [PubMed] [Google Scholar]
- 18.Xu T, Wang Q, Shi Q, Fang Q, Guo Y, Zhou B. Bioconcentration, metabolism and alterations of thyroid hormones of Tris(1,3-dichloro-2-propyl) phosphate (TDCPP) in Zebrafish. Environ Toxicol Pharmacol. 2015;40(2):581–6. doi: 10.1016/j.etap.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Lai NL, Wang X, Guo Y, Lam PK, Lam JC et al. Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environ Sci Technol. 2015;49(8):5123–32. doi: 10.1021/acs.est.5b00558. [DOI] [PubMed] [Google Scholar]
- 20.Gu Y, Yang Y, Wan B, Li M, Guo LH. Inhibition of O-linked N-acetylglucosamine transferase activity in PC12 cells - A molecular mechanism of organophosphate flame retardants developmental neurotoxicity. Biochem Pharmacol. 2018;152:21–33. doi: 10.1016/j.bcp.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119(7):900–7. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klocke C, Sethi S, Lein PJ. The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): similarities and differences. Environ Sci Pollut Res Int. 2019. doi: 10.1007/s11356-019-06723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zieminska E, Ruszczynska A, Lazarewicz JW. Tetrabromobisphenol A disturbs zinc homeostasis in cultured cerebellar granule cells: A dual role in neurotoxicity. Food Chem Toxicol. 2017;109(Pt 1):363–75. doi: 10.1016/j.fct.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Shi X, Zha J, Wen B, Zhang S. Diastereoisomer-specific neurotoxicity of hexabromocyclododecane in human SH-SY5Y neuroblastoma cells. Sci Total Environ. 2019;686:893–902. doi: 10.1016/j.scitotenv.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Costa LG, de Laat R, Tagliaferri S, Pellacani C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett. 2014;230(2):282–94. doi: 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ait Bamai Y, Bastiaensen M, Araki A, Goudarzi H, Konno S, Ito S et al. Multiple exposures to organophosphate flame retardants alter urinary oxidative stress biomarkers among children: The Hokkaido Study. Environ Int. 2019;131:105003. doi: 10.1016/j.envint.2019.105003. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int. 2014;63:169–72. doi: 10.1016/j.envint.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosarac I, Kubwabo C, Foster WG. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1014:24–30. doi: 10.1016/j.jchromb.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Castorina R, Butt C, Stapleton HM, Avery D, Harley KG, Holland N et al. Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere. 2017;179:159–66. doi: 10.1016/j.chemosphere.2017.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin MY, Lee S, Kim HJ, Lee JJ, Choi G, Choi S et al. Polybrominated Diphenyl Ethers in Maternal Serum, Breast Milk, Umbilical Cord Serum, and House Dust in a South Korean Birth Panel of Mother-Neonate Pairs. Int J Environ Res Public Health. 2016;13(8). doi: 10.3390/ijerph13080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stasinska A, Heyworth J, Reid A, Callan A, Odland JO, Trong Duong P et al. Polybrominated diphenyl ether (PBDE) concentrations in plasma of pregnant women from Western Australia. Sci Total Environ. 2014;493:554–61. doi: 10.1016/j.scitotenv.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Adetona O, Horton K, Sjodin A, Jones R, Hall DB, Aguillar-Villalobos M et al. Concentrations of select persistent organic pollutants across pregnancy trimesters in maternal and in cord serum in Trujillo, Peru. Chemosphere. 2013;91(10):1426–33. doi: 10.1016/j.chemosphere.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vizcaino E, Grimalt JO, Lopez-Espinosa MJ, Llop S, Rebagliato M, Ballester F. Polybromodiphenyl ethers in mothers and their newborns from a non-occupationally exposed population (Valencia, Spain). Environ Int. 2011;37(1):152–7. doi: 10.1016/j.envint.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Meijer L, Weiss J, Van Velzen M, Brouwer A, Bergman A, Sauer PJ. Serum concentrations of neutral and phenolic organohalogens in pregnant women and some of their infants in The Netherlands. Environ Sci Technol. 2008;42(9):3428–33. doi: 10.1021/es702446p. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73(7):1036–41. doi: 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 37.Junque E, Garcia S, Martinez MA, Rovira J, Schuhmacher M, Grimalt JO. Changes of organochlorine compound concentrations in maternal serum during pregnancy and comparison to serum cord blood composition. Environ Res. 2019;182:108994. doi: 10.1016/j.envres.2019.108994. [DOI] [PubMed] [Google Scholar]
- 38.Tang J, Zhai JX. Distribution of polybrominated diphenyl ethers in breast milk, cord blood and placentas: a systematic review. Environ Sci Pollut Res Int. 2017;24(27):21548–73. doi: 10.1007/s11356-017-9821-8. [DOI] [PubMed] [Google Scholar]
- 39.Ding J, Xu Z, Huang W, Feng L, Yang F. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci Total Environ. 2016;554–555:211–7. doi: 10.1016/j.scitotenv.2016.02.171. [DOI] [PubMed] [Google Scholar]
- 40.Ma J, Zhu H, Kannan K. Organophosphorus Flame Retardants and Plasticizers in Breast Milk from the United States. Environ Sci Technol Lett. 2019;6(9):525–31. doi: 10.1021/acs.estlett.9b00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeKoning EP, Karmaus W. PCB exposure in utero and via breast milk. A review. J Expo Anal Environ Epidemiol. 2000;10(3):285–93. doi: 10.1038/sj.jea.7500090. [DOI] [PubMed] [Google Scholar]
- 42.Grandjean P, Budtz-Jorgensen E, Barr DB, Needham LL, Weihe P, Heinzow B. Elimination half-lives of polychlorinated biphenyl congeners in children. Environ Sci Technol. 2008;42(18):6991–6. doi: 10.1021/es800778q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosen DH, Flanders WD, Friede A, Humphrey HE, Sinks TH. Half-life of polybrominated biphenyl in human sera. Environ Health Perspect. 1995;103(3):272–4. doi: 10.1289/ehp.95103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geyer HJ, Schramm K, Darnerud PO, Aune M, Feicht EA, Fried KW et al. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 2004;66. [Google Scholar]
- 45.Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114(2):176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parry E, Zota AR, Park JS, Woodruff TJ. Polybrominated diphenyl ethers (PBDEs) and hydroxylated PBDE metabolites (OH-PBDEs): A six-year temporal trend in Northern California pregnant women. Chemosphere. 2018;195:777–83. doi: 10.1016/j.chemosphere.2017.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P et al. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ Health Perspect. 2017;125(8):086001. doi: 10.1289/EHP1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Horm Behav. 2018;101:94–104. doi: 10.1016/j.yhbeh.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect. 2008;116(10):1416–22. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobson JL, Jacobson SW. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology. 1997;18(2):415–24. [PubMed] [Google Scholar]
- 51.Chen YC, Guo YL, Hsu CC. Cognitive development of children prenatally exposed to polychlorinated biphenyls (Yu-Cheng children) and their siblings. J Formos Med Assoc. 1992;91(7):704–7. [PubMed] [Google Scholar]
- 52.Gascon M, Verner MA, Guxens M, Grimalt JO, Forns J, Ibarluzea J et al. Evaluating the neurotoxic effects of lactational exposure to persistent organic pollutants (POPs) in Spanish children. Neurotoxicology. 2013;34:9–15. doi: 10.1016/j.neuro.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ Health Perspect. 2012;120(6):904–9. doi: 10.1289/ehp.1104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143(6):780–8. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- 55.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335(11):783–9. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 56.Jacobson JL, Jacobson SW, Humphrey HE. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J Pediatr. 1990;116(1):38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- 57.Tatsuta N, Nakai K, Murata K, Suzuki K, Iwai-Shimada M, Kurokawa N et al. Impacts of prenatal exposures to polychlorinated biphenyls, methylmercury, and lead on intellectual ability of 42-month-old children in Japan. Environ Res. 2014;133:321–6. doi: 10.1016/j.envres.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 58.Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ Health. 2010;9:51. doi: 10.1186/1476-069X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patandin S, Lanting CI, Mulder PG, Boersma ER, Sauer PJ, Weisglas-Kuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J Pediatr. 1999;134(1):33–41. doi: 10.1016/s0022-3476(99)70369-0. [DOI] [PubMed] [Google Scholar]
- 60.Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol Teratol. 2003;25(1):11–22. [DOI] [PubMed] [Google Scholar]
- 61.Gladen BC, Rogan WJ. Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethene on later development. J Pediatr. 1991;119(1 Pt 1):58–63. doi: 10.1016/s0022-3476(05)81039-x. [DOI] [PubMed] [Google Scholar]
- 62.Gray KA, Klebanoff MA, Brock JW, Zhou H, Darden R, Needham L et al. In utero exposure to background levels of polychlorinated biphenyls and cognitive functioning among school-age children. Am J Epidemiol. 2005;162(1):17–26. doi: 10.1093/aje/kwi158. [DOI] [PubMed] [Google Scholar]
- 63.Boucher O, Bastien CH, Saint-Amour D, Dewailly E, Ayotte P, Jacobson JL et al. Prenatal exposure to methylmercury and PCBs affects distinct stages of information processing: an event-related potential study with Inuit children. Neurotoxicology. 2010;31(4):373–84. doi: 10.1016/j.neuro.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 2010;171(5):593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plusquellec P, Muckle G, Dewailly E, Ayotte P, Begin G, Desrosiers C et al. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology. 2010;31(1):17–25. doi: 10.1016/j.neuro.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol Teratol. 2005;27(6):771–80. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Vreugdenhil HJ, Mulder PG, Emmen HH, Weisglas-Kuperus N. Effects of perinatal exposure to PCBs on neuropsychological functions in the Rotterdam cohort at 9 years of age. Neuropsychology. 2004;18(1):185–93. doi: 10.1037/0894-4105.18.1.185. [DOI] [PubMed] [Google Scholar]
- 68.Chen YC, Yu ML, Rogan WJ, Gladen BC, Hsu CC. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am J Public Health. 1994;84(3):415–21. doi: 10.2105/ajph.84.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N et al. Prenatal exposure to environmental contaminants and behavioural problems at age 7–8years. Environ Int. 2013;59:225–31. doi: 10.1016/j.envint.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 70.Newman J, Behforooz B, Khuzwayo AG, Gallo MV, Schell LM, Akwesasne Task Force on the E. PCBs and ADHD in Mohawk adolescents. Neurotoxicol Teratol. 2014;42:25–34. doi: 10.1016/j.ntt.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A et al. Gestational Exposure to Endocrine-Disrupting Chemicals and Reciprocal Social, Repetitive, and Stereotypic Behaviors in 4- and 5-Year-Old Children: The HOME Study. Environ Health Perspect. 2014. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E et al. Response inhibition and error monitoring during a visual go/no-go task in inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environ Health Perspect. 2012;120(4):608–15. doi: 10.1289/ehp.1103828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo YL, Chen YC, Yu ML, Hsu CC. Early development of Yu-Cheng children born seven to twelve years after the Taiwan PCB outbreak. Chemosphere. 1994;29(9–11):2395–404. doi: 10.1016/0045-6535(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 74.Rogan WJ, Gladen BC. PCBs, DDE, and child development at 18 and 24 months. Ann Epidemiol. 1991;1(5):407–13. doi: 10.1016/1047-2797(91)90010-a. [DOI] [PubMed] [Google Scholar]
- 75.Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121(2):257–62. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chopra V, Harley K, Lahiff M, Eskenazi B. Association between phthalates and attention deficit disorder and learning disability in U.S. children, 6–15 years. Environ Res. 2014;128:64–9. doi: 10.1016/j.envres.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjodin A et al. Prenatal Polybrominated Diphenyl Ether Exposures and Neurodevelopment in U.S. Children through 5 Years of Age: The HOME Study. Environ Health Perspect. 2014;122(8):856–62. doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M et al. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int. 2011;37(3):605–11. doi:S0160–4120(10)00250–3 [pii] 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Adgent MA, Hoffman K, Goldman BD, Sjodin A, Daniels JL. Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatr Perinat Epidemiol. 2014;28(1):48–57. doi: 10.1111/ppe.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gump BB, Yun S, Kannan K. Polybrominated diphenyl ether (PBDE) exposure in children: possible associations with cardiovascular and psychological functions. Environ Res. 2014;132:244–50. doi: 10.1016/j.envres.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.•.Berghuis SA, Van Braeckel K, Sauer PJJ, Bos AF. Prenatal exposure to persistent organic pollutants and cognition and motor performance in adolescence. Environ Int. 2018;121(Pt 1):13–22. doi: 10.1016/j.envint.2018.08.030.. [DOI] [PubMed] [Google Scholar]; The only study on maternal HBCD exposure and child cognitive function and motor performance in adolescence.
- 83.Caspersen IH, Aase H, Biele G, Brantsaeter AL, Haugen M, Kvalem HE et al. The influence of maternal dietary exposure to dioxins and PCBs during pregnancy on ADHD symptoms and cognitive functions in Norwegian preschool children. Environ Int. 2016;94:649–60. doi: 10.1016/j.envint.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 84.•.Ruel MVM, Bos AF, Soechitram SD, Meijer L, Sauer PJJ, Berghuis SA. Prenatal exposure to organohalogen compounds and children’s mental and motor development at 18 and 30 months of age. Neurotoxicology. 2019;72:6–14. doi: 10.1016/j.neuro.2019.01.003. [DOI] [PubMed] [Google Scholar]; The only study on maternal HBCD exposure and early childhood mental and motor function.
- 85.Rosenquist AH, Hoyer BB, Julvez J, Sunyer J, Pedersen HS, Lenters V et al. Prenatal and Postnatal PCB-153 and p,p’-DDE Exposures and Behavior Scores at 5–9 Years of Age among Children in Greenland and Ukraine. Environ Health Perspect. 2017;125(10):107002. doi: 10.1289/EHP553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verner MA, Hart JE, Sagiv SK, Bellinger DC, Altshul LM, Korrick SA. Measured Prenatal and Estimated Postnatal Levels of Polychlorinated Biphenyls (PCBs) and ADHD-Related Behaviors in 8-Year-Old Children. Environ Health Perspect. 2015;123(9):888–94. doi: 10.1289/ehp.1408084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verner MA, Plusquellec P, Desjardins JL, Cartier C, Haddad S, Ayotte P et al. Prenatal and early-life polychlorinated biphenyl (PCB) levels and behavior in Inuit preschoolers. Environ Int. 2015;78:90–4. doi: 10.1016/j.envint.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Neugebauer J, Wittsiepe J, Kasper-Sonnenberg M, Schoneck N, Scholmerich A, Wilhelm M. The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: results from the Duisburg Birth Cohort Study. Int J Hyg Environ Health. 2015;218(1):153–62. doi: 10.1016/j.ijheh.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Sovcikova E, Wimmerova S, Stremy M, Kotianova J, Loffredo CA, Murinova LP et al. Simple reaction time in 8–9-year old children environmentally exposed to PCBs. Neurotoxicology. 2015;51:138–44. doi: 10.1016/j.neuro.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Bernardo BA, Lanphear BP, Venners SA, Arbuckle TE, Braun JM, Muckle G et al. Assessing the Relation between Plasma PCB Concentrations and Elevated Autistic Behaviours using Bayesian Predictive Odds Ratios. Int J Environ Res Public Health. 2019;16(3). doi: 10.3390/ijerph16030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nowack N, Wittsiepe J, Kasper-Sonnenberg M, Wilhelm M, Scholmerich A. Influence of Low-Level Prenatal Exposure to PCDD/Fs and PCBs on Empathizing, Systemizing and Autistic Traits: Results from the Duisburg Birth Cohort Study. PLoS One. 2015;10(6):e0129906. doi: 10.1371/journal.pone.0129906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC et al. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology. 2017;62:192–9. doi: 10.1016/j.neuro.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.•.Vuong AM, Yolton K, Xie C, Webster GM, Sjodin A, Braun JM et al. Childhood polybrominated diphenyl ether (PBDE) exposure and neurobehavior in children at 8 years. Environ Res. 2017;158:677–84. doi: 10.1016/j.envres.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; This prospective birth cohort reported that childhood PBDE concentrations were associated with decreased FSIQ as well as more externalizing behaviors, utilizing multiple informant models that took into account prenatal PBDE concentrations.
- 94.Zhang H, Yolton K, Webster GM, Sjodin A, Calafat AM, Dietrich KN et al. Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Environ Health Perspect. 2017;125(4):746–52. doi: 10.1289/EHP478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim S, Eom S, Kim HJ, Lee JJ, Choi G, Choi S et al. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2years of age- CHECK cohort study. Sci Total Environ. 2018;624:377–84. doi: 10.1016/j.scitotenv.2017.12.058. [DOI] [PubMed] [Google Scholar]
- 96.Donauer S, Chen A, Xu Y, Calafat AM, Sjodin A, Yolton K. Prenatal exposure to polybrominated diphenyl ethers and polyfluoroalkyl chemicals and infant neurobehavior. J Pediatr. 2015;166(3):736–42. doi: 10.1016/j.jpeds.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oulhote Y, Tremblay E, Arbuckle TE, Fraser WD, Lemelin JP, Seguin JR et al. Prenatal exposure to polybrominated diphenyl ethers and predisposition to frustration at 7months: Results from the MIREC study. Environ Int. 2018;119:79–88. doi: 10.1016/j.envint.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 98.Ji H, Liang H, Wang Z, Miao M, Wang X, Zhang X et al. Associations of prenatal exposures to low levels of Polybrominated Diphenyl Ether (PBDE) with thyroid hormones in cord plasma and neurobehavioral development in children at 2 and 4years. Environ Int. 2019;131:105010. doi: 10.1016/j.envint.2019.105010. [DOI] [PubMed] [Google Scholar]
- 99.Forns J, Mandal S, Iszatt N, Polder A, Thomsen C, Lyche JL et al. Novel application of statistical methods for analysis of multiple toxicants identifies DDT as a risk factor for early child behavioral problems. Environ Res. 2016;151:91–100. doi: 10.1016/j.envres.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 100.•.Lipscomb ST, McClelland MM, MacDonald M, Cardenas A, Anderson KA, Kile ML. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ Health. 2017;16(1):23. doi: 10.1186/s12940-017-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; First cross-sectional study using silicone wristbands to examine OPFR exposure and child behavioral development.
- 101.Cowell WJ, Lederman SA, Sjodin A, Jones R, Wang S, Perera FP et al. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3–7 years. Neurotoxicol Teratol. 2015;52(Pt B):143–50. doi: 10.1016/j.ntt.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.•.Sagiv SK, Kogut K, Gaspar FW, Gunier RB, Harley KG, Parra K et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicol Teratol. 2015;52(Pt B):151–61. doi:S0892–0362(15)30019–2 [pii] 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this propsective birth cohort, repeated measures of PBDEs from gestation to school-age were examined in relation to attention at ages 9–12. Only prenatal exposure to PBDEs were adversely associated with measures of attention.
- 103.Vuong AM, Yolton K, Poston KL, Xie C, Webster GM, Sjodin A et al. Prenatal and postnatal polybrominated diphenyl ether (PBDE) exposure and measures of inattention and impulsivity in children. Neurotoxicol Teratol. 2017;64:20–8. doi: 10.1016/j.ntt.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding G, Yu J, Cui C, Chen L, Gao Y, Wang C et al. Association between prenatal exposure to polybrominated diphenyl ethers and young children’s neurodevelopment in China. Environ Res. 2015;142:104–11. doi: 10.1016/j.envres.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 105.Wang BL, Pang ST, Zhang XL, Li XL, Sun YG, Lu XM et al. Levels of polybrominated diphenyl ethers in settled house dust from urban dwellings with resident preschool-aged children in Nanjing, China. Arch Environ Contam Toxicol. 2015;68(1):9–19. doi: 10.1007/s00244-014-0065-z. [DOI] [PubMed] [Google Scholar]
- 106.••.Margolis AE, Banker S, Pagliaccio D, De Water E, Curtin P, Bonilla A et al. Functional connectivity of the reading network is associated with prenatal polybrominated diphenyl ether concentrations in a community sample of 5 year-old children: A preliminary study. Environ Int. 2020;134:105212. doi: 10.1016/j.envint.2019.105212. [DOI] [PMC free article] [PubMed] [Google Scholar]; The second preliminary study to investigate the relationship between prenatal PBDEs and neuroimaging. Findings support an adverse relationship with poorer word reading, as evidenced by the obsevered inverse association between PBDEs and global efficiency in a particular area of the brain that plays a role in reading development.
- 107.••.de Water E, Curtin P, Zilverstand A, Sjodin A, Bonilla A, Herbstman JB et al. A preliminary study on prenatal polybrominated diphenyl ether serum concentrations and intrinsic functional network organization and executive functioning in childhood. J Child Psychol Psychiatry. 2019;60(9):1010–20. doi: 10.1111/jcpp.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]; The findings indicate that prenatal PBDE exposure is positively associated with global efficiency in an area of the brain where higher global efficiency is related with more executive function impairments.
- 108.••.Castorina R, Bradman A, Stapleton HM, Butt C, Avery D, Harley KG et al. Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere. 2017;189:574–80. doi: 10.1016/j.chemosphere.2017.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; First comprehensive assessment of maternal OPFR exposure and child neurodevelopment in a longitudinal birth cohort. The findings suggest developmental neurotoxicity of in utero OPFR exposure in school age children.
- 109.••.Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD et al. Prenatal exposure to organophosphate esters and cognitive development in young children in the Pregnancy, Infection, and Nutrition Study. Environ Res. 2019;169:33–40. doi: 10.1016/j.envres.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large birth cohort study of maternal OPFR exposure during pregnancy and child cognitive development at 2–3 years, suggesting impairments in cognitive composite and vocabulary.
- 110.••.Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD et al. Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study. Neurotoxicology. 2019;73:150–60. doi: 10.1016/j.neuro.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; A prospective investigation of maternal OPFR exposure during pregnancy and child behavior in early pregnancy. Findings suggest adverse impact on Behavioral Symptoms Index and externalizing problems.
- 111.Choi G, Kim S, Kim S, Kim S, Choi Y, Kim HJ et al. Occurrences of major polybrominated diphenyl ethers (PBDEs) in maternal and fetal cord blood sera in Korea. Sci Total Environ. 2014;491–492:219–26. doi: 10.1016/j.scitotenv.2014.02.071. [DOI] [PubMed] [Google Scholar]
- 112.•.National Academies of Sciences E, and Medicine. A Class Approach to Hazard Assessment of Organohalogen Flame Retardants. Washington, DC: The National Academies Press; 2019. [PubMed] [Google Scholar]; Comprehensive report by an expert panel on approaches to evaluate organohalogen flame retardants in general. This report provides the advantages and disadvantages of subcategorizing organohalogen flame retardants in risk assessment.
- 113.Greaves AK, Letcher RJ. A Review of Organophosphate Esters in the Environment from Biological Effects to Distribution and Fate. Bull Environ Contam Toxicol. 2017;98(1):2–7. doi: 10.1007/s00128-016-1898-0. [DOI] [PubMed] [Google Scholar]
- 114.Schreder ED, Uding N, La Guardia MJ. Inhalation a significant exposure route for chlorinated organophosphate flame retardants. Chemosphere. 2016;150:499–504. doi: 10.1016/j.chemosphere.2015.11.084. [DOI] [PubMed] [Google Scholar]
- 115.Ali N, Dirtu AC, Van den Eede N, Goosey E, Harrad S, Neels H et al. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere. 2012;88(11):1276–82. doi: 10.1016/j.chemosphere.2012.03.100. [DOI] [PubMed] [Google Scholar]
- 116.Ali N, Ali L, Mehdi T, Dirtu AC, Al-Shammari F, Neels H et al. Levels and profiles of organochlorines and flame retardants in car and house dust from Kuwait and Pakistan: implication for human exposure via dust ingestion. Environ Int. 2013;55:62–70. doi: 10.1016/j.envint.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Ali N, Eqani S, Ismail IMI, Malarvannan G, Kadi MW, Albar HMS et al. Brominated and organophosphate flame retardants in indoor dust of Jeddah, Kingdom of Saudi Arabia: Implications for human exposure. Sci Total Environ. 2016;569–570:269–77. doi: 10.1016/j.scitotenv.2016.06.093. [DOI] [PubMed] [Google Scholar]
- 118.Percy Z, La Guardia MJ, Xu Y, Hale RC, Dietrich KN, Lanphear BP et al. Concentrations and loadings of organophosphate and replacement brominated flame retardants in house dust from the home study during the PBDE phase-out. Chemosphere. 2019;239:124701. doi: 10.1016/j.chemosphere.2019.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Persson J, Wang T, Hagberg J. Organophosphate flame retardants and plasticizers in indoor dust, air and window wipes in newly built low-energy preschools. Sci Total Environ. 2018;628–629:159–68. doi: 10.1016/j.scitotenv.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 120.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45(12):5323–31. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43(19):7490–5. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bergman A, Ryden A, Law RJ, de Boer J, Covaci A, Alaee M et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ Int. 2012;49:57–82. doi: 10.1016/j.envint.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao Z, Xu F, Covaci A, Wu M, Wang H, Yu G et al. Distribution patterns of brominated, chlorinated, and phosphorus flame retardants with particle size in indoor and outdoor dust and implications for human exposure. Environ Sci Technol. 2014;48(15):8839–46. doi: 10.1021/es501224b. [DOI] [PubMed] [Google Scholar]
- 124.Liagkouridis I, Cousins AP, Cousins IT. Physical-chemical properties and evaluative fate modelling of ‘emerging’ and ‘novel’ brominated and organophosphorus flame retardants in the indoor and outdoor environment. Sci Total Environ. 2015;524–525:416–26. doi: 10.1016/j.scitotenv.2015.02.106. [DOI] [PubMed] [Google Scholar]
- 125.Cooper EM, Covaci A, van Nuijs AL, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(7):2123–32. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van den Eede N, Neels H, Jorens PG, Covaci A. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J Chromatogr A. 2013;1303:48–53. doi: 10.1016/j.chroma.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 127.Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol. 2014;48(17):10432–8. doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- 128.Doherty BT, Hammel SC, Daniels JL, Stapleton HM, Hoffman K. Organophosphate Esters: Are These Flame Retardants and Plasticizers Affecting Children’s Health? Curr Environ Health Rep. 2019;6(4):201–13. doi: 10.1007/s40572-019-00258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu X, Cai Y, Wang Y, Xu S, Ji K, Choi K. Effects of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish. Ecotoxicol Environ Saf. 2019;170:25–32. doi: 10.1016/j.ecoenv.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 130.Preston EV, McClean MD, Claus Henn B, Stapleton HM, Braverman LE, Pearce EN et al. Associations between urinary diphenyl phosphate and thyroid function. Environ Int. 2017;101:158–64. doi: 10.1016/j.envint.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Choo G, Cho HS, Park K, Lee JW, Kim P, Oh JE. Tissue-specific distribution and bioaccumulation potential of organophosphate flame retardants in crucian carp. Environ Pollut. 2018;239:161–8. doi: 10.1016/j.envpol.2018.03.104. [DOI] [PubMed] [Google Scholar]
- 132.Lazarevic N, Barnett AG, Sly PD, Knibbs LD. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ Health Perspect. 2019;127(2):26001. doi: 10.1289/EHP2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE et al. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann N Y Acad Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 135.Shaw SD, Blum A, Weber R, Kannan K, Rich D, Lucas D et al. Halogenated flame retardants: do the fire safety benefits justify the risks? Rev Environ Health. 2010;25(4):261–305. doi: 10.1515/reveh.2010.25.4.261. [DOI] [PubMed] [Google Scholar]


