Abstract
Background
The aims of this study were (1) to compare the prevalence of myocardial diastolic dysfunction (DD) in antiretroviral therapy (ART)–naive people living with human immunodeficiency virus (PLWH) to human immunodeficiency virus (HIV)–uninfected adults in East Africa and (2) to determine the association between serum concentration of the cardiac biomarkers ST2 and DD.
Methods
In this cross-sectional study, we enrolled PLWH and uninfected adults at a referral HIV clinic in Mwanza, Tanzania. Standardized history, echocardiography, and serum were obtained. Regression models were used to quantify associations.
Results
We enrolled 388 ART-naive PLWH and 461 HIV-uninfected adults with an average age of 36.0 ± 10.2 years. Of PLWH in the third, fourth, and fifth decades of life, 5.0%, 12.5%, and 32.7%, respectively, had DD. PLWH had a higher prevalence of DD (adjusted odds ratio, 2.71 [95% confidence interval, 1.62–4.55]; P < .0001). PLWH also had a higher probability of dysfunction with one or fewer traditional risk factors present. Serum ST2 concentration was associated with dysfunction in PLWH but not uninfected participants (P = .04 and P = .90, respectively).
Conclusions
In a large population of young adults in sub-Saharan Africa, DD prevalence increased starting in the third decade of life. HIV was independently associated with dysfunction. Serum ST2 concentration was associated with DD in PLWH but not HIV-uninfected participants. This pathway may provide insight into the mechanisms of HIV-associated dysfunction.
The prevalence of myocardial diastolic dysfunction was 2-fold higher in young HIV-infected, antiretroviral therapy–naive Tanzanian adults as compared to those uninfected. Serum concentration of the biomarker ST2 was associated with dysfunction in HIV-infected but not uninfected participants.
Cardiovascular disease is a major complication of human immunodeficiency virus (HIV) infection [1]. People living with HIV (PLWH) have a 2-fold higher incidence of myocardial infarction and stroke and a 4-fold higher incidence of sudden cardiac death [1–3]. PLWH also share a disproportionate burden of heart failure, with studies indicating a nearly 2-fold increased incidence [4, 5]. Cardiovascular disease in PLWH has tripled worldwide over the past 25 years and is now a leading cause of death globally. A recent American Heart Association position statement notes that HIV-attributable cardiovascular disease risk is highest in Africa where heart failure is one of the most common cardiovascular manifestations of HIV [6, 7]. The reason for this increased risk is likely due to a complex interaction between traditional risk factors, antiretroviral therapy (ART), HIV-induced inflammation, and immune activation [6].
Asymptomatic myocardial diastolic dysfunction, due to inappropriate relaxation of the myocardium, is the most prevalent myocardial disorder worldwide and is a precursor to heart failure with preserved ejection fraction. In studies from the United States and Europe, up to one-quarter of adults over the age of 40 years have diastolic dysfunction [8, 9]. Risk factors include hypertension, obesity, and advanced age [10]. In cross-sectional studies, PLWH also have a ~2-fold higher prevalence of diastolic dysfunction [11–17]. There are few data on prevalence of diastolic dysfunction in PLWH and HIV-uninfected adults in Africa, where the prevalence of HIV is highest and risk factors may be different [18].
The medications that successfully treat heart failure with reduced ejection fraction do not improve outcomes in those with heart failure with preserved ejection fraction [19–21]. This failure is likely due to different underlying pathophysiologies [22]. Data from both animal and human studies support the paradigm that activation of immune pathways leading to myocardial fibrosis is critical in development of diastolic dysfunction [23–26]. Alterations in immune pathways have recently been implicated in both diastolic dysfunction and subsequent heart failure [27]. One of the innate immune pathways implicated is the interleukin 33 (IL-33)/ST2 pathway [28].
Soluble ST2 (soluble suppression of tumorigenicity 2 [sST2]) is a receptor for the cytokine IL-33 [29]. IL-33 is released after cell injury and alerts the immune system to tissue injury (ie, an alarmin cytokine) [30]. IL-33 is cardioprotective and decreases cardiac fibrosis and improves diastolic function [23, 31]. The binding of IL-33 by sST2 ablates the cardioprotective antifibrotic effects of IL-33. Serum ST2 concentration is associated with HIV infection and is highest early in disease course [32]. ST2 is associated with diastolic dysfunction in PLWH on long-term ART [6, 33]. Whether this association is present before ART initiation is unknown.
We conducted a cross-sectional study in both PLWH and HIV-uninfected adults in Tanzania. The objectives were to determine the prevalence and characteristics of asymptomatic myocardial diastolic dysfunction prior to ART initiation. We also determined the association of serum sST2 concentration and diastolic dysfunction within these groups. We hypothesized that HIV infection would be associated with a 2-fold increased prevalence of asymptomatic diastolic dysfunction prior to initiating ART. We also hypothesized that serum ST2 concentration would be significantly higher in both PLWH and uninfected adults with diastolic dysfunction as compared to controls.
METHODS
Study Design
This was a cross-sectional study of PLWH and HIV-uninfected Tanzanian adults.
Study Population
HIV-infected participants were enrolled from the referral HIV clinic of Bugando Medical Centre (BMC) in the city of Mwanza, Tanzania. HIV-uninfected adults were selected from treatment supporters for these PLWH. In Tanzania, treatment supporters are HIV-uninfected peers of PLWH (close friends or relatives) who accompany them during their clinic visits and are required by Tanzanian national guidelines [34].
HIV-uninfected adult inclusion criteria were age 18–65 years; willingness to be contacted by cell phone; Tanzanian citizenship; and willingness to provide written informed consent. Inclusion criteria for HIV-infected adults also included ART naive and willingness to initiate ART within 1 month. Exclusion criteria for both groups included previous history of cardiovascular events (heart failure, stroke, myocardial infarction, pulmonary embolism), any medical condition with a prognosis of <12 months, and plans to move from Mwanza in the next 4 years.
Study Setting
Study participants were enrolled at the outpatient HIV clinic of BMC in Mwanza, Tanzania. Mwanza has a population of approximately 1 million and is the second largest city in Tanzania. BMC is the referral hospital for northwestern Tanzania, serving a population of approximately 15 million people. The prevalence of HIV in the catchment area for BMC is approximately 6%, which is similar to the national average of 5.1% [35]. At the time of the study, BMC was providing outpatient care to 15 000 HIV-infected adults.
Study Procedures
Potential participants were identified during their initial clinic visit by the attending HIV physician. They were then screened for eligibility by a study nurse and invited to participate. If agreeable, participants provided written informed consent after review in Kiswahili by study staff.
At enrollment, the study staff administered a standardized questionnaire based on the World Health Organization’s (WHO) Stepwise Approach to Surveillance (STEPS) for risk factors for cardiovascular disease [36]. The STEPS questionnaire has been translated into the local language (Kiswahili) and validated in East Africa. It includes questions about socioeconomic status, diet, exercise, and standard protocols for anthropomorphic measurements. Additional questions were included regarding previous HIV diagnosis and exposure to ART. After completing the questionnaire, the study staff conducted a standardized physical examination (including weight, height, and waist circumference).
Echocardiography
Echocardiograms were performed in a private clinic room dedicated for this purpose. Echocardiography was performed using a Sonosite M-turbo with an advanced cardiac calculation package. The study staff were credentialed in echocardiography, and examinations and measurements were based on American Society of Echocardiography (ASE) guidelines [37, 38]. Diastolic dysfunction was diagnosed and graded according to 2009 ASE guidelines [39]. Specifically, diastolic dysfunction was diagnosed if 2 of the following criteria were met: septal e′ <8 cm/second, lateral e′ <10 cm/second, and left atrial index ≥34 mL/m2. Structural and functional disease such as myocardial hypertrophy, systolic function, and valvular disease was classified according to ASE disease-specific guidelines. All diagnoses were confirmed by 2 blinded, independent reviewers. In the case of disagreement, a third reviewer was consulted for final diagnosis.
Laboratory Procedures
Routine laboratory tests included complete blood count and urinalysis. Point-of-care CD4+ T-cell count and hemoglobin level were additionally measured using an automated BD FACS Calibur Machine (BD Biosciences). Blood glucose was measured from a fingerpick sample (OneTouch Select, LifeScan). Participants with a random blood glucose level of ≥7 mmol/L (126 mg/dL) had a follow-up fasting measurement to confirm the diagnosis of diabetes mellitus [40]. Anemia was defined as <12 g/dL for women and <13 g/dL for men according to the WHO definition [41].
The clinic phlebotomists drew blood between 9 am and 12 am from study participants at enrollment. This included 10 mL collected in an anticoagulant-free tube. Blood was transported to the laboratory of the Tanzanian National Institute for Medical Research, which is approximately 1 mile from the study site, for processing. Serum aliquots were then stored in the –80°C freezer within 6 hours from the time of the sample collection. Serum was transferred to the Weill Cornell Center for Global Health laboratory on dry ice with continuous temperature monitoring for further analysis.
ST2 Concentration
Serum ST2 concentration was measured using R&D Systems Quantikine enzyme-linked immunosorbent assay (catalog number DST200). Samples were tested in duplicate and mean values were reported. Standard controls (R&D Systems Quantikine Immunoassay Control Set: 1063) were also measured on each plate to ensure intraplate consistency. The absorbance at 570 nm and 450 nm was measured with an EMax Plus Microplate Reader (Molecular Devices), and concentration was calculated with SoftMax Pro software by generation of a log/log standard curve.
Data Analysis
Data were entered into a secure OpenClinica (OpenClinica LLC) database by the Mwanza Intervention Trials Unit data management personnel. Data analysis was performed using Stata 15 software (StataCorp LLC). Continuous variables were summarized by mean and standard deviation. Categorical variables were summarized by frequency and percentage. Logistic and linear regression models were used for binary and continuous variables, respectively. All regression models were controlled for age, sex, blood pressure, and body mass index (BMI), given their known associations with diastolic dysfunction. Unadjusted values were also reported where appropriate. Kolmogorov–Smirnov diagrams were used to further compare sST2 values between groups.
Ethical Considerations
This study was carried out in accordance with Good Clinical Practice. Compliance with these standards provides public assurance that the rights, safety, and well-being of study participants are protected, consistent with the principles that have their origin in the Declaration of Helsinki and the Belmont Report. The study and consent forms were approved by the institutional review boards of Weill Cornell Medicine, the Tanzanian National Institute for Medical Research, and BMC.
RESULTS
Enrollment
During the period from November 2016 to October 2019, 1427 adults attending the BMC HIV clinic were screened for enrollment. Of these, 1080 (75.7%) met the study eligibility criteria and 996 of the 1080 (92.2%) gave informed consent for study inclusion. Of these 996, 147 participants (108 HIV-infected and 39 uninfected) declined echocardiography. Therefore, a total of 849 study participants (388 HIV-infected, ART naive adults and 461 HIV-negative controls) were included in this analysis.
Baseline Characteristics
The baseline characteristics of the 2 groups are described in Table 1. Compared to the HIV-uninfected control group, HIV-infected adults had significantly higher heart rate (76.9 vs 71.6 beats per minute), lower systolic (115.5 vs 124.7 mm Hg) and diastolic blood pressure (75.8 vs 79.4 mm Hg), less hypertension (19.1% vs 34.8%), less central obesity (32.2% vs 41.2%), and similar BMI (23.0 vs 23.3 kg/m2). HIV-infected participants were also more likely to use alcohol (8.3% vs 2.8%) and consume more dietary vegetables (2.9 vs 2.5 servings/wk). The HIV-infected group had fewer participants with education past primary school than uninfected controls (19.4% vs 26.3%). The 2 groups had similar age, sex, fasting blood glucose, diagnosis of diabetes, tobacco use, and water source.
Table 1.
Baseline Characteristics of the 849 Tanzanian Adult Study Participants
| Variable | HIV-Uninfected (n = 461) | PLWH, ART-Naive (n = 388) | P Value |
|---|---|---|---|
| Age, y, mean (SD) | 35.9 (10.9) | 36.1 (9.3) | .756 |
| Female sex | 306 (66.5%) | 278 (72.0%) | .085 |
| Current CD4 T-cell count, cells/μL, mean (SD) | 898.6 (275.8) | 438.7 (300.1) | <.0001 |
| ART duration, mo | NA | NA | |
| Heart rate, bpm, mean (SD) | 71.6 (14.0) | 76.9 (14.6) | <.0001 |
| BMI, kg/m2, mean (SD) | 23.3 (4.7) | 23.0 (5.4) | .350 |
| <18.5 | 55 (12.0%) | 68 (17.6%) | .014 |
| 18.5–24.9 | 253 (55.1%) | 219 (56.7%) | |
| 25–29.9 | 107 (23.3%) | 54 (14.0%) | |
| ≥30 | 44 (9.6%) | 45 (11.7%) | |
| Waist circumference, cm, mean (SD) | 82.0 (11.2) | 80.7 (10.9) | .076 |
| Central obesity (IDFa) | 190 (41.2%) | 125 (32.2%) | .007 |
| Systolic BP, mm Hg, mean (SD) | 124.7 (18.5) | 115.5 (17.0) | <.0001 |
| Diastolic BP, mm Hg, mean (SD) | 79.4 (11.8) | 75.8 (10.9) | <.0001 |
| Hypertensionb | 159 (34.8%) | 71 (19.1%) | <.0001 |
| Fasting blood glucose, mmol/L, mean (SD) | 7.6 (4.4) | 8.9 (6.5) | |
| Diabetes | 5 (1.1%) | 8 (2.1%) | .588 |
| Diet | .246 | ||
| Vegetables, servings/week | 2.5 (2.6) | 2.9 (2.7) | |
| Fruits, servings/week | 4.6 (2.6) | 4.4 (2.7) | .028 |
| Current smoker | 26 (5.6%) | 16 (4.1%) | .263 |
| Current alcohol use | 13 (2.8%) | 32 (8.3%) | .310 |
| Exerciseb, days/wk, mean (SD) | 5.1 (2.1) | 5.2 (2.3) | <.0001 |
| Education | .833 | ||
| Primary school or less | 339 (73.7%) | 311 (80.6%) | .024 |
| Secondary school | 104 (22.6%) | 61 (15.8%) | |
| College or university | 17 (3.7%) | 14 (3.6%) | |
| Water source | |||
| Running water in house | 212 (46.1%) | 181 (46.9%) | .815 |
| Other | 248 (53.9%) | 205 (53.1%) |
Data are presented as No. (%) unless otherwise indicated. Linear regression for continuous variables, logistic regression for binary, and ordinal logistic regression for hierarchical groups (P < .05 in bold).
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; BP, blood pressure; HIV, human immunodeficiency virus; IDF, International Diabetes Federation; NA, not applicable; PLWH, people living with human immunodeficiency virus; SD, standard deviation.
aIDF: >80 cm and >94 cm for women and men, respectively.
bHypertension: American Heart Association/American College of Cardiology, systolic >130 mm Hg and/or diastolic >80 mm Hg (average of 2 separate readings).
cExercise: at least 10 minutes per day.
Echocardiographic Characteristics
Echocardiographic characteristics of both groups are shown in Table 2 and Supplementary Table 1.
Table 2.
Echocardiographic Parameters of 849 People Living With Human Immunodeficiency Virus and Uninfected Study Participants
| Variable | HIV-Uninfected (n = 461) | PLWH, ART-Naive (n = 388) | P Valuea |
|---|---|---|---|
| Systolic measures | |||
| Left ventricular function | |||
| Ejection fraction, % | 66.4 (8.9) | 66.4 (8.8) | .720 |
| End diastolic volume, mL | 61.2 (20.0) | 61.4 (19.5) | .154 |
| End diastolic volume index, mL/m2 | 36.0 (11.4) | 36.7 (11.4) | .108 |
| End systolic volume, mL | 20.6 (9.2) | 20.6 (9.0) | .484 |
| End systolic volume index, mL/m2 | 12.1 (5.4) | 12.3 (5.4) | .427 |
| Right ventricular function | |||
| TAPSE, cm | 2.2 (0.4) | 2.2 (0.4) | .874 |
| Structural measures | |||
| Left atrial/ventricular chamber size | |||
| Diastolic diameter, cm | 4.2 (0.5) | 4.2 (0.5) | .833 |
| LV mass, g | 119.5 (32.3) | 122.0 (34.5) | <.0001 |
| Relative wall thickness | 0.43 (0.13) | 0.45 (0.13) | .105 |
| Left atrial volume, mL | 33.3 (12.7) | 33.7 (20.0) | .108 |
| Left atrial volume index, mL/m2 | 19.5 (7.2) | 20.1 (12.8) | .077 |
| Diastolic measures | |||
| Pulse Doppler at mitral valve | |||
| E wave velocity, m/s | 0.79 (0.17) | 0.76 (0.16) | .066 |
| A wave velocity, m/s | 0.53 (0.17) | 0.55 (0.16) | .001 |
| E wave deceleration time, ms | 195.3 (43.4) | 196.1 (46.7) | .638 |
| E/A ratio | 1.62 (0.6) | 1.47 (0.5) | <.0001 |
| Tissue Doppler | |||
| Lateral e′ wave, cm/s | 14.4 (3.2) | 14.0 (3.1) | .003 |
| Septal e′ wave, cm/s | 10.7 (2.6) | 10.0 (2.4) | <.0001 |
| Average E/e′ | 6.7 (1.8) | 6.8 (1.8) | .006 |
Data are presented as mean (standard deviation).
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PLWH, people living with human immunodeficiency virus; TAPSE, tricuspid annular plane systolic excursion.
aLinear regression (adjusted for age, sex, systolic blood pressure, and body mass index) (P < .05 in bold).
Systolic Measures
HIV-infected participants and uninfected controls had similar left and right ventricular systolic function: left ventricular ejection fraction, a measure of left systolic function (66.4% for both groups), and tricuspid annular plane systolic excursion, a measure of right systolic function (2.2 cm for both groups).
Structural Measures
HIV-infected participants had significantly higher left ventricular mass (122.0 vs 119.5 g; P < .0001) and left atrial volume index (20.1 vs 19.5 mL/m2; P = .077) as compared to uninfected controls. HIV-infected participants also had a trend toward higher relative wall thickness (P = .105) as compared to controls. Left ventricular chamber volumes were similar between groups.
HIV-infected, dysfunction-positive participants had higher relative wall thickness compared to their dysfunction-negative controls (P = .052) (Supplementary Table 1). Additionally, HIV-infected participants with concurrent diastolic dysfunction had significantly higher relative wall thickness (P = .036) as compared to HIV-uninfected participants with dysfunction.
Diastolic Measures
HIV-infected participants had significantly lower lateral e′ (14.0 vs 14.4 cm/second; P = .003) and septal e′ (10.0 vs 10.7 cm/second; P < .0001) tissue Doppler velocities, both markers of left ventricular wall relaxation. HIV-infected participants also had lower E/A ratio (1.47 vs 1.62; P < .0001), a blood flow surrogate measure of left ventricular relaxation, as compared to HIV-uninfected controls. Additionally, HIV-infected participants had higher average E/e′ ratio (6.8 vs 6.7; P = .006).
HIV-infected and uninfected participants with dysfunction had significantly lower lateral e′ and septal e′ tissue Doppler velocities (P < .0001 for both) compared to their dysfunction-negative control groups (Supplementary Table 1).
Prevalence of Left Ventricular Diastolic Dysfunction
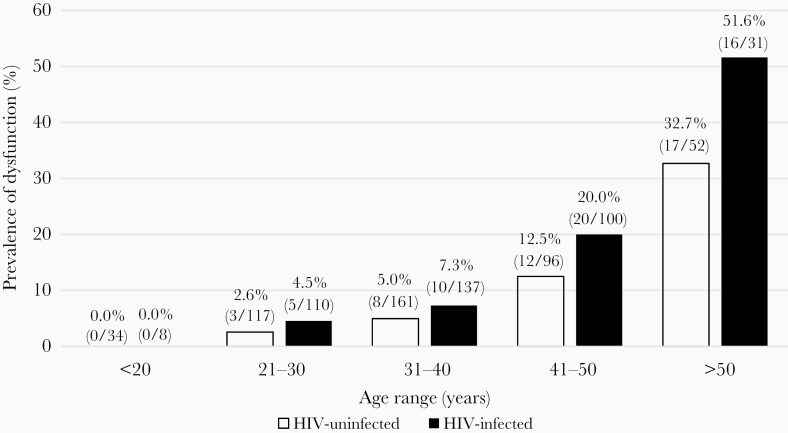
HIV-infected participants had a significantly higher prevalence of left ventricular diastolic dysfunction as compared to uninfected controls (51/388 [13.2%] vs 40/461 [8.7%]; P < .0001) (Table 3). HIV-infected participants with diastolic dysfunction also had a significantly higher prevalence of more severe grade II/III dysfunction (38/51 [74.5%] vs 21/40 [52.5%]; P = .028). Intraobserver agreement for diastolic dysfunction grade was 96.7% (88/91). When stratified by age, HIV-infected participants had a higher prevalence of diastolic dysfunction at younger ages than the HIV-uninfected controls (P = .016 for linear trend across groups) (Figure 1). Starting in the second and third decades of life, HIV-infected participants trended toward a higher prevalence of diastolic dysfunction (adjusted odds ratio [aOR], 1.56 [95% confidence interval {CI}, .33–7.40] and 1.71 [95% CI, .64–4.60], respectively), which was statistically significant by the fourth decade (3.24 [95% CI, 1.29–8.15]. By the fifth decade of life, HIV-infected adults had a 1.6-fold increased prevalence of diastolic dysfunction as compared to uninfected controls (51.6% vs 32.7%; aOR, 5.73 [95% CI, 1.69–19.35]; P = .005).
Table 3.
Prevalence of Diastolic Dysfunction and Grade in People Living With Human Immunodeficiency Virus and Uninfected Participants
| Diastolic Dysfunction Grade | HIV-Uninfected (n = 461) | PLWH, ART Naive (n = 388) | OR (95% CI) | P Value |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Diastolic dysfunctiona | 40 (8.7%) | 51 (13.2%) | 2.71 (1.62–4.55) | <.0001a |
| Grade I | 19/40 (47.5%) | 13/51 (25.5%) | … | .028b |
| Grade II/III | 21/40 (52.5%) | 38/51 (74.5%) | … |
Abbreviation: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; PLWH, people living with human immunodeficiency virus.
aLogistic regression (adjusted for age, sex, systolic blood pressure, and body mass index) (P = .036 unadjusted).
bOrdinal logistic regression (adjusted for age, sex, systolic blood pressure, and body mass index) (P = .031 unadjusted).
Figure 1.
Age-stratified prevalence of diastolic dysfunction in people living with human immunodeficiency virus (HIV) and uninfected study participants. Logistic regression: P = .016 for linear trend across age groups.
We conducted a secondary, ad hoc analysis of moderation by sex. Sex disaggregated data revealed similar prevalence of diastolic dysfunction by sex and HIV status. Prevalence was lowest in HIV-uninfected males (8/146 [5.5%]) and highest in HIV-infected males (16/92 [17.4%]; aOR, 3.37 [95% CI, 1.24–9.18]; P = .018) adjusted for age, blood pressure, and BMI. The prevalence of dysfunction was also higher in HIV-infected females as compared to those uninfected (35/243 [14.4%] vs 32/274 [11.7%]; aOR, 2.56 [95% CI, 1.39–4.73]; P = .003). Supplementary Figure 1 displays the prevalence and 95% CI for each group.
Factors Associated With Left Ventricular Diastolic Dysfunction
In a multivariate model, systolic blood pressure (OR 1.03 [95% CI, 1.02–1.04]), HIV infection (2.71 [1.62–4.55]), and age (1.10 [1.07–1.13]) were significantly associated with echocardiographic diagnosis of diastolic dysfunction (Supplementary Table 2A).
In a univariate model, factors associated with dysfunction in both HIV-infected and uninfected participants were hypertension, systolic blood pressure, age, and BMI (Supplementary Table 2B). Abdominal obesity was significantly associated with dysfunction only in HIV-infected participants, whereas obesity by body mass was significantly associated only in HIV-uninfected participants. Of note, female sex trended toward significance in the HIV-uninfected group (P = .06).
Left Ventricular Diastolic Dysfunction Risk Factors
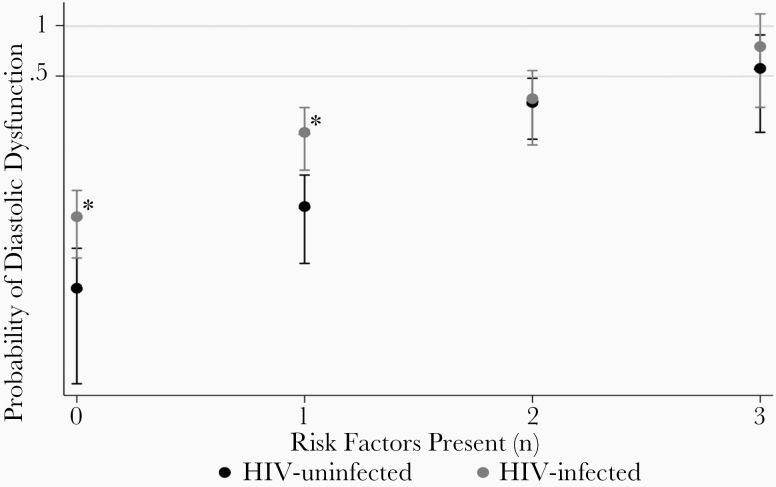
Figure 2 displays the proportion of HIV-infected vs uninfected participants with diastolic dysfunction according to the number of traditional risk factors present (hypertension, age ≥50 years, and BMI ≥30 kg/m2). HIV-infected participants without any traditional risk factors (7.3% vs 2.7%; odds ratio [OR], 2.80 [95% CI, 1.16–6.79]; P = .022) and those with only 1 traditional risk factor present (21.1% vs 8.3%; OR, 3.30 [95% CI, 1.50–7.28]; P = .003) had a significantly higher probability of diastolic dysfunction as compared to HIV-uninfected controls. The probability of dysfunction was the same in HIV-infected and uninfected participants if 2 or more traditional risk factors were present (OR, 1.09 [95% CI, .42–2.83]; P = .867 and 2.40 [95% CI, .18–32.88]; P = .512, respectively).
Figure 2.
Contribution of traditional risk factors to diastolic dysfunction in people living with human immunodeficiency virus (HIV) and uninfected participants. Risk factors: hypertension, age ≥50 years, and body mass index >30 kg/m2. *Logistic regression (P < .05).
Serum sST2 Concentration
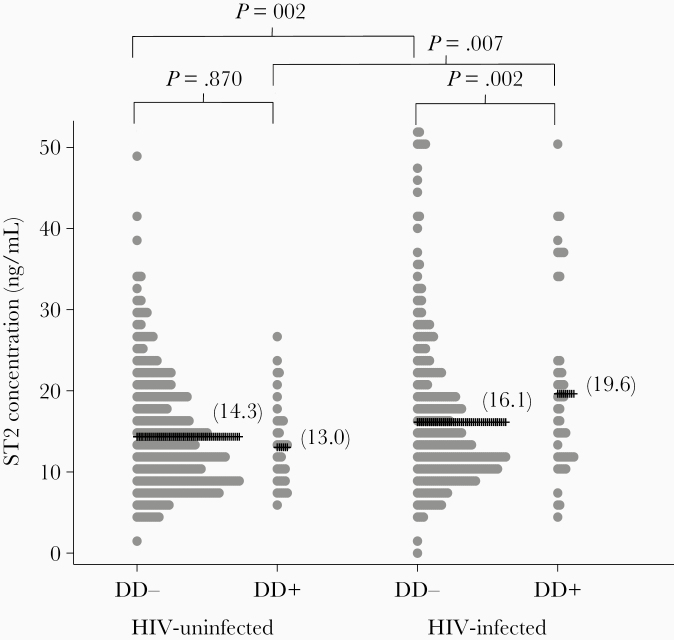
Serum sST2 concentration in HIV-infected and uninfected participants with and without myocardial diastolic dysfunction is shown in Figure 3. HIV-infected participants had statistically higher serum sST2 than uninfected controls (16.6 vs 14.2 ng/mL; P < .0001). Additionally, HIV-infected adults with diastolic dysfunction had higher sST2 than HIV-infected adults without dysfunction (19.6 vs 16.1 ng/mL; P = .002); however, this association was not observed in HIV-uninfected adults (13.0 vs 14.3 ng/mL; P = .870).
Figure 3.
Serum ST2 concentration in people living with human immunodeficiency virus (HIV) and uninfected participants with and without myocardial diastolic dysfunction (DD). Linear regression (adjusted for age, sex, systolic blood pressure, and body mass index).
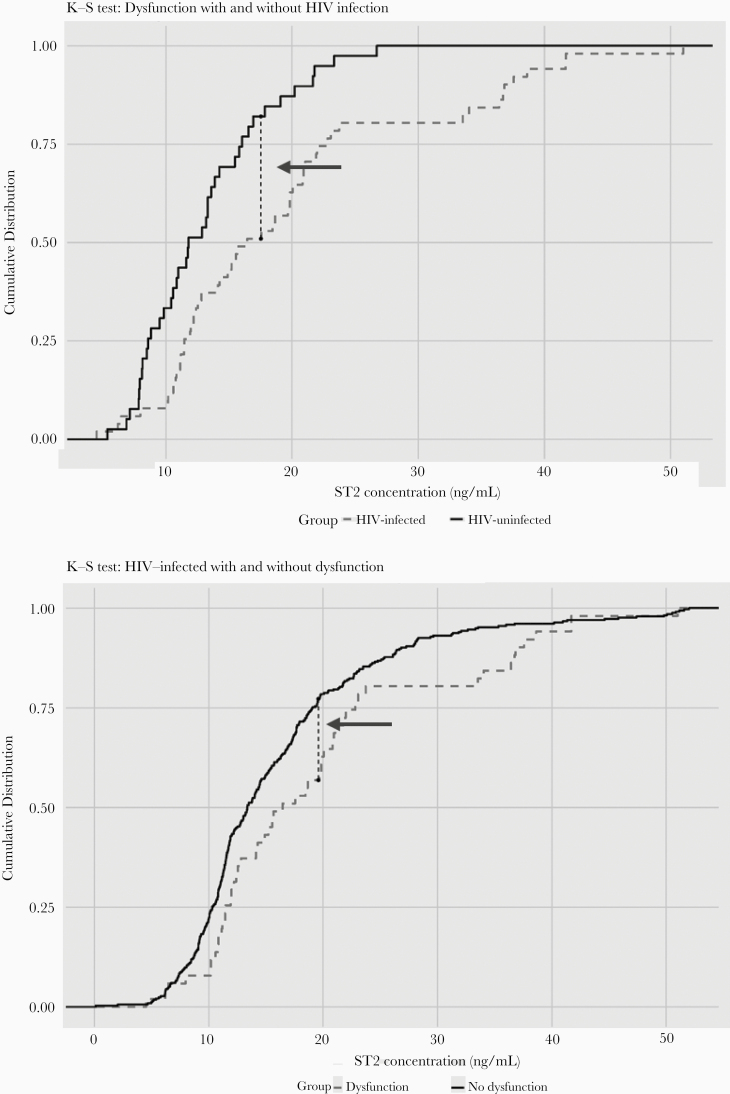
Figure 4 depicts Kolmogorov-Smirnov diagrams comparing sST2 distribution among groups. HIV-infected adults with dysfunction had statistically higher levels of sST2 as compared to uninfected controls with dysfunction (P = .024; Figure 4A). The point of maximal sST2 difference was 18 ng/mL; 82.5% of HIV-infected adults with diastolic dysfunction had sST2 >18 ng/mL vs 52.9% of HIV-uninfected controls. Additionally, HIV-infected participants with dysfunction had higher levels of sST2 as compared to HIV-infected participants without dysfunction (P = .04; Figure 4B). The point of maximal sST2 difference was 19.5 ng/mL; 75.4% of HIV-infected adults with diastolic dysfunction had an sST2 >19.5 ng/mL vs 56.9% of HIV-infected adults without diastolic dysfunction.
Figure 4.
Kolmogorov-Smirnov (K-S) diagrams for comparing participants with diastolic dysfunction with or without human immunodeficiency virus (HIV) infection (A) and participants with HIV infection with or without diastolic dysfunction (B). Arrow: point of maximal difference. K-S test: P = .02 (A) and P = .04 (B).
Sex-disaggregated data revealed similar serum ST2 values in male HIV-uninfected and infected participants with and without dysfunction and HIV-uninfected females with or without dysfunction. HIV-infected females with diastolic dysfunction had significantly higher serum ST2 concentration than HIV-infected females without dysfunction (14.0 vs 20.7 ng/mL; P < .0001). Supplementary Figure 2 displays the distribution and mean for each group.
DISCUSSION
We report that asymptomatic myocardial diastolic dysfunction, a precursor to heart failure with preserved ejection fraction, is nearly 2 times more common in HIV-infected Tanzanian adults as compared to uninfected controls. This dysfunction occurs as early as 20–30 years of age, which is at least 2 decades before that observed in the United States and Europe. Serum concentration of the cardiac biomarker sST2 is significantly associated with diastolic dysfunction in HIV-infected adults prior to ART exposure.
HIV-infected, ART-naive adults had a 1.5-fold higher prevalence of asymptomatic diastolic dysfunction (13.2%) as compared to HIV-uninfected treatment partners (8.7%). This finding persisted even after adjustment for the traditional risk factors of blood pressure, age, sex, and BMI. An increased prevalence of diastolic dysfunction in HIV-infected adults has previously been reported in those ART experienced in high-income countries [12–16, 42]. Our study indicates that this increased prevalence exists at the time of HIV diagnosis in East Africa. We also report that HIV infection independently imparts an elevated risk of myocardial diastolic dysfunction at the time of diagnosis and that this risk is independent of the other traditional risk factors (hypertension, age, and BMI). In fact, our data indicate that HIV-infected adults without these risk factors have a 3-fold higher odds ratio of dysfunction as compared to HIV-uninfected adults. However, if 2 of the traditional risk factors are present, the probability of dysfunction is similar in both HIV-infected and uninfected adults. Therefore, HIV alone is equivalent to one of the major traditional risk factors.
We further report that diastolic dysfunction is present in young Tanzanian adults with and without HIV infection. The prevalence of diastolic dysfunction increased with age with patients diagnosed as early as 21–30 years of age (4.5% vs 2.6%, respectively). The rates in both HIV-infected and uninfected persons increased 1.5- to 2-fold each decade with the difference between infected and uninfected achieving significance at age 40. After age 50, the prevalence was >50% in HIV-infected and 33% in uninfected adults. Our finding that 18.4% (29/148) of HIV-uninfected adults in the fifth and sixth decades of life have diastolic dysfunction is consistent with a study from South Africa, which reported a prevalence of 23.5% in an HIV-uninfected cohort with an average age of 45 years [43]. This study was not stratified by age and excluded HIV-infected persons. We expand on this finding by reporting that this disease burden is not limited to middle age in sub-Saharan Africa, but begins in early adulthood. This contrasts to the United States and Europe where dysfunction is typically prevalent only after the sixth decade of life [8, 44].
We determined that serum soluble ST2 concentration was significantly associated with asymptomatic diastolic dysfunction in ART-naive, HIV-infected adults but not in HIV-uninfected controls. HIV-infected adults with dysfunction had significantly higher ST2 concentration than HIV-infected persons without dysfunction (19.6 vs 16.1 ng/mL; P = .002). The association between sST2 and diastolic dysfunction was independent of blood pressure, age, and BMI. A previous study indicated that diastolic dysfunction was associated with elevated sST2 concentration in HIV-infected males on ART with an average age of 49 years in the United States [33]. Higher ST2 concentration in patients with diastolic dysfunction predicts worse outcomes [45]. This is particularly true for adults of African descent [46]. To our knowledge, this present study is the first to report this association either in sub-Saharan Africa or in the pre-ART period.
Soluble ST2 is an immunologically active decoy receptor for the cardioprotective antifibrotic cytokine IL-33. Elevated ST2 concentrations decrease IL-33 resulting in fibrosis and subsequent heart failure. Although the mechanism of the cardioprotection conferred by IL-33 remains unknown, preclinical studies suggest that IL-33 plays a central role in monocyte activation and that its antifibrotic effects may be monocyte mediated [47, 48]. Monocytes have recently been shown to be necessary for the development of diastolic dysfunction and failure [49]. We hypothesize that elevated serum sST2 in HIV-infected adults with dysfunction may be reflective of both lower myocardial protection from IL-33 and altered peripheral monocyte phenotype and that this may be most pronounced in females. Supporting this theory, a recent study reported that in a small cohort (n = 20) of HIV-infected women on ART an average of 18 years, the number of circulating intermediate monocytes expressing the surface homing marker CCR2 was associated with diastolic dysfunction [50]. The temporal course describing monocyte phenotype and associated immune markers in HIV-associated diastolic dysfunction, however, remains unknown.
There are limitations to the current study. First, this was a cross-sectional study. Therefore, whether the dysfunction identified at the time of HIV diagnosis persists, worsens, or regresses during ART initiation and maintenance in this population remains unknown. While participants did not have a history of heart failure or meet Framingham criteria, it is possible that a small fraction had subclinical symptomatology. HIV-infected adults with systolic myocardial dysfunction might have been excluded from this study due to symptoms of heart failure; therefore, conclusions regarding systolic dysfunction cannot be inferred from these data. Additionally, while statistically significant differences in echocardiographic parameters are reported, some of these may not translate into clinical significance. It is possible that pre-ART dysfunction is mediated by HIV-associated altered immunity, direct viral–myocardial interaction, or other processes, and represents an independent pathophysiology from that of long-term ART–associated dysfunction. Furthermore, while we report that sST2 levels are associated with dysfunction in the pre-ART period, whether this imparts alterations in immune cell phenotype or other pathways remains unknown. Delineation of the broader ST2 pathway may provide further insight into possible pathophysiologic disease targets.
In conclusion, we report that in a large cohort of asymptomatic East African adults, myocardial diastolic dysfunction is present at least 2 decades earlier than that observed in high-income settings. Furthermore, HIV infection is associated with an approximately 2-fold higher prevalence of dysfunction, and diastolic dysfunction is present in nearly 1 of 8 people with HIV at the time of HIV diagnosis. Serum ST2 levels were significantly associated with this dysfunction in HIV-infected, ART-naive but not HIV-uninfected adults, implying that ST2 may play a role in the pathogenesis of HIV-associated diastolic dysfunction and may represent a potential target for intervention. Taken together, these data provide insights into the trends and possible underlying immunological mechanisms of HIV-associated diastolic dysfunction in a geographic area with the highest HIV-attributable cardiovascular risk burden globally.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by a gift from Joan and Sanford I. Weill and the Weill Family Foundation. Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (grant number K24-AI098627); the Fogarty International Center (grant number K01 TW010281); and the NIH/Weill Cornell Clinical and Translational Science Center (grant number KL2-TR-002385).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep 2013; 10:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnes RP, Lacson JC, Bahrami H. HIV infection and risk of cardiovascular diseases beyond coronary artery disease. Curr Atheroscler Rep 2017; 19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freiberg MS, Chang CH, Skanderson M, et al. . Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol 2017; 2:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Janjua SA, Triant VA, Addison D, et al. . HIV infection and heart failure outcomes in women. J Am Coll Cardiol 2017; 69:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feinstein MJ, Hsue PY, Benjamin LA, et al. . Characteristics, Prevention, and Management of Cardiovascular Disease in People Living with HIV: A Scientific Statement From the American Heart Association. Circulation 2019; 140(2):e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bloomfield GS, Alenezi F, Barasa FA, Lumsden R, Mayosi BM, Velazquez EJ. Human immunodeficiency virus and heart failure in low- and middle-income countries. JACC Heart Fail 2015; 3:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kane GC, Karon BL, Mahoney DW, et al. . Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011; 306:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuznetsova T, Herbots L, López B, et al. . Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail 2009; 2:105–12. [DOI] [PubMed] [Google Scholar]
- 10. Ather S, Chan W, Bozkurt B, et al. . Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012; 59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsue PY, Hunt PW, Ho JE, et al. . Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail 2010; 3:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schuster I, Thöni GJ, Edérhy S, et al. . Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am J Cardiol 2008; 101:1213–7. [DOI] [PubMed] [Google Scholar]
- 13. Fontes-Carvalho R, Mancio J, Marcos A, et al. . HIV patients have impaired diastolic function that is not aggravated by anti-retroviral treatment. Cardiovasc Drugs Ther 2015; 29:31–9. [DOI] [PubMed] [Google Scholar]
- 14. Nayak G, Ferguson M, Tribble DR, et al. . Cardiac diastolic dysfunction is prevalent in HIV-infected patients. AIDS Patient Care STDS 2009; 23:231–8. [DOI] [PubMed] [Google Scholar]
- 15. Mondy KE, Gottdiener J, Overton ET, et al. . SUN Study Investigators High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis 2011; 52:378–86. [DOI] [PubMed] [Google Scholar]
- 16. Esser S, Gelbrich G, Brockmeyer N, et al. . Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol 2013; 102:203–13. [DOI] [PubMed] [Google Scholar]
- 17. Cerrato E, D’Ascenzo F, Biondi-Zoccai G, et al. . Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J 2013; 34:1432–6. [DOI] [PubMed] [Google Scholar]
- 18. Erqou S, Lodebo BT, Masri A, et al. . Cardiac dysfunction among people living with HIV: a systematic review and meta-analysis. JACC Hear Fail 2019; 7:98–108. [DOI] [PubMed] [Google Scholar]
- 19. Pitt B, Pfeffer MA, Assmann SF, et al. . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370:1383–92. [DOI] [PubMed] [Google Scholar]
- 20. Paulus WJ, van Ballegoij JJM. Treatment of heart failure with normal ejection fraction. An inconvenient truth! J Am Coll Cardiol 2010; 55:526–37. [DOI] [PubMed] [Google Scholar]
- 21. Pfeffer MA, Swedberg K, Granger CB, et al. . CHARM Investigators and Committees Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–66. [DOI] [PubMed] [Google Scholar]
- 22. Shah SJ, Kitzman DW, Borlaug BA, et al. . Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016; 134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veeraveedu PT, Sanada S, Okuda K, et al. . Ablation of IL-33 gene exacerbate myocardial remodeling in mice with heart failure induced by mechanical stress. Biochem Pharmacol 2017; 138:73–80. [DOI] [PubMed] [Google Scholar]
- 24. Suetomi T, Willeford A, Brand CS, et al. . Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca2+/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 2018; 138:2530–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hulsmans M, Sager HB, Roh JD, et al. . Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018; 215:423–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuwahara F, Kai H, Tokuda K, et al. . Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 2004; 43:739–45. [DOI] [PubMed] [Google Scholar]
- 27. Glezeva N, Baugh JA. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail Rev 2014; 19:681–94. [DOI] [PubMed] [Google Scholar]
- 28. Farcaş AD, Anton FP, Goidescu CM, Gavrilă IL, Vida-Simiti LA, Stoia MA. Serum soluble ST2 and diastolic dysfunction in hypertensive patients. Dis Markers 2017; 2017:2714095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmitz J, Owyang A, Oldham E, et al. . IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005; 23:479–90. [DOI] [PubMed] [Google Scholar]
- 30. Cayrol C, Girard JP. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev 2018; 281:154–68. [DOI] [PubMed] [Google Scholar]
- 31. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007; 117:1538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu X, Li Y, Song CB, et al. . Increased expression of sST2 in early HIV infected patients attenuated the IL-33 induced T cell responses. Front Immunol 2018; 9:2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Secemsky EA, Scherzer R, Nitta E, et al. . Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in HIV-infected individuals. JACC Heart Fail 2015; 3:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. et al. National guidelines for the management of HIV and AIDS 2017. https://www.nacp.go.tz/guidelines/.
- 35. Kingery JR, Alfred Y, Smart LR, et al. . Short-term and long-term cardiovascular risk, metabolic syndrome and HIV in Tanzania. Heart 2016; 102:1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riley L, Guthold R, Cowan M, et al. . The world health organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health 2016; 106:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lang RM, Badano LP, Mor-Avi V, et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 38. Nagueh SF, Appleton CP, Gillebert TC, et al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–33. [DOI] [PubMed] [Google Scholar]
- 39. Nagueh SF, Appleton CP, Gillebert TC, et al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009; 10:165–93. [DOI] [PubMed] [Google Scholar]
- 40. International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014; 104:1–52. [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva, Switzerland: WHO,2011. [Google Scholar]
- 42. Hsue PY, Hunt PW, Ho JE, et al. . Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail 2010; 3:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peterson VR, Woodiwiss AJ, Libhaber CD, Raymond A, Sareli P, Norton GR. Cardiac diastolic dysfunction is associated with aortic wave reflection, but not stiffness in a predominantly young-to-middle-aged community sample. Am J Hypertens 2016; 29:1148–57. [DOI] [PubMed] [Google Scholar]
- 44. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA 2003; 289:194–202. [DOI] [PubMed] [Google Scholar]
- 45. Savvoulidis P, Snider JV, Rawal S, et al. . Serum ST2 and hospitalization rates in Caucasian and African American outpatients with heart failure. Int J Cardiol 2020; 304:116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parikh RH, Seliger SL, Christenson R, Gottdiener JS, Psaty BM, deFilippi CR. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community-dwelling population. J Am Heart Assoc 2016; 5:e003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kurowska-Stolarska M, Stolarski B, Kewin P, et al. . IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol 2009; 183:6469–77. [DOI] [PubMed] [Google Scholar]
- 48. Joshi AD, Oak SR, Hartigan AJ, et al. . Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol 2010; 11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hulsmans M, Sager HB, Roh JD, et al. . Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018; 215:423–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zanni MV, Awadalla M, Toribio M, et al. . Immune correlates of diffuse myocardial fibrosis and diastolic dysfunction among aging women with human immunodeficiency virus. J Infect Dis 2019; 221:1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.