Introduction:
Many published accounts have shown that quality improvement (QI) initiatives within medical practice settings can increase vaccination rates. Project ECHO is a telementoring platform that uses video conferencing technology to educate and support healthcare professionals through case-based learning and brief lectures. This manuscript explores the results of a learning collaborative focused on combining QI and Project ECHO to increase human papillomavirus (HPV) vaccination rates within pediatric practices.
Methods:
The American Academy of Pediatrics (AAP) recruited 3 AAP chapters that then recruited individual pediatricians and their practices for participation. Participants responded to surveys regarding chapter and pediatrician experience and satisfaction. Impact on HPV immunization rates (HPV initiation, series completion, and missed opportunities to vaccinate during visits) was measured using practice reports of chart reviews to AAP’s data aggregator, which produced run charts.
Results:
Thirty-four pediatricians within 8 practices completed the project; 1 practice withdrew. Physicians self-reported increased confidence in communicating with vaccine-hesitant families and implementing QI activities. We analyzed practice run charts utilizing QI run chart rules and found nonrandom change towards improvement for aggregate missed opportunities to vaccinate but not for HPV vaccine initiation or series completion.
Conclusions:
An HPV QI learning collaborative improved participant confidence in HPV vaccine communication and QI skills and decreased missed opportunities to vaccinate. Future projects should consider a more extended project period or more frequent data collection to reduce data variability to make it easier to spot nonrandom changes.
INTRODUCTION
Human papillomavirus (HPV) infection is associated with more than 43,000 new cancers in the United States annually, including cervical, vaginal, vulvar, penile, anal, and oropharyngeal cancers.1 To prevent these cancers, the Advisory Committee on Immunization Practices recommends HPV vaccination for all adolescents at age 11 or 12 years.2 While rates for HPV vaccination have continued to rise since 2008, national rates remain well below the Healthy People 2020 goal of 80% for completion of the HPV vaccine series.3 As of 2019, 71% of adolescents ages 13–17 years had started the series, and 54.2% were up-to-date.4 There are significant geographic disparities in HPV vaccination rates. The HPV vaccination rate of adolescents living in non-metropolitan statistical areas was almost ten percentage points lower than that of peers living in metropolitan statistical areas principal cities.4
Quality improvement (QI) initiatives can increase HPV vaccination rates.5,6 From 2014 to 2019, the American Academy of Pediatrics (AAP) implemented HPV vaccination QI projects across the United States through its network of chapters. In 2018, the Extension for Community Healthcare Outcomes (ECHO) model was integrated into existing QI projects, which aimed to teach pediatric providers QI methodology to improve HPV vaccination rates.
ECHO is a telementoring platform that uses video conferencing technology to educate and support healthcare professionals through case-based learning and brief lectures on various health conditions. Unlike telemedicine, this model does not establish a provider-patient relationship but builds primary care providers’ capacity to manage health conditions encountered within their scope of practice. The model started as a way to treat Hepatitis C in New Mexico and has expanded to address more than 100 diseases and conditions across 37 countries.7,8
In their 2018 report, the National Vaccine Advisory Committee recommended using telementoring platforms to improve HPV vaccine provider education in rural and underserved communities.9 This manuscript is the first description of the ECHO model’s adaptation to assess HPV vaccination rates utilizing QI methodology in primary care. This description of the ECHO QI learning collaborative on HPV vaccination (1) examines the feasibility, acceptability, and sustainability of Project ECHO for immunization QI work within a multi-state learning collaborative; (2) analyzes the impact of participation on HPV vaccination outcomes; (3) identifies lessons learned that could be applied to future projects using ECHO for QI.
METHODS
Learning Collaborative Structure
The AAP is a membership organization of 67,000 pediatricians focused on improving all children’s health. The national AAP works closely with 59 United States and 7 Canadian independent AAP chapters. AAP chapters are individually incorporated organizations, usually defined by state boundaries, and tasked with furthering the AAP mission and addressing state-specific child health challenges. From 2014 to 2019, the AAP Hub and Spoke Initiative focused on Improving HPV Vaccination Rates has been supported by a Centers for Disease Control and Prevention cooperative agreement to focus on increasing HPV vaccination rates across the country.10 As part of the HPV QI Initiative, the national AAP served as the “hub” to support QI projects while AAP chapters served as “spokes,” implementing the QI projects in their respective states. A critical goal was to provide training and experience in QI to chapters for HPV vaccination, leading to sustainability beyond the funding period.
Chapter and Practice Recruitment, Ethics
Recruitment took place in 2 phases. First, the national AAP recruited AAP chapters through a competitive application process. Selected AAP chapters then recruited pediatric practices from their respective states or jurisdictions, utilizing their contacts with local practices from previous AAP projects, and list-serves to recruit participants. Practices had to agree to identify a practice leader for the project, complete monthly data collection and case forms, attend 9 virtual meetings, and hold 8 team meetings to discuss project outcomes and goal progress. Recruited practices that met program requirements completed a memorandum of understanding to enroll in the project. Chapters offered participating practices $1,000–$2,000 to offset their costs for a 9-month project. Participating pediatricians were eligible to receive 25 American Board of Pediatrics Maintenance of Certification (MOC) Part 4 credits at no cost. The project received an exemption from the AAP Institutional Review Board.
Project Design and Curriculum
Multidisciplinary faculty, including pediatricians, immunization education experts, an obstetrician/gynecologist with HPV vaccination expertise, and a QI coach served as hub faculty. Hub faculty developed a 9-session curriculum (Table 1) to teach QI processes and evidence-based guidelines around HPV vaccination, including a strong provider recommendation, reminder/recall messaging, and provider prompts.11,12
Table 1.
The Quality Improvement Learning Collaborative Curriculum Topics, By Session
| Session 1 | Overview of HPV and the ECHO model |
| Session 2 | Quality improvement basics: PDSA cycles, aim statements, change concepts |
| Session 3 | Understanding your data: annotated run charts, baseline data, measure sets (outcome, process, balancing), data collection tools |
| Session 4 | HPV vaccine communication |
| Session 5 | Office logistics for improving vaccination rates |
| Session 6 | Centers for Disease Control and Prevention update: HPV vaccine |
| Session 7 | HPV vaccine: effectiveness and safety |
| Session 8 | Maintaining momentum: sustaining your quality improvement project |
| Session 9 | Applying spread principles |
Practices chose which vaccination strategies to implement and test in plan-do-study-act (PDSA) cycles (described below). Each month for 9 months, program participants convened for 1 hour via video conferencing. The authors taught the curriculum twice each month to facilitate participation across multiple time zones and fit busy providers’ needs. If a provider missed the monthly live sessions, the opportunity to view an archived recording was available. Each session included a 15–20-minute educational lesson covering critical topics in HPV vaccination and QI, followed by a practice-led de-identified case presentation and group discussion. In the first few months, the cases focused on individual patient encounters (eg, how to engage with parents with specific questions). As the QI work advanced, practices presented cases on specific change ideas (eg, vaccinating patients starting at age 9 years versus age 11 or 12 years) that providers had tested within their clinic settings. Run charts showing both aggregate and practice level data also were discussed during the video conferences.
QI Methodology
The improvement science model used and taught to participants for this work was the Model for Improvement. Langley13 developed this model, described in the Improvement Guide: A Practical Approach to Enhancing Organizational Performance. The model emphasizes 3 questions: What are we trying to accomplish? How will we know that a change is an improvement? What changes can we make that will result in improvement? The aim statement for this project was: to improve practice’s HPV vaccination initiation, completion, and missed opportunity rates with patients aged 11–14 by 20% by the end of the 9-month project. We taught practices to use “change ideas”—evidence-based strategies, preferably shown to work elsewhere, to test in their current setting. Participants used rapid PDSA cycles to test change ideas. As the office-based teams gained confidence that their change ideas were feasible and would lead to improvement, they implemented the changes, that is, made them standard practice.13,14 Practices chose which strategies to test in rapid PDSA cycles, how many strategies to try, and which to implement. They could adjust strategies throughout the project in response to improvements seen. Thus, for each data cycle (1 month of data collection), many PDSA cycles were performed.
Outcome: Program Participation and Experience
AAP staff tracked fulfillment of project requirements, including monthly teleconference attendance, submission of cases, and submission of chart review data to AAP’s data aggregator. Participating AAP chapters reported on their project experience twice (mid-way and post-completion), and answered 12 questions to identify project updates, feedback, challenges, successes, current reach, and project partnerships.
Practice participants reported on their project experience each month throughout the project utilizing a post-video conference survey on the session’s speakers, pace, and desired future topics. Each month practices also submitted information on the change ideas that they were testing that month using rapid PDSA cycles. For the first half of the project, each month, practices submitted a case presentation form that described a specific case of an 11–14-year-old patient and whether or not they received the HPV vaccine at that visit. Upon completing the project, practice participants answered a 27-item survey that included questions on their overall project experience, HPV vaccination and QI practices, and the demographic data for themselves and their practices. We assessed participant confidence in their QI skills by asking them to rate their ability to serve as a consultant on HPV vaccination QI efforts within (1) their clinic, and (2) their community. For each of the 2 questions, they could rate themselves as novice, competent, or expert. Questions focused on vaccination practices and confidence change were analyzed in SPSS statistical analysis software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0, Armonk, N.Y.) using the McNemar-Bowker test for statistical significance.
Outcome: HPV Vaccination
For 8 of the 9 project months, practices uploaded chart review data on HPV vaccination outcomes into AAP’s Quality Improvement Data Aggregator (QIDA). QIDA is an AAP developed and owned web-based data aggregation system that allows individual providers to securely enter practice-level data and view real-time data reports. Practices conducted retrospective chart reviews and collected data from charts of a minimum of 15 eligible patients age 11–14 seen consecutively within that data cycle (30 days). For each visit, practice personnel recorded the visit type and the patient’s age, sex, if they were due for the HPV vaccine, if they received the HPV vaccine at that visit (if applicable), and the reason for not getting vaccinated (if applicable). If a practice did not see at least 15 age-eligible patients during the month, practice personnel entered the data on all eligible patients seen that month. Using the QIDA system, practices could produce run charts on their monthly rates of HPV vaccination initiation, completion, and missed opportunities to vaccinate.
For this project, we defined outcomes as follows. HPV vaccination initiation was the number of patients who received their first dose at the time of the visit, divided by the number of patients who were due for the first dose. HPV vaccination completion was the number of patients who received dose 2 or 3 (depending on schedule) at the time of the visit, divided by the number of patients who were due for their last dose at the visit. Missed opportunities to vaccinate was the number of patients who did not receive the HPV vaccine during their visit, divided by the number of patients due for an HPV vaccine dose. For this analysis, the authors applied run chart rules to identify trends in HPV vaccination rates at the aggregate and practice level. Some run chart rules require a median for interpretation, and experts recommend having at least 10 data points. However, Provost and Murray14 note that it is acceptable to plot medians with fewer data points. Data will cross the median frequently as part of normal random variation, without having any QI-driven changes introduced to the process. A reduction in median crossings is, therefore, likely related to the team’s tests of change. We interpreted fewer than 3 crossings of the median for our 8 data points to indicate a meaningful change for this project.
RESULTS
Feasibility, Participation, and Satisfaction
Chapter Participation and Sustainability
Four AAP chapters applied, and 3 were selected: Arizona, New Jersey, and Oregon. Each chapter recruited between 2 and 4 practices to participate. Video conference sessions were held monthly from January to September 2018. To build capacity and enhance sustainability among the 3 AAP chapters, each chapter’s staff received training in the ECHO model, participated in all ECHO sessions, and organized and managed 1 of the 9 sessions.
On a 5-point Likert scale (very successful, somewhat successful, neutral, somewhat unsuccessful, and not successful), all 3 chapters rated the project very or somewhat successful. Chapters mentioned several aspects that made this learning collaborative successful, including gaining QI methodology skills, strengthening relationships with the participating pediatric practices and providers, and creating and disseminating HPV vaccination resources among the learning collaborative. All 3 chapters strongly agreed that this project increased their capacity to implement QI activities. Chapters shared that the advanced QI tools and methodology gained in this learning collaborative transferred over to their other QI projects and, in 1 case, precipitated the establishment of a QI committee within their state. In November 2018, after completing this learning collaborative, all 3 participating chapters launched an HPV immunization learning collaborative as ECHO hubs, recruiting between 5 and 10 additional practices each for participation.
Practice/Provider Participation and Experience
Chapters recruited 9 practices, among which 8 (5 suburban, 2 urban, and 1 a combination of suburban and urban) completed the project. One practice withdrew in the first few months, due to a staff emergency. Two to 8 individuals per practice participated, including pediatricians, nurse practitioners, and office staff (total = 42, pediatricians = 34). Pediatrician participation varied by practice, with a range of 50%–100% of a practice’s providers participating (Fig. 1). The average attendance at each video conference session was 34. After 1 practice withdrew from the project, the remaining 34 pediatricians completed the project.
Fig. 1.
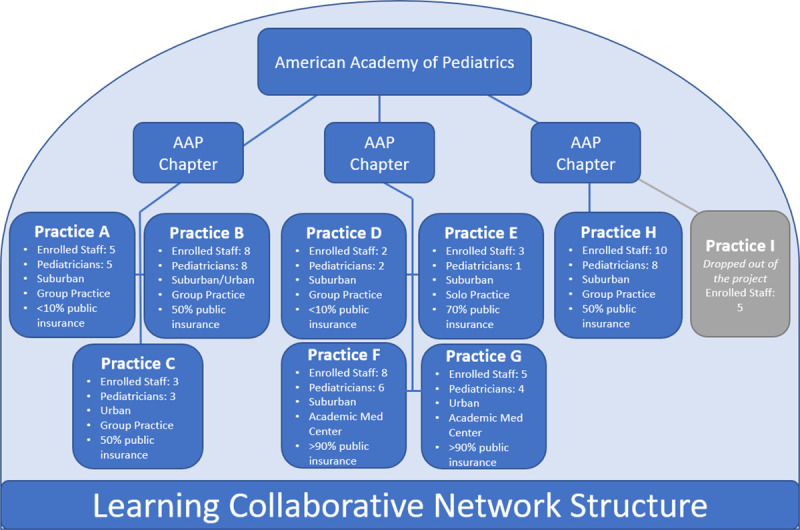
The learning collaborative structure. The American Academy of Pediatrics (AAP) provided training, tools, technical assistance, and project oversight. Three AAP chapters recruited practice sites and verified that practices within their state met requirements for data collection and attendance. Nine sites participated, and their characteristics are listed.
In the post-project survey, providers (physicians and nurse practitioners) reported increased knowledge and confidence (along a continuum from novice to competent to expert) on HPV vaccine communication with hesitant families. Similarly, participants were more likely to report they were “competent” or “expert” to serve as a consultant for HPV vaccination QI efforts in their clinic and community (Table 2). Providers also shared that they enjoyed learning from others in the collaborative, the interactive design, the curriculum, and the presenters of the educational material. Some providers reported a preference for a shorter project period and shorter conference sessions (Table 3).
Table 2.
Post-project Clinical Staff Self-assessment of HPV Vaccination Learning Collaborative Competencies (Survey results from September 2018) (n = 31)
| Before Project | After Project | McNemar Bowker Test | |
|---|---|---|---|
| Frequency, n (%) | Frequency, n (%) | ||
| Educate my staff about the importance of the HPV vaccine | |||
| Novice | 10 (32.2) | 0 (0.0) | 0.000* |
| Competent | 19 (61.3) | 14 (45.2) | |
| Expert | 2 (6.5) | 17 (54.8) | |
| Introduce HPV vaccine in a way that optimizes parents’ vaccine confidence | |||
| Novice | 17 (54.8) | 0 (0.0) | 0.000* |
| Competent | 11 (35.5) | 15 (48.4) | |
| Expert | 3 (9.7) | 16 (51.6) | |
| Communicate with families and caregivers who are hesitant about HPV vaccination | |||
| Novice | 20 (64.5) | 0 (0.0) | 0.000* |
| Competent | 6 (19.4) | 18 (58.1) | |
| Expert | 5 (16.1) | 13 (41.9) | |
| Communicate with families and caregivers who decline/delay HPV vaccination | |||
| Novice | 23 (74.2) | 1 (3.2) | 0.000* |
| Competent | 7 (22.6) | 22 (71) | |
| Expert | 1 (3.2) | 8 (25.8) | |
| Serve as a consultant within my clinic for HPV vaccination quality improvement efforts | |||
| Novice | 17 (54.8) | 1 (3.2) | 0.000* |
| Competent | 13 (41.9) | 19 (61.3) | |
| Expert | 1 (3.2) | 11 (35.5) | |
| Serve as a consultant within my community for HPV vaccination quality improvement efforts | |||
| Novice | 20 (64.5) | 2 (6.5) | 0.000* |
| Competent | 11 (35.5) | 24 (77.4) | |
| Expert | 0 (0.0) | 5 (16.1) | |
| Implement methods to increase HPV vaccination rates by increasing visit attendance (ie, reminders, recall) | |||
| Novice | 14 (45.2) | 3 (9.7) | 0.001* |
| Competent | 16 (51.6) | 18 (58.1) | |
| Expert | 1 (3.2) | 10 (32.2) | |
| Implement methods to increase HPV vaccination rates by increasing captured opportunities (ie, standing orders, practitioner prompts) | |||
| Novice | 17 (54.8) | 1 (3.2) | 0.000* |
| Competent | 13 (41.9) | 21 (67.7) | |
| Expert | 1 (3.2) | 9 (29) |
Table 3.
Qualitative Comments Participants Shared About the Learning Collaborative, From Retrospective Survey
| What providers enjoyed about the learning collaborative |
| Increase in confidence |
| “I felt much more confident speaking with parents about the HPV vaccine because of this program.” |
| “Our HPV vaccination rates have increased, and our staff is more game to answer parents’ questions and concerns about the HPV vaccine.” |
| “This project changed my confidence and approach. I used to recommend the vaccine but now I offer it as a lifesaving cancer eliminating vaccine.” |
| “This project has reshaped the way we offer HPV vaccine in our office for the better. We have better ways to introduce the vaccine and educate families.” |
| The collaborative learning network |
| “The interaction with other practices to hear what they are doing for their PDSA cycles was the best part.” |
| “I liked sharing ideas with other practices.” |
| “I learned from the many offices participating across the United States.” |
| “Interaction with other practices was invaluable.” |
| The interactive design |
| “Everyone could ask questions.” |
| “The discussions were great.” |
| The curriculum and speakers |
| “Learning about the quality improvement method was helpful.” |
| “The more we understand the diseases the better we can educate families on the importance of vaccinating.” |
| “The clear presentations and guest speakers were the best part of this project.” |
| “My favorite aspect was learning how to understand our data better.” |
| What participants would improve about the learning collaborative |
| The length of the project or monthly session |
| It could be fewer weeks.” |
| “Shorter time period.” |
| “There were too many sessions and cycles.” |
| “Shorter sessions like 30 minutes rather than an hour.” |
| Too much time spent on introductions each session |
| “Avoid prolonged introductions which allows for more discussion time.” |
| “I recommend not having everyone introduce themselves in the beginning. That took a very long time.” |
| Repetition of project curriculum |
| “The quality improvement education seemed redundant.” |
| “Some of the material was repetitive.” |
Implementation Strategies
Practices chose various evidence-based change ideas to increase HPV vaccination rates and then tested those ideas using PDSA cycles (Table 4). Most practices chose to offer HPV vaccine at both sick (88%) and follow-up visits (75%), conducted all-staff training sessions (75%), implemented a provider communication strategy focused on the bundled recommendation and cancer prevention (75%), and developed processes to review charts and identify patients due for HPV vaccine before the physician encounter (75%). Four practices (50%) began offering the HPV vaccine at younger ages (9 or 10 years) than before the project. Two practices implemented a reminder/recall system, and 1 practice developed standing orders for HPV vaccination. Practices reported testing between 4 and 7 different change ideas over the 9-month project period.
Table 4.
Number of Practices that Tested Specific Change Ideas, as Described in Monthly Practice Reports (Number of Practices = 8)
| Change Idea | Number of Practices, N (%) |
|---|---|
| Offer HPV vaccine at follow-up visits | 6 (75) |
| Offer HPV vaccine at sick visits | 7 (86) |
| All staff training session | 6 (75) |
| Communication strategy: bundle recommendation, cancer prevention message | 6 (75) |
| Checking charts pre-visit to identify patients due for HPV | 6 (75) |
| Handout patient education materials | 5 (63) |
| Offer the vaccine at age 9 or 10 (instead of 11 or 12) | 4 (50) |
| Reminder/recall | 2 (25) |
| Offer vaccine at age 11 (instead of 12) | 1 (13) |
| Standing orders for HPV vaccine | 1 (13) |
| Scheduling next HPV vaccine dose | 1 (13) |
HPV Vaccination Outcomes
Throughout the 8-month data collection period, aggregate rates of missed opportunities to vaccinate at all visit types fell by 13 percentage points (38%–25%). Aggregate HPV vaccine initiation rates for patients ages 11–14 years increased by 15 percentage points (54%–69%), and HPV vaccination completion rates decreased by 2 percentage points (88%–86%). Utilizing a QI run chart rule14 that less than 3 crossings of the median suggest nonrandom change, the aggregate missed opportunities run chart data crossed the median once, suggesting a nonrandom change towards improvement. However, the aggregate HPV vaccination initiation data crossed the median line 3 times, and aggregate HPV vaccination completion data crossed the median 4 times, indicating no change during the project (Fig. 2).
Fig. 2.
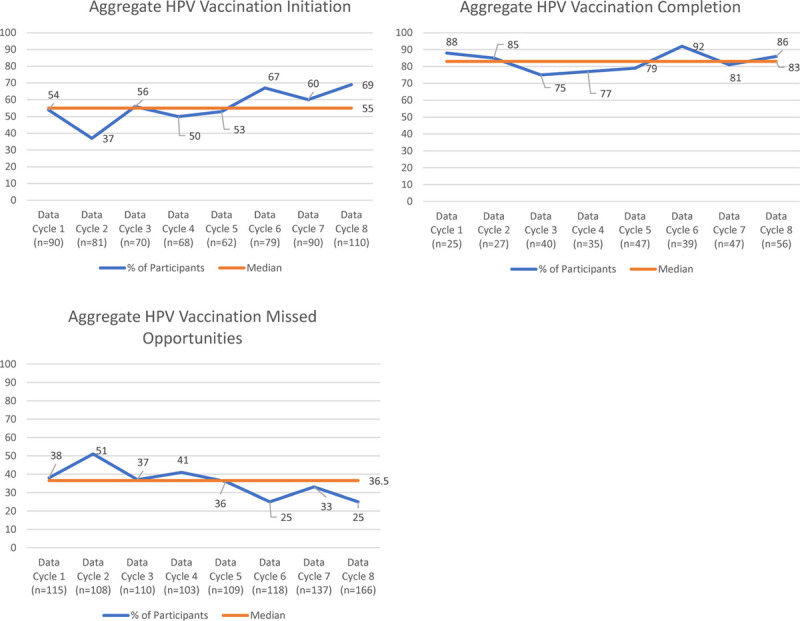
The learning collaborative aggregate level run chart data of HPV vaccination initiation, completion, and missed opportunities during the 8 monthly project data collection.
Three practices used run chart rules to achieve improvement in 1 or more project measures at the practice level. One practice met the rule for series initiation, series completion and missed opportunities, 1 for initiation and completion, and 1 for completion alone. One practice had nearly a steady worsening across all 3 measurements. The remaining 6 practices had fluctuations in their data.
DISCUSSION AND LESSONS LEARNED
Volunteer practices showed the feasibility of this 9-month AAP-organized ECHO QI learning collaborative focused on HPV vaccination using video-conferencing during monthly, live, interactive sessions. Aggregate data on missed opportunities showed an improvement. Three of the final 8 practices showed improvement in at least 1 of 3 key measures (series initiation, series completion, missed opportunities).
Feasibility, Acceptability, and Sustainability
This project demonstrates that the Project ECHO model can be used for a multi-state QI learning collaborative. Utilizing a hub and spoke model, the AAP was able to recruit chapters from diverse states. One of the benefits of this model is that it enhanced the likelihood of sustainability of ongoing learning collaboratives. All participating chapters mastered the integrated Project ECHO/QI process through their participation, including their required facilitation of a live session; all rated the project as successful. Having AAP chapters act as a “spoke” (participant) before serving as a “hub” (leader) was novel to the AAP ECHO program structure. All 3 chapters have since gone on to lead their own HPV vaccination QI learning collaboratives.
One limitation of the project identified by chapters was the challenge with practice recruitment. This difficulty occurred despite the incentive of MOC Part 4 credit for physicians and funding for practices to offset participation costs, including data reporting. The limited timeframe for recruitment (6 weeks) may have been a factor. Future ECHO QI iterations should allow more time for practice recruitment. One of the ECHO model’s strengths is the ability, through video conferencing, to reach rural practices that may not otherwise have access to subject matter experts. The impact of the ECHO model in rural locations has been previously demonstrated in the areas of hepatitis C treatment, and behavioral health.7,15 While rural areas were not a specific focus of this project (and no rural practices enrolled) and given lower rates of HPV vaccination in rural areas, this could be a focus for future recruitment. Of note, chapters mentioned above that went on to lead their own collaboratives were able to recruit 3 rural practices to participate. We suspect having more time for recruitment facilitated this success.
Provider and Staff Level
Despite challenges with recruitment, pediatricians had high retention, with more than 88% completing the project. MOC Part 4 credit, frequently used as an incentive for participation in pediatric QI programs, probably played a role in project completion among pediatricians and should continue to be used in future projects.5,6,16 We did not offer any tangible incentive to the nurses and staff in these pediatricians’ offices, and they were less likely than pediatricians to attend the calls, attending only 1 or 2 meetings. Offering a greater diversity of credits could incentivize various medical professionals to achieve multidisciplinary teams in future projects.
The use of video conference technology in learning settings, which has become extremely popular because of the COVID-19 pandemic, can allow for fuller engagement and enhanced communication and satisfaction among participants.17,18 In this project, some participants either did not have video capability or chose to participate via audio rather than video, which may have negatively affected engagement. Faculty leading the monthly learning sessions felt that future ECHO projects should ensure broad video participation. Allowing more time for familiarization with the technology, in the first session or during onboarding, may increase the use of the video component.
Impact on HPV Immunization
While only offering 8 monthly data points, the project did allow for examining the impact on HPV immunization rates. In the run chart analysis, extrapolating on the fewer than 3 crossings of the median rule for our 8 data points, the only aggregate missed opportunities improved over this project period. If this project continued for more than 8 months, we would expect improvements in initiation and completion would follow, given this decrease in missed opportunities. Future projects should consider a more extended project period or more frequent data collection to reduce data variability to make it easier to spot nonrandom changes. Given that some participants reported they felt the project duration was too long, more frequent data collection might be the most acceptable solution.
In the pre/post-analysis, improvements were seen in aggregate missed opportunities and initiation rates, but not series completion, which remained stable at around 80%. This result is likely due to a combination of improvement being more difficult when rates are already high and the project’s short duration. Other HPV vaccination QI projects have demonstrated improvements across all 3 measures, including series completion, but their initial completion rates were lower.5,6,16 Additionally, several participating practices tested the recommendation to offer the HPV vaccine starting at 9–10 years of age. As a result of this change, improved immunization rates would not have been captured in the data, as we included only charts of patients age 11–14 years of age. Future projects should consider collecting data starting at age 9 years as pediatricians move towards earlier HPV vaccination in alignment with AAP recommendations.19,20
Only 3 out of the 8 practices demonstrated positive HPV immunization results throughout the collaborative. These practices did not differ in the type or number of evidence-based changes implemented compared to other practices. They may have differed in the degree to which they fully implemented changes across the entire practice. The practice that demonstrated improvement across all 3 measures (initiation, completion, and missed opportunities) was a solo practitioner. Future collaboratives should identify and address practice barriers to system-level change.
CONCLUSIONS
This learning collaborative aimed to engage multiple AAP chapters and pediatric practices to teach QI skills and improve HPV vaccination rates using the Project ECHO telementoring platform. At the chapter level, the collaborative demonstrated success in satisfaction and in teaching the necessary skills to allow all participating chapters to go on to lead HPV QI learning collaboratives of their own. At the practice level, participants gained confidence in HPV vaccine communication and QI skills. The collaborative demonstrated early improvements in reducing missed opportunities to vaccinate, while the impact on HPV vaccination initiation and completion lagged. Future projects should be extended for more than 8 data cycles or use more frequent data collection to capture this change and further identify barriers to system-level change.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online December 28, 2020
Supported by the Centers for Disease Control and Prevention (CDC) under cooperative agreement number - 5H23IP000952. Its contents are solely the authors’ responsibility and do not necessarily represent the official views of the CDC or the Department of Health and Human Services.
To cite: Oliver K, Beskin K, Noonan L, Shah A, Perkins R, Humiston S. A Quality Improvement Learning Collaborative for Human Papillomavirus Vaccination. Pediatr Qual Saf 2021;6:e377.
REFERENCES
- 1.Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in Human Papillomavirus-Associated Cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018; 67:918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016; 65:1405–1408 [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020. 2019. Available at https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed August 6, 2019
- 4.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020; 69:1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rand CM, Tyrrell H, Wallace-Brodeur R, et al. A learning collaborative model to improve human papillomavirus vaccination rates in primary care. Acad Pediatr. 2018; 18:S46–S52 [DOI] [PubMed] [Google Scholar]
- 6.Bonville CA, Domachowske JB, Suryadevara M. A quality improvement education initiative to increase adolescent human papillomavirus (HPV) vaccine completion rates. Hum Vaccin Immunother. 2019; 15:1570–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011; 364:2199–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Project ECHO. 2020. Accessed January 28, 2020
- 9.National Vaccine Advisory Committee on June 25, 2018. Strengthening the effectiveness of national, state, and local efforts to improve HPV vaccination coverage in the United States: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2018; 133:543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell K, Saraiya M, Bhatt A. Increasing HPV vaccination rates through national provider partnerships. J Womens Health (Larchmt). 2019; 28:747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver K, Frawley A, Garland E. HPV vaccination: population approaches for improving rates. Hum Vaccin Immunother. 2016; 12:1589–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewer NT, Hall ME, Malo TL, et al. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics. 2017; 139:e20161764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langley GJ. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2009; xxi2nd ed San Francisco, Calif.: Jossey-Bass; 490 p [Google Scholar]
- 14.Provost LP, Murray SK. Chapter 3: understanding variation using run charts. The Health Care Data Guide Learning from Data for Improvement. 2011; xxviii1st ed San Francisco, Calif.: Jossey-Bass; 445 p, 2 p [Google Scholar]
- 15.Komaromy M, Madden EF, Hager B, et al. Improvement in behavioral health symptoms and functioning among rural patients cared for by primary care teams using the extension for community health care outcomes model. J Rural Ment Health. 2019; 43:73–80 [Google Scholar]
- 16.Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015; 33:1223–1229 [DOI] [PubMed] [Google Scholar]
- 17.Knapp NF. Increasing interaction in a flipped online classroom through video conferencing. Techtrends. 2018; 62:618–624 [Google Scholar]
- 18.Wilson SF, Marks R, Collins N, et al. Benefits of multidisciplinary case conferencing using audiovisual compared with telephone communication: a randomized controlled trial. J Telemed Telecare. 2004; 10:351–354 [DOI] [PubMed] [Google Scholar]
- 19.Kimberlin DW; American Academy of Pediatrics, Committee on Infectious Diseases. Red book: 2018-2021 Report of the Committee on Infectious Diseases. 2018. 31st ed. American Academy of Pediatrics; 1 online resource (xlix, 1213 pages) [Google Scholar]
- 20.Goleman MJ, Dolce M, Morack J. Quality improvement initiative to improve human papillomavirus vaccine initiation at 9 years of age. Acad Pediatr. 2018; 18:769–775 [DOI] [PubMed] [Google Scholar]


