Supplemental Digital Content is available in the text.
Introduction:
Sickle cell disease is a complex chronic disorder associated with increased morbidity and early mortality. The Pediatric Quality Measures Program has developed new sickle cell-specific quality measures focused on hydroxyurea (HU) counseling and annual transcranial Doppler (TCD) screening; however, these measures have not been used in a clinical setting to inform quality improvement (QI) efforts.
Methods:
From 2017 to 2018, 9 sickle cell subspecialty clinics from the Pacific Sickle Cell Regional Collaborative conducted a year-long QI collaborative focused on improving the percentage of patients with HU counseling and TCD screening based on the new quality measures. After an initial kick-off meeting, the 9 sites participated in monthly conference calls. We used run charts annotated with plan-do-study-act cycle activities to track each site’s monthly progress and the overall mean percentage for the entire collaborative.
Results:
There was an overall improvement in the aggregate HU counseling from 85% to 98% (P < 0.01). For TCD screening, referral frequency changed from 85% to 90% (P = 0.76). For both measures, the variation in frequencies decreased over the year.
Conclusion:
Over 1 year, we found that a regional QI collaborative increased HU counseling. Although referral for TCD screening increased, there was no overall change in TCD completion. Overall, this QI report’s findings can help clinicians adopt and implement these quality measures to improve outcomes in children.
INTRODUCTION
Sickle cell disease includes a group of inherited, chronic disorders [including sickle cell anemia (SCA—HbSS, HbSβ0 thalassemia), HbSC, HbSβ+-thalassemia] which are associated with increased morbidity and early mortality.1 There are evidence-based interventions that can improve pediatric outcomes. For example, hydroxyurea (HU) therapy can decrease complications related to SCA, such as pain, acute chest syndrome and anemia.2 Annual transcranial Doppler (TCD) ultrasound screening for children with SCA, when coupled with chronic transfusion therapy for those at highest risk, can decrease the incidence of stroke.3
Despite evidence-based recommendations for the management of SCA,4 there are gaps in current care. For example, based on Medicaid administrative data from 6 states, only 18% of children with SCA were noted to have consistent HU therapy, based on prescription refill records.5 Also, annual TCD screening rates for children with SCA are estimated to range from only 22% to 44%.6
To improve the quality of pediatric SCA care, the Pediatric Quality Measures Program has developed 2 new quality measures for SCA that focus on (1) counseling regarding the benefits and risks of HU, and (2) annual TCD screening.7 Though the American Academy of Pediatrics recommends that the new pediatric quality measures “are useful for local quality improvement (QI) activities as well as for reporting to state and federal agencies and other regulatory bodies,”8 there are limited data about the usability of these new measures. Our objective was to examine if a multi-state QI collaborative composed of sickle cell subspecialty clinics could utilize these new PQMP quality measures for SCA care.
METHODS
Context
The Pacific Sickle Cell Regional Collaborative (PSCRC) includes 15 clinical sites in the Western United States. It is 1 of 5 regional collaboratives funded by the Department of Health and Human Services Health Resources and Services Administration Sickle Cell Disease Treatment Demonstration Program.9 PSCRC sites include community hospitals and clinics, academic medical centers, and federally qualified health centers.
PSCRC leadership includes clinicians with regional collaboration experience to standardize, improve, and refine sickle cell disease care. Previous projects include tracking barriers to HU uptake, developing HU educational materials, and creating state action plans to improve sickle cell disease care. PSCRC site leads present lectures and clinical case presentations on monthly telementoring videoconferences, aligned with the Extension for Community Healthcare Outcomes Project model that brings best-practice care to underserved areas.10
The existing collaborative structures provided a venue for the QI collaborative work to test the SCA measures usability. The QI collaborative started with a 1-day in-person meeting. It continued for a year, with monthly conference calls, plan-do-study-act (PDSA) cycles, and data collection every other month.
Case Identification
Clinic staff at each site used manual chart reviews every 2 months to generate data on performance for both measures for children with SCA based on provider documentation. For the HU measure, participating clinics identified patients 9 months and older with SCA seen in the clinic during the previous 2 months. For the TCD measure, participating clinics identified children with SCA 2–15 years of age seen during the preceding 2 months. Participating clinicians collected data on the percentage of eligible children who had received anticipatory guidance regarding the risks and benefits of treatment with HU within the last 12 months. Also, each site collected data on the percentage of children referred for TCD and the percentage of children who had documentation of a completed TCD within the last 12 months in the medical record.
If a patient was currently prescribed HU, “counseling” was defined as a documented discussion of the risks and benefits of continued therapy, reviewing labs and discussing the results with the patient, a parent or guardian, or any other family member, and/or reviewing the patient’s adherence. If a patient was not currently prescribed HU, “counseling” was defined as a discussion of the rationale for HU treatment with the patient, a parent or guardian, or any other family member, and/or a review of the patient’s rationale for declining HU and/or a review of the patient’s medical reasons for ineligibility.
TCD referral for screening (“TCD referral”) was defined as any documentation by a healthcare provider of patient referral for TCD screening to decrease stroke risk. Completed TCD screening (“TCD completion”) was defined as documentation of TCD screening results noted in the patient’s medical record.
Interventions
We used the Model for Improvement as the QI framework for the project.11 Participating PSCRC practices were asked to identify a team from their practice. Practice teams typically included 1 or more clinicians, an office manager, and clinic staff (medical assistant or RN). Four sites included a family partner (eg, parent of a child in the practice with SCA). The family partner provided additional ideas from a family perspective for clinic interventions, particularly concerning educational materials.
In August 2017, we conducted an 8-hour face-to-face QI kick-off meeting over 2 days within the PSCRC annual meeting in Sacramento, CA. This initial meeting included a review of SCA care evidence, goals of clinical QI measures, a practical review of QI theory and methods, and a review of baseline data that was collected on current performance on HU counseling and TCD referral measures. During the meeting, based on baseline data, the collaborative team members developed specific collaborative goals to increase the percentage of patients meeting the measures. Each site also developed a PDSA cycle for a test of change tailored to their site. Some PDSA cycles attempted to address the specific clinic characteristics (eg, availability of radiology and staffing), changes in staff workflow, or interventions to modify patient behavior (eg, patient reminders).
From August 2017 to September 2018, teams at each site conducted a minimum of at least 3 PDSA cycles, with most completing at least 6 cycles (one every 2 months). Every other month, teams at each site collected data on at least 10 patients or all patients if the volume was less than 10, via manual chart review as described above. Some patients were evaluated more than once if they had multiple visits in the same year. During the 12-month intervention period, the PSCRC collaborative conducted ten 1-hour webinar conferences that occurred approximately once per month. The webinars were hosted by a moderator who was familiar with QI techniques. During the monthly webinars, the collaborative members reviewed individual site data and overall collaborative performance. Leaders at each of the sites shared their lessons learned from their PDSA cycles and were encouraged to apply lessons learned at other sites to their sites, as applicable.12
Analysis
We used run charts (percentage of charts with documented TCD referral and HU counseling within the last 12 months), annotated with PDSA cycle activities, to track the results monthly for each site, and the mean percentage for the entire collaborative. We tracked the range of frequencies reported by the sites every other month.
Based on consensus and a review of baseline data, the collaborative participants felt that achieving benchmarks of 90% of patients with documented for HU counseling and 90% for TCD referral within the last 12 months would be a clinically significant goal. Since referral does not always guarantee that a test will be completed, we also collected data on TCD completion percentage documented in the medical record.
Due to the small number of patients at many sites, reflecting the low prevalence of SCA in any given geographical area, we did not collect monthly data, nor were we able to construct statistical process control charts. However, we used chi-square and Fisher Exact tests to compare the percentage of patients counseled for HU, referred for TCD screening, and completed TCD screening at the baseline and final cycle.
Maintenance of Certification credit was provided to qualified participants who completed the QI collaborative requirements. The Institutional Review Boards at the University of California, San Francisco, and at the Albert Einstein College of Medicine approved this project.
RESULTS
Participation
From August 2017 to July 2018, 9 PSCRC clinics participated in the QI collaborative (Table 1). Of the 9 clinics, 3 clinics treated only adult patients, 5 treated only pediatric patients, and 1 treated both adult and pediatric patients. Since HU counseling recommendations applied to adult and pediatric patients, all 3 adult-only clinics focused on the HU counseling measure and only supplied data regarding HU counseling frequencies. All 6 of the pediatric clinics provided data on both HU counseling and TCD referral.
Table 1.
Participating Clinics
| Clinic | Population | Quality Focus | Annual Sickle Cell Patient Volume* | |
|---|---|---|---|---|
| Pediatric | Adult | |||
| A | Peds and adult | HU | 34 | 36 |
| B | Peds | HU, TCD | 267 | — |
| C | Peds | TCD | 251 | — |
| D | Peds | HU, TCD | 129 | — |
| E | Peds | HU, TCD | 48 | — |
| F | Peds | HU, TCD | 27 | — |
| G | Adult | HU | — | 234 |
| H | Adult | HU | — | 142 |
| I | Adult | HU | — | 33 |
Peds indicates pediatric.
*Based on 2016–2018 data. Pediatric volumes reflects patients 0–19 years of age. Adult volumes reflect patients greater than 19 years old for clinics that include both adult and pediatric patients. All clinics were subspecialty clinics in hematology.
PDSA Cycles to Improve HU Counseling
For the HU measure, there was an overall improvement in the aggregate data. At baseline, 85% of patients had documented HU counseling in the last 12 months. The counseling percentage gradually increased each cycle to 98% (P < 0.01 for comparison of beginning to the end of the collaborative performance). Also, there was more consistency in reporting over time. The reported clinic counseling percentages varied by 60 points (40%–100%) at the beginning of the intervention to a range of 10% age points (90%–100%) at the end. Individually, 4 clinics showed an improvement, 3 showed no improvement, and 2 showed a decline in the percentage of patients with a history of HU counseling. In the 2 clinics with a decline, both had a baseline counseling rate of 100% that declined to 90%.
Figure 1 shows the changes in the HU counseling percentage and ranges with the different PDSA interventions initiated by the sites. The individual clinics tested various interventions throughout the year. Many clinics first implemented the changes with only one provider or panel of patients before expanding the interventions or training other providers.
Fig. 1.
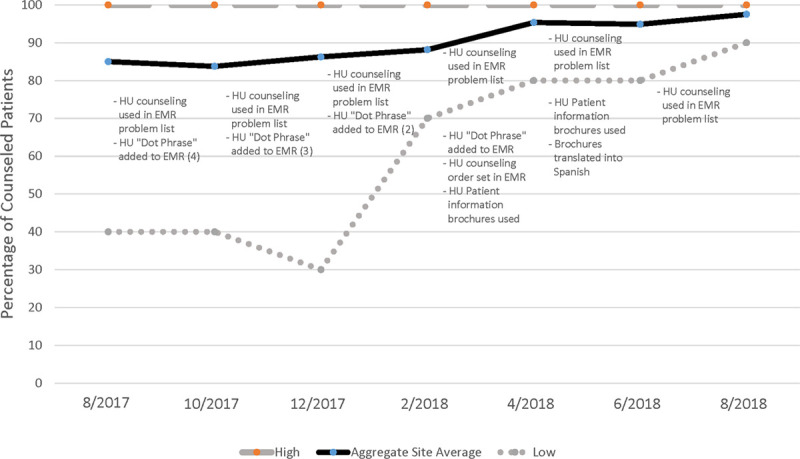
Changes in HU counseling with PDSA interventions initiated. Numbers in parentheses indicate the number of clinics that attempted the intervention during the cycle.
Several different interventions focused on the use of the electronic medical record (EMR). For example, several clinics had EMRs that allowed clinicians to modify an individual patient’s problem list. One intervention was to add “HU counseling” to the patient problem list to remind clinicians to counsel about HU. In 1 case, the clinic incorporated a standard order set for HU counseling into the EMR. Another clinic implemented an order set in the EMR to coordinate HU counseling with other clinic personnel.
Because the EMR could also be modified to create a standardized set of extended text for clinical documentation, this provided the impetus for clinicians to create HU counseling documentation which could be easily imported into a note, using an abbreviated word or phrase (eg, a “dot phrase” in the EPIC EHR; Epic Systems, Verona, Wis.). Early on, the QI collaborative developed a consensus on what constituted adequate counseling in specific circumstances (eg, initial counseling of HU, discussion of HU for patients who have previously declined HU) and standard language to describe HU counseling (See Appendix 1, Supplemental Digital Content 1, http://links.lww.com/PQ9/A231). The development of this dot-phrase created standardization of practice and documentation. It increased the ease of medical record review. Multiple clinics applied this intervention during the year.
Finally, many clinics were already utilizing patient education information and brochures to facilitate the discussion of different topics. One clinic initiated the development of an HU patient information brochure for use in HU counseling.
PDSA Cycles to Improve TCD Referral Frequency
At baseline, for the TCD referral measure, 85% of patients had been referred for TCD screening in the last 12-month period. This percentage e fluctuated over the 12-month intervention period, from a low of 80% to a high of 97%, with a final mean of 90% (P = 0.75). As with HU counseling, more consistency was observed with the range of reported percentages. The range of values decreased from 40% age points (60%–100%) to 20% age points (80%–100%) at the end of the intervention period. Individually, 3 clinics showed an improvement; 1 showed no improvement, and 2 showed a temporary improvement and then a decline in the frequency of TCD referral.
Figure 2 shows the changes in the percentages of patients referred for TCD in the last 12-month period and reported ranges with the different PDSA interventions initiated by the sites. Several interventions focused on reminders for scheduling the TCD test. Initially, these interventions focused on reminding the patient’s family via telephone 1 week before the clinic visit to schedule a TCD test before the clinic visit. However, over the year, these interventions evolved to clinics assisting patients in scheduling the TCD test at the end of the visit, to clinic staff scheduling the TCD test on behalf of the patient. Towards the latter part of the intervention year, clinic nurses and scheduling staff in 2 clinics began tracking patients with overdue TCD test and addressing follow-up issues. In 1 clinic, the physician and an RN navigator would discuss which patients needed reminders and referrals for TCD testing in the coming month. Besides, 1 clinic provided additional training to clinic staff regarding the need for TCD testing and the testing interval. Reminders were also directed at physicians. One site modified the EMR by adding “TCD screening” and due date to the patient problem list to remind clinicians to discuss and order TCD testing.
Fig. 2.
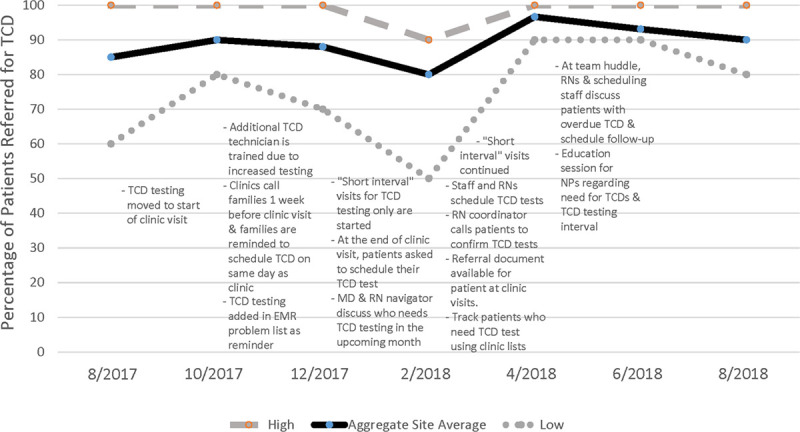
Changes in TCD Screening with PDSA Interventions Initiated. Numbers in parentheses indicate the number of clinics that attempted the intervention during the cycle.
One site had the capability and staff to conduct TCD testing as part of its routine clinical service (Fig. 3). During the first PDSA cycle, this clinic re-organized the visit workflow to begin with the TCD test. In the second PDSA cycle, this clinic added a second ultrasound TCD technician to the clinic staff to decrease missed screenings if the sole technician was not available. Towards the end of the intervention period, the clinic noted that patients were not obtaining TCD screening within 1 year. As a result, clinic visits only for TCD testing were initiated. These “short interval” visits ensured that TCD testing could occur within a 1-year interval. Both the frequency of referrals for TCD and the completion of TCDs increased in this clinic.
Fig. 3.
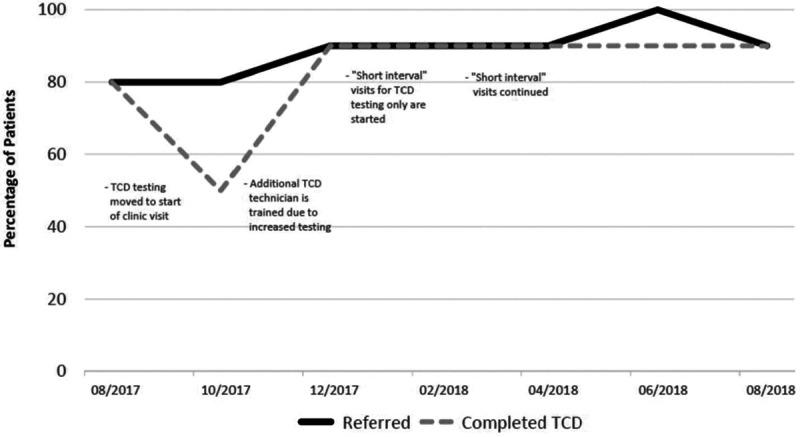
Run chart for the clinic with “in-house” TCD showing changes in TCD screening and completion.
Although the mean percentage of patients with documented TCD referrals increased, there was no change in the mean percentage of patients with a completed TCD screening documented in the medical record for the collaborative (Fig. 4). The baseline percentage of TCD completion documented in the medical record was 82%, fluctuated from 70% to 90% during the year, and ended at 82% (P = 1.00). The range of completed TCD percentages decreased from 60% age points (40%–100%) to 20% age points (70%–90%). Individually, 4 clinics showed an improvement, 1 showed no improvement, and 1 showed an overall decline in the percentage of documented completed TCD screening. For the single clinic with TCD testing capability, the percentage of documented completed TCD screening increased from 80% to 90% during the intervention period (Fig. 3).
Fig. 4.
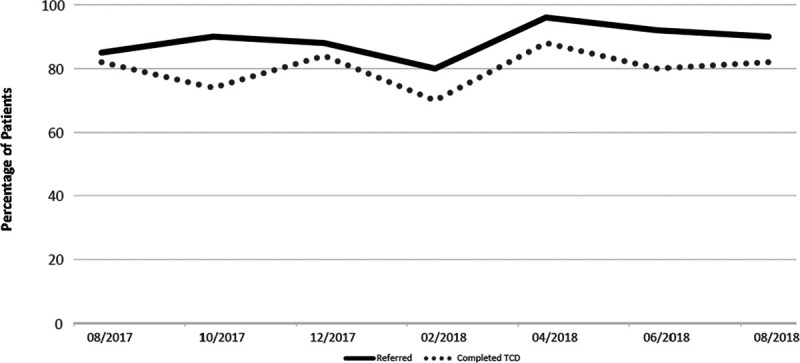
Mean percentage of TCD referral and TCD completion for collaborative.
DISCUSSION
Although sickle cell disease is the most common inherited blood disorder in the United States, few pediatric practice collaboratives focus exclusively on this condition. We found that a regional collaborative was able to improve the likelihood of HU counseling with SCA over 1 year. There was a change in aggregate HU counseling from 85% to 98%. However, for TCD referral, there was no statistically significant increase (85%–90%). Also, there was no change in the frequency of completed TCD testing documented in the medical record, which remained 82%.
There were variations of 3 successful interventions (eg, standardized documentation, EMR reminders or prompts, education materials) that were applied using PDSA cycles to improve HU counseling. Interventions focused on leveraging the EMR to remind clinicians to counsel about HU therapy and refer for TCD. EMR prompts have been used as reminders to enhance clinical practice for ordering vaccinations, screening tests, and referrals. Investment in EMR-based reminders may result in sustainable interventions.13–15
Another EMR intervention was to standardize the documentation of HU counseling. However, 1 issue with the HU counseling quality measure is that the definition of counseling can differ from clinician to clinician and by the patient situation. Clinical practice guidelines for the management of sickle cell disease state that clinicians should “educate” about HU therapy; however, the guidelines provide little detail about what should be discussed.4 At the start of the collaborative, the PSCRC developed a consensus regarding what constituted counseling based on different clinical situations. For example, the topics discussed during annual HU counseling may differ for a patient who is initially learning about HU versus a patient who continues to take HU; it may also differ by the patient’s age and sex. The collaborative structure facilitated achieving group consensus through monthly calls and clinicians testing the use of dot phrases in interval months.
To help sustain HU counseling, the standardization of HU documentation can help clinicians more quickly communicate the HU counseling status and what topics have already been discussed. Similarly, placement of HU counseling in a prominent place in the EMR can serve as a reminder and make the HU counseling documentation routine.
We noted that many interventions for TCD screening focused not on physician TCD referral, but also on facilitating patient adherence for TCD completion. For instance, interventions to improve TCD completion were initially designed to remind patients who were already scheduled for the clinic to set up their TCD testing individually. Later interventions focused on the clinic staff monitoring, of which patients needed TCD testing with active staff reminders for patient follow-up. Over the year, the interventions evolved from patient reminders to systems to track and engage patients.
There was a discordance in the percentage of patients referred for TCD compared to patients with a completed TCD. In most situations, patients with SCA need to go to a different clinical site or building for TCD testing. Few pediatric hematologists have on-site TCD available at the clinic. This situation adds further patient burden and time away from work. A national survey on TCD screening also noted that the distance to travel for testing was a barrier to completing TCD screening.16 Successful TCD screening requires multi-disciplinary care, which can be difficult to coordinate across different settings.
The one high-volume clinic in the collaborative with TCD testing available in the clinic improved TCD testing completion frequency. This experience supports having in-clinic access to TCD as a method to improve screening rates. This approach could potentially be used in other settings to justify the expense associated with this additional resource. For lower volume clinics, this approach may not be sustainable and may be more challenging to justify. These clinics may need to partner with health plans to coordinate a population health management and preventive care approach for children with sickle cell disease. The additional expenses would need to be balanced by the savings in costs and quality of life through stroke episodes averted through TCD screening. However, this is an area for additional research.
Limitations
It is difficult to compare the frequency of HU counseling and TCD referral with other published reports. For this QI report, we only included children seen in the clinic in the last 2 months versus including every child followed at each of the centers in our denominators. This difference likely explains why there was such a high proportion of children at baseline who had been offered HU and referred for a TCD compared to other studies.5,6
The PSCRC is a unique collaborative; however, the providers work in clinical sites that provide general pediatric hematology care, similar to clinics in large medical centers throughout the United States. The overall baseline performance of the PSCRC was higher than other clinics, based on other published reports.5,6 There may have been a “ceiling effect,” as the performance approached 100%. For example, several clinics already had high HU counseling levels, and the 2 sites that declined went from 100% to 90%. The overall effect of the different interventions may be more significant in other settings with lower baseline performance. Alternatively, the success may have been due to the characteristics of these high-performing clinics. Overall, the general strategies, such as reminder systems, staff engagement, and patient engagement, can be applied in any setting. For clinics that see lower volumes of patients with SCA, some interventions, such as additional staff for in-clinic TCD screening, may not be cost-effective.
The HU measure focused on documentation of provider counseling. Although this can be measured quantitatively, the measure does not assess counseling effectiveness, patient understanding, or patient adherence. Further work is needed to understand better how to measure and improve these subsequent facets of SCA care. Finally, the outcomes were based on medical record audit and were not externally validated by administrative claims data or by parent-report of actual physician counseling. However, data from the medical record are sufficient to assess the success of small tests of change through PDSA cycles.11
CONCLUSIONS
Both HU counseling and TCD screening strategies are highlighted in current practice guideline recommendations for SCA. We found that these quality measures can be used in QI initiatives in clinics that care for children with SCA. However, we did not see a significant change in TCD referral or TCD completion frequency. The limited ability of the clinics without TCD testing within the clinic to improve on the TCD completion measure suggests that TCD testing completion as a quality measure may be more appropriate at a health plan level. Overall, this QI report’s findings can help clinicians adopt and implement these quality measures to improve sickle cell disease outcomes in children.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGEMENTS
This work was funded by the Agency for Health Care Research & Quality (AHRQ) (U18HS025297) and the Health Resources and Services Administration (U1EMC27862).
Supplementary Material
Footnotes
Published online December 28, 2020
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
Selections from this manuscript were presented at the Pediatric Academic Societies Meeting, April 27, 2019, Baltimore, MD.
To cite: Cabana MD, Marsh A, Treadwell M, Stemmler P, Rowland M, Bender MA, Bhasin N, Chung JH, Hassell K, Nik Abdul Rashid NF, Wong TE, Bardach NS. Improving Preventive Care for Children With Sickle Cell Anemia: A Quality Improvement Initiative. Pediatr Qual Saf 2021;6:e379.
REFERENCES
- 1.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018; 4:18010. [DOI] [PubMed] [Google Scholar]
- 2.Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev. 2017; 4:CD002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MT, Piomelli S, Granger S, et al. ; STOP Study Investigators. Stroke prevention trial in sickle cell anemia (STOP): extended follow-up and final results. Blood. 2006; 108:847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Heart Lung and Blood Institute. Evidence-based Management of Sickle Cell Disease: Expert Panel Report, 2014. 2014. Bethesda, Md.: National Heart Lung and Blood Institute. United States Department of Health and Human Services, National Institutes of Health [Google Scholar]
- 5.Reeves SL, Jary HK, Gondhi JP, et al. Hydroxyurea use among children with sickle cell anemia. Pediatr Blood Cancer. 2019; 66:e27721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves SL, Madden B, Freed GL, et al. Transcranial Doppler screening among children and adolescents with sickle cell anemia. JAMA Pediatr. 2016; 170:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agency for Healthcare Research and Quality (AHRQ). Pediatric Quality Measures Program: Management of Chronic Conditions. https://www.ahrq.gov/pqmp/measures/management-of-chronic-conditions.html. Accessed May 18, 2019
- 8.Adirim T, Meade K, Mistry K; AAP Council on Quality Improvement and Patient Safety. A new era in quality measurement: the development and application of quality measures. Pediatrics. 2017; 139:e20163442. [DOI] [PubMed] [Google Scholar]
- 9.Pacific Sickle Cell Regional Collaborative. https://pacificscd.org/. Accessed May 18, 2019
- 10.Arora S, Kalishman S, Thornton K, et al. Project ECHO (project extension for community healthcare outcomes): a national and global model for continuing professional development. J Contin Educ Health Prof. 2016; 36(suppl 1):S48–S49 [DOI] [PubMed] [Google Scholar]
- 11.Lloyd R. On Demand: an Introduction to the Model for Improvement. 2016. Boston, MA: Institute for Healthcare Improvement [Google Scholar]
- 12.Nembhard IM. Learning and improving in quality improvement collaboratives: which collaborative features do participants value most? Health Serv Res. 2009; 44(2 Pt 1):359–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soon R, Sung S, Cruz MR, et al. Improving human papillomavirus (HPV) vaccination in the postpartum setting. J Community Health. 2017; 42:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hihi E, Shankweiler C, Stricklen D, et al. Electronic medical record alert improves HCV testing for baby boomers in primary care setting: adults born during 1945-1965. BMJ Open Qual. 2017; 6:e000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magill MK, Day J, Mervis A, et al. Improving colonoscopy referral rates through computer-supported, primary care practice redesign. J Healthc Qual. 2009; 31:43–52 [DOI] [PubMed] [Google Scholar]
- 16.Reeves SL, Fullerton HJ, Dombkowski KJ, et al. Physician attitude, awareness, and knowledge regarding guidelines for transcranial Doppler screening in sickle cell disease. Clin Pediatr (Phila). 2015; 54:336–345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


