Abstract
Background
Chronic hepatitis B and D virus (HBV/HDV) infections can cause cancer. Current HBV therapy using nucleoside analogues (NAs) is life-long and reduces but does not eliminate the risk of cancer. A hallmark of chronic hepatitis B is a dysfunctional HBV-specific T-cell response. We therefore designed an immunotherapy driven by naive healthy T cells specific for the HDV antigen (HDAg) to bypass the need for HBV-specific T cells in order to prime PreS1-specific T cells and PreS1 antibodies blocking HBV entry.
Methods
Ten combinations of PreS1 and/or HDAg sequences were evaluated for induction of PreS1 antibodies and HBV- and HDV-specific T cells in vitro and in vivo. Neutralization of HBV by PreS1-specific murine and rabbit antibodies was evaluated in cell culture, and rabbit anti-PreS1 were tested for neutralization of HBV in mice repopulated with human hepatocytes.
Results
The best vaccine candidate induced T cells to PreS1 and HDAg, and PreS1 antibodies blocking HBV entry in vitro. Importantly, adoptive transfer of PreS1 antibodies prevented, or modulated, HBV infection after a subsequent challenge in humanized mice.
Conclusions
We here describe a novel immunotherapy for chronic HBV/HDV that targets viral entry to complement NAs and coming therapies inhibiting viral maturation.
Keywords: chronic hepatitis B, immunotherapy, PreS1 antibodies, T-cell tolerance, hepatitis D antigen
The paper shows a completely new immunotherapy for chronic hepatitis B and potentially also hepatitis D. The immunotherapy induced neutralizing antibodies and T cells targeting both HBV and HDV. Importantly, the induced antibodies neutralized HBV in vitro and in vivo.
Despite preventive vaccines and antiviral therapies, chronic hepatitis B virus (HBV) infection currently affects over 250 million people across the globe [1]. One million chronic carriers die every year due to the liver-related complications caused by HBV, such as liver cirrhosis and eventually hepatocellular carcinoma (HCC) [2–4]. The hepatitis D virus (HDV), an RNA satellite virus to HBV that “steals” the surface antigen from HBV (HBsAg), coinfects 15–25 million of the HBV carriers globally and worsens disease progression [3, 5]. As yet, there is no effective functional cure for chronic HBV or HDV infection. The current standard of care therapy for HBV consists of nucleoside analogues (NAs) that inhibit the reverse transcriptase (RT) function of HBV polymerase. This prevents viral maturation by blocking the synthesis of the partially double-stranded DNA inside the capsid [6]. Thus, NAs only suppress the viral replication during therapy. This is due to the fact that blocking RT neither affects protein production (including HBsAg) and release, nor synthesis of the covalently closed circular DNA, the main cause of HBV persistence [7]. Life-long NA therapy reduces, but does not eliminate, the risk of HCC [8, 9]. At least 1-year pegylated interferon-α (IFN-α) is the currently recommended treatment for chronic HDV; however, sustained response rates are low [3, 10]. Combination treatment with pegylated IFN-α and NAs has been shown to have limited efficacy against HDV [11] and HBV [12].
HBV uses several strategies in order to evade the host immune response. The chronic presence of HBV proteins induces a T-cell dysfunction [13–17]. HBV-infected cells overproduce subviral HBsAg particles, mainly containing small HBsAg [18], to block the neutralizing antibody population directed to small HBsAg [19]. This ensures survival of viral particles whose surfaces are denser in the middle HBsAg (containing S and PreS2) and large HBsAg (containing S, PreS2, and PreS1) proteins [18, 20]. Importantly, the PreS1 domain is responsible for binding to the Na+-taurocholate cotransporting polypeptide (NTCP) receptor for HBV on hepatocytes [21, 22]. Thus, an obvious way to target infectious HBV particles and prevent the infection of new hepatocytes would be to raise antibodies to the PreS1 domain of the virus.
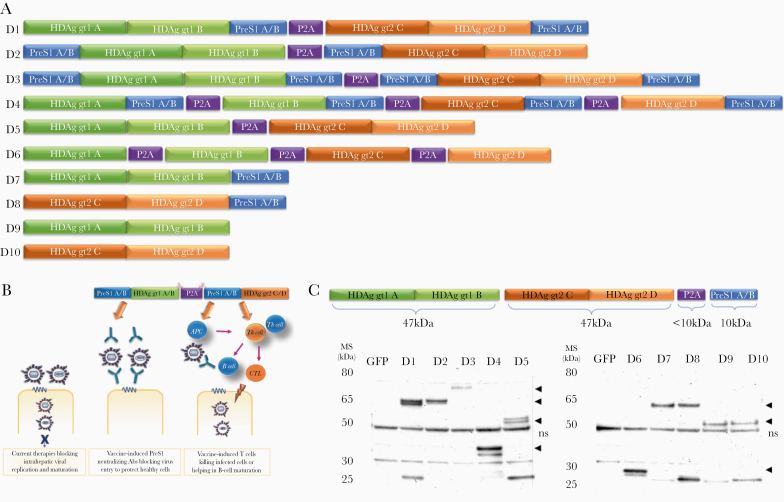
To build an immunotherapy targeting both infections able to induce PreS1 antibodies and T cells to HBV and HDV we generated chimeric genes containing PreS1 and the large HDV antigen (HDAg) in different combinations (Figure 1). The advantage of linking PreS1 to HDAg is that HDAg will act as a heterologous T-cell epitope carrier in patients monoinfected by HBV [21, 22]. Thus, these HDAg-specific T cells support a sustained endogenous production of PreS1 antibodies that block viral entry and bypass the need for HBV-specific T cells. In fact, >90% of HBV carriers are monoinfected with HBV [5], and in these the heterologous HDAg will prime healthy naive T cells that support priming of HBV-specific responses. In addition, it is likely the HDAg-specific T cells and PreS1 antibodies may also prevent HDV superinfection in these patients. To induce both neutralizing antibodies and T cells, we used genetic immunization, as this strategy has been shown to activate immune responses to HBV [23–25]. Overall, this virus entry-blocking strategy complements the maturation inhibitors and capsid assembly inhibitors currently under development [26, 27] in order to achieve sustainable off-therapy responses.
Figure 1.
Vaccine design, analysis of expression of HBV/HDV proteins from vaccine constructs, and therapy concept. A, A total of 10 vaccine constructs (D 1–10) were designed containing HBV and HDV sequences according to the design concept (B). C, The DNA vaccine candidates were characterized by direct western blot for their ability to be translated into the expected proteins of interest (arrow heads). The gene constructs consisted of different combinations of PreS1A/B consensus sequences linked to the HDAg of genotype 1 and/or genotype 2 from 4 different clinical isolates (A/B, genotype 1; C/D, genotype 2). The self-cleaving peptide P2A (approximately 10k Da) was included in some of the constructs for cleavage of the fusion proteins at different loci of PreS1A/B-HDAg sequences to test if and how this affects the functionality of the constructs. Depending on where the P2A cleaved the fusion proteins, bands of the respective fragments corresponded to the expected size range (ie, HDAg genotype 1 A/B at 47 kDa, HDAg genotype 2 C/D at 47 kDa, PreS1A/B at 10 kDa, and P2A at 10 kDa). Bands at around 50 kDa are nonspecific (ns). GFP was used as negative control. Detailed analysis of each construct and the sizes of the respective fragments are given in Supplementary Table 1. Abbreviations: GFP, green fluorescent protein; HBV, hepatitis B virus; HDAg, HDV antigen; HDV, hepatitis D virus.
METHODS
Animals
Female C57BL/6 (H-2b) mice were obtained from Charles River Laboratories, Sulzfeld, Germany. HLA-A2 transgenic HHD mice were bred inhouse. All mice were 8–10 weeks old at the start of the experiments and maintained under standard conditions at Preclinical laboratory (PKL), Karolinska University Hospital Huddinge, Sweden. uPA+/+-SCID mice with humanized liver were produced as previously described [28, 29] and maintained at the central animal facility of the Faculty of Medicine and Health Sciences, Ghent University. New Zealand White rabbits were purchased from commercial vendors and kept at Astrid Fagreus Facility at Karolinska Institute. All animal procedures were approved by local ethical committees for animal research in Sweden and Belgium.
DNA Plasmids
Plasmids encoding genotypes 1 and 2 of the L-HDAg and the PreS1 domain (aa 2–48) of the HBsAg were used in this study as fusion constructs, consisting of different combinations of HDAg/PreS1 sequences cleaved by P2A (Figure 1). The HDAg sequences of genotypes 1 and 2 were obtained from 4 different clinical isolates: US-2 and CB, and 7/18/83 and TW2476, respectively. All genes were cloned into the pVAX1 backbone (Invitrogen) using restriction sites EcoRI and HindIII. Plasmids were grown in TOP10 Escherichia coli cells (Life Technologies) and purified for in vivo injections using Qiagen Endofree DNA purification kit (Qiagen) following manufacturer’s instructions. The correct gene size was confirmed by restriction enzyme digests using EcoRI and HindIII (Fast Digest; Thermo Fisher Scientific).
Western Blot
Western blot was essentially performed as described previously [30, 31]. Hela cells were transfected with each pVAX1 D 1-10 DNA plasmid and pVAX1 with the reporter gene green fluorescent protein (GFP) as control (Figure 1), using Lipofectamine 3000 Transfection Reagent (Thermo Fisher Scientific). For proteins detection, serum from the D4 vaccinated rabbit diluted 1:1000 (primary antibody) and goat anti-rabbit immunoglobulins HRP 0.25g/L (DAKO) diluted 1:4000 (secondary antibody) were used. For chemiluminescence detection, Pierce ECL Plus Western Blotting Substrate was used and images were collected with the Gel Doc XR+ system (Biorad) (Supplementary Material).
Peptides
A total of 168 HDAg 15mer peptides with 10 amino acid (aa) overlap were purchased from Sigma-Aldrich. The 168 peptides were divided in 8 pools each containing 20 or 21 peptides (Supplementary Table 2). Four pools corresponded to genotype 1 (pool 11–21, pool 222–42, pool 343–63, and pool 464–84) and 4 pools corresponded to genotype 2 (pool 11–21, pool 222–42, pool 343–63, and pool 464–84) for sequences A, B, C, and D, with each sequence referring to each clinical isolate.
Two consensus sequences of the PreS1 HBsAg (PreS1A and PreS1B) consisting of 47 aa and 20mer PreS1peptides with 10 aa overlap for HBV (sub) genotypes A1, A2, B, B2, C, D1, E1, and F (Supplementary Table 3) were purchased from Sigma-Aldrich. All peptides passed quality control (Sigma-Aldrich PEPscreen Directory) and had purity >70%. OVA 257–264 CTL (SIINFEKL) and OVA 323–339 Th (ISQAVHAAHAEINEAGR) were used as negative peptide controls, while concanavalin A (Sigma Aldrich) was used as a positive control at final concentration 0.5μg/μL.
Immunization Protocols for Evaluating Immunogenicity of HBV/HDV Plasmids in Mice and Rabbits
To evaluate the immunogenicity of the constructs in vivo, mice and rabbits were immunized essentially as described [25, 30–32], boosted at monthly intervals, and sacrificed 2 weeks later for spleens and blood collection. In brief, female C57BL/6 mice (5 per group) were immunized intramuscularly in the tibialis cranialis anterior muscle with 50 μg plasmid DNA in a volume of 50 μL in sterile phosphate-buffered saline (PBS) by regular needle (27G) injection followed by in vivo electroporation using a Cliniporator 2 device (IGEA). During in vivo electroporation a 1-ms 600-V/cm pulse followed by a 400-ms 60-V/cm pulse pattern was used to facilitate better uptake of the DNA. Prior to vaccine injections, mice were given analgesic and kept under isoflurane anesthesia during the vaccinations. For studies in rabbits, 2 New Zealand White rabbits per group were immunized with 900 μg D3 and D4 DNA vaccines. Vaccines were administered by intramuscular injection in 300 μL sterile PBS to the right tibialis cranialis anterior muscle followed by in vivo electroporation.
Detection of IFN-γ–Producing T Cells by Enzyme-Linked Immunospot Assay
Two weeks after the last vaccination, splenocytes from each immunized group of mice were pooled (5 mice/group) and tested for their ability to induce HBV/HDV-specific T cells based on IFN-γ secretion after peptide stimulation for 48 hours as described previously [25, 30, 31] using a commercially available enzyme-linked immunospot (ELISpot) assay (Mabtech).
Antibody Detection by ELISA
Detection of mouse and rabbit immunoglobulin G (IgG) against PreS1 consensus and overlapping 20mer peptides (10 μg/mL) was performed following a previously described protocol [32]. Antibody titers were determined as endpoint serum dilutions at which the OD value at 405 nm was at least twice the OD of the negative control (nonimmunized or control animal serum) at the same dilution.
Neutralization Effect of PreS1 Antibodies by an HBV In Vitro Assay
HepG2-NTCP-A3 is a selected cell clone derived from the HepG2 cell expressing human NTCP as described previously [22]. It was cultivated in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin. During and after inoculation, 2.5% dimethyl sulfoxide (DMSO) was added to the medium to enhance HBV infection and replication. HBV virus stock used for infection was prepared from HepAD38 cells by PEG precipitation as described [33]. Cell culture media between day 3 and 6 postinfection were collected and diluted 1:5 with PBS for enzyme-linked immunosorbent assay (ELISA) analysis of hepatitis B e antigen (HBeAg) quantification using commercial antibodies (Fitzgerald) as described previously [34].
HBV Neutralization Assay in Human Liver–uPA-SCID Mouse Model
IgG antibodies were purified (Pierce Protein A kit; Thermo Fisher Scientific) from D4-vaccinated rabbit serum and concentrated (Amicon Ultra device; Millipore) at a final concentration of 50 mg/mL as determined spectrophotometrically at 280 nm. A total of 15 mg vaccine-induced IgG and IgG from nonimmunized rabbit was administered to human liver chimeric uPA-SCID mice [29, 35]. Three days after intraperitoneal administration of IgG, mice were challenged with an HBV dose of 106 IU/mouse. DNA levels were measured at weeks 1, 2, 3, 4, 6, and 8 using the Abbott RealTime HBV assay (Abbott Laboratories) to detect whether IgG administration could protect against HBV infection.
Statistical Analysis
Data was analyzed using GraphPad Prism v.5 and v.8 software and Microsoft Excel v.16.13.1.
RESULTS
Expression Products of HBV/HDV Plasmids
The 10 different HBV/HDV plasmids were correctly translated into the predicted proteins in vitro as confirmed by direct western blot (Figure 1 and Supplementary Table 1).
Immunization With HDAg Induces Genotype-Specific T Cells
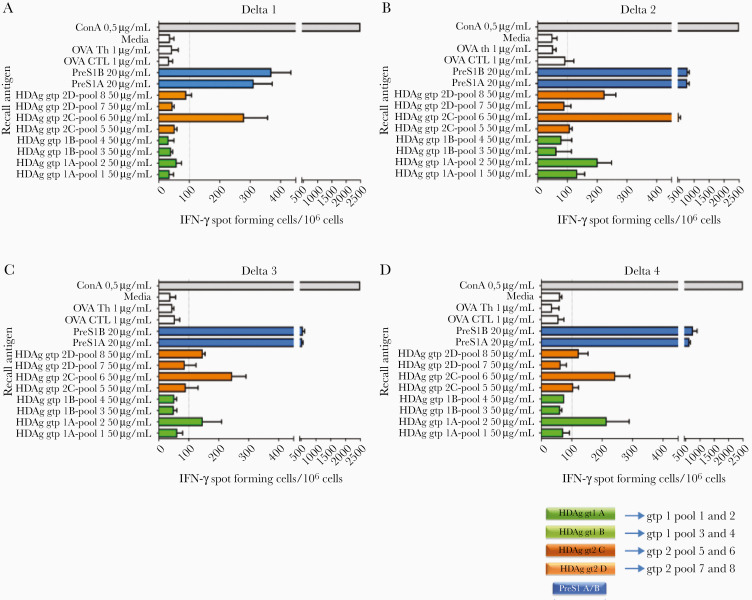
Vaccine primed HDV-specific T cells secrete high levels of IFN-γ in an HDAg genotype-specific pattern as determined by ELISpot, shown in Figure 2 and Supplementary Figure 1. In addition, strong T-cell responses to PreS1 were also seen (Figure 2). Notably, in D3 and D4-treated groups a slight reduction in HDV T-cell responses was observed compared to constructs that contained only HDAg (D5, D6, D9, and D10; Supplementary Figure 1), which might be attributable to epitope recognition competition with the simultaneous priming of PreS1-specific T cells. Overall, this shows that active immunization is able to induce functional T cells to PreS1 and HDAg antigens and suggests that a broadly functional immunotherapy should contain both HDV genotypes 1 and 2 to ensure induction of specific T cells.
Figure 2.
HBV/HDV IFN-γ–secreting T cells are genotype specific. Graphs show the mean number of IFN-γ–secreting cells per million for 4 representative vaccinated mice groups with D1–D4 constructs after stimulation with the respective antigens, as determined by ELISpot. Each color corresponds to the respective sequences of the constructs shown in Figure 1 (ie, green, HDAg genotype 1; orange, HDAg genotype 2; blue, PreS1 A/B). Mice were sacrificed 6 weeks after first immunization and pooled splenocytes from each group were stimulated for 48 hours with the HDV peptide pools 1–8 corresponding to genotype 1 (pools 1–4) and genotype 2 (pools 5–8). Pools 1 and 2 of genotype 1 refer to sequence/isolate A while pools 3 and 4 correspond to sequence/isolate B. Similarly, for HDV genotype 2, pools 5 and 6 refer to sequence/isolate C and pools 7 and 8 to sequence/isolate D (Supplementary Table 2). Each pool contained 20 or 21 (for pools 1 and 5) 15mer peptides with 10-aa overlap. PreS1A and PreS1B peptides refer to the consensus sequences (total 47 aa; region 2–48 aa) while OVA CTL and OVA Th (1 μg/mL) were negative controls. ConA (0.5 μg/mL) was a positive control. Each peptide-stimulated group was run in triplicate and bars show the mean number of IFN-γ SFC per 106 cells with standard error. Cutoff was set at 100 SFCs/106 splenocytes. Abbreviations: aa, amino acid; ConA, concanavalin A; ELISpot, enzyme-linked immunospot; gpt, genotype; HBV, hepatitis B virus; HDAg, HDV antigen; HDV, hepatitis D virus; IFN-γ, interferon-γ; SFC, spot forming cell.
DNA Immunization Induces Broadly Cross-Reactive PreS1 Antibodies and HLA-A2–Restricted T Cells
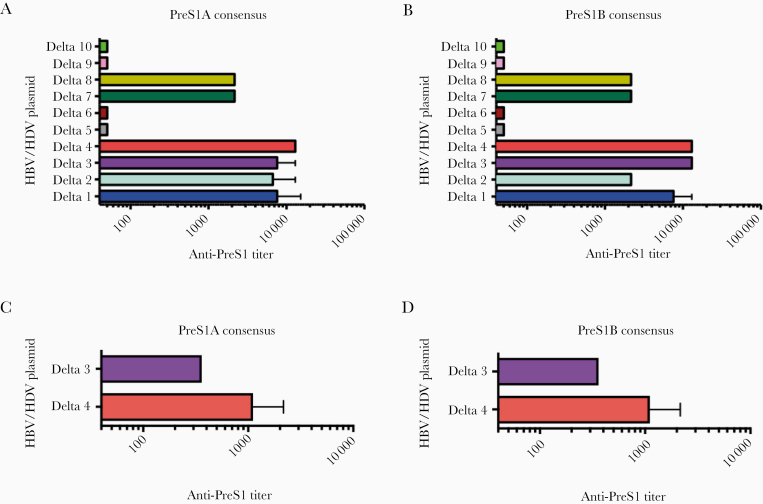
The ability of the candidate immunotherapies to induce PreS1 antibodies was determined in sera from immunized mice and rabbits. Antisera were tested for reactivity against PreS1A and PreS1B consensus peptides (aa 2–48) and for cross-reactivity against HBV (sub) types A1, A2, B, B2, C, D1, E1, and F using pools of 20mer PreS1-peptides (Supplementary Figure 2 and Figure3). As shown in Figure 3, the DNA plasmids expressing constructs D3 and D4 induced antibody titers >104 in mice, and lower titers with constructs D1, D2, D7, and D8. Importantly, antisera from D4 and D7 immunized mice effectively cross-reacted with all tested HBV types (Supplementary Figure 2). The data from the D3 and D4 immunized rabbits followed the same pattern, with the D4 plasmid inducing antibodies >103 (Figure 3C and 3D).
Figure 3.
Detection of PreS1-specific IgG by ELISA. IgG antibodies from 6-week vaccinated mouse and rabbit sera were measured by ELISA, as previously described [32]. All 10 DNA constructs (D 1–10) were tested for IgG antibodies against (A) PreS1A and (B) PreS1B 2-48 amino acid consensus sequences in mice (5 mice per group); only constructs containing PreS1 were further evaluated for cross-reactivity against HBV (sub) genotypes A1, A2, B, B2, C, D1, E1, and F (Supplementary Figure 2). Similarly, rabbits (2 per group) were vaccinated with D3 and D4 DNA constructs and 6 weeks after first immunization sera were collected and tested by ELISA against (C) PreS1A and (D) B consensus peptides. The vaccinated rabbit antisera were also tested for cross-reactivity to HBV (sub) genotypes A1, A2, B, B2, C, D1, E1, and F (Supplementary Figure 3). Bars show the mean anti-PreS1 titers for each group, determined as the last serum dilution giving an OD 405nm 3 times higher than the OD of nonimmunized sera at the same dilution. Sera were titrated serially with 6-fold dilutions starting at 1:60. A and B, Mean ± SD from 2 independent experiments. C and D, Mean from 1 representative experiment. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HBV, hepatitis B virus; HDV, hepatitis D virus; IgG, immunoglobulin G.
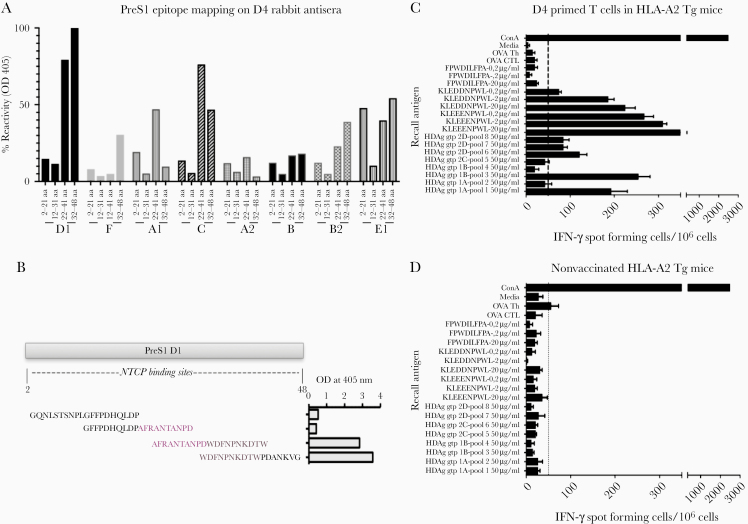
The fine specificity of the rabbit D4 antisera was determined using individual 20mer PreS1 peptides of HBV types A1, A2, B, B2, C, D1, E1, and F (Supplementary Table 3 and Figure 4). This mapped the epitopes to PreS1 region 22–48 aa of genotype D1, as indicated by the higher reactivity, with lower reactivity to genotypes C, E1, and A1 (Figure 4). This overlaps with the NTCP binding site and partly with previously identified epitopes recognized by neutralizing antibodies [36]. Finally, immunization of HLA-A2 Tg mice with D4 induced “human-like” HLA-A2–restricted T cells (Figure 4).
Figure 4.
Percentage reactivity of D4-vaccinated rabbit antisera against PreS1 of different HBV (sub) genotypes. A and B, Six-week D4-vaccinated rabbit antisera were tested for reactivity (OD 405 nm) against HBV (sub) genotypes D1, F, A1, C, A2, B, B2, and E1 by ELISA using individual 20mer PreS1 peptides with 10-aa overlap corresponding to each HBV type of aa 2–21, 12–31, 22–41, and 32–48 (Supplementary Table 3), neutralizing epitopes mainly localized at aa 22–41 and 32–48 of genotype D1 as indicated by the highest percentage of reactivity, and (sub) types C, E1, and A1 at the same aa region. C and D, DNA vaccination with the D4 construct in HHD mice transgenic for the HLA-A2.1 molecule. Splenocytes were restimulated with MHC class I restricted peptide pools described in Figure 2 and 3 individual 9mer peptides chosen based on NetCTL scoring.
Abbreviations: aa, amino acid; ELISA, enzyme-linked immunosorbent assay; HBV, hepatitis B virus; IFN-γ, interferon-γ; NTCP, Na+-taurocholate cotransporting polypeptide.
PreS1 Antibodies From Immunized Mice and Rabbits Neutralize HBV In Vitro
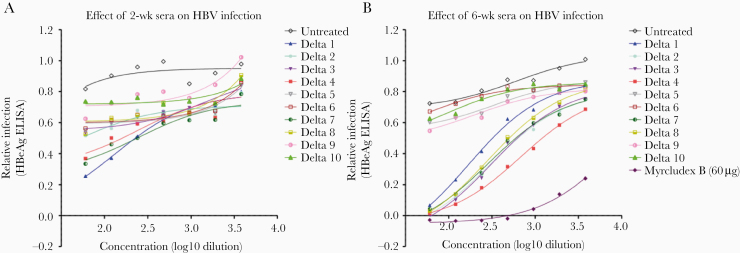
Sera from immunized mice obtained 2 weeks (mice) and 6 weeks (mice and rabbits) after first immunization were tested for ability to prevent HBV infection of HepG2 cells expressing Human NTCP (Figure 5 and Figure 6A). Neutralization of HBV was determined by detection of secreted HBeAg in culture supernatants. The immunogens D3 and D4 induced the strongest neutralizing antibodies (Figure 5), suggesting that these PreS1 antibodies indeed block HBV entry. In rabbits, only the D4 plasmid induced cross-reactive PreS1 antibodies (Figure 3), and we noted that anti-PreS1 levels by ELISA of >103 were required for achieving in vitro neutralization of HBV (Figure 6A).
Figure 5.
In vitro neutralization against HBV from 2-week (A) and 6-week (B) vaccinated mouse antisera. All the vaccinated mouse antisera (D 1–10) were evaluated for their functionality by an in vitro neutralization assay against HBV on HepG2 cells expressing Human NTCP. Quantification of HBV infection was determined by measuring secreted HBeAg using ELISA, presented as relative infectivity (% HBeAg). Standard curve was generated from HBV infected but untreated (negative control) group diluted with phosphate-buffered saline. All groups vaccinated with constructs containing PreS1 showed neutralization activity at 2 weeks after first immunization (A) while the D4 vaccine construct had the strongest neutralization effect as indicated by the lowest relative infectivity at 6 week, followed by construct D3 (B). All samples from both week 2 and week 6 time points were run at the same time and Myrcludex B (60 μM) was used as positive control in the assay. Abbreviations: HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; ELISA, enzyme-linked immunosorbent assay; NTCP, Na+-taurocholate cotransporting polypeptide.
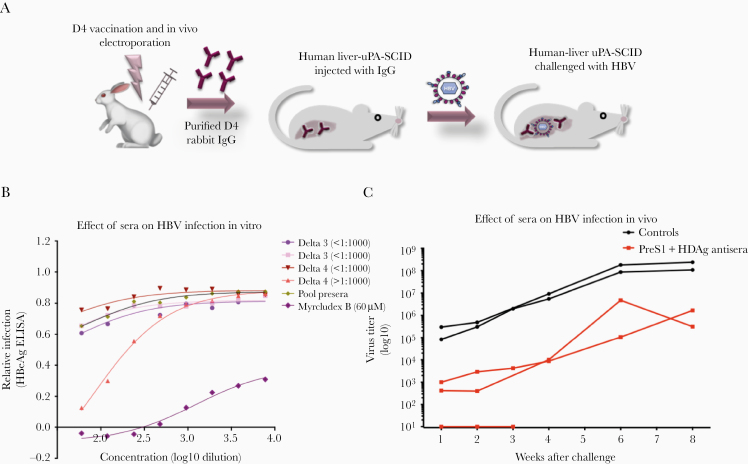
Figure 6.
HBV in vitro and in vivo neutralization assays of 6-week antirabbit sera. A, Two rabbits per group were immunized twice with D3 and D4 vaccine constructs, sacrificed, and whole blood collected. IgG antibodies from the vaccinated sera were evaluated by HBV in vitro neutralization assay as described previously. The effect of rabbit antisera on HBV infection was dependent on the antibody titers. B, Only the D4 vaccine construct, which induced higher antibody end titers at >103, neutralized HBV, as indicated by the lowest relative infectivity (secreted HBeAg) by ELISA, while the others had titers <103 and a nonneutralization effect was observed for these. D4-induced IgG antibodies from this rabbit and nonspecific IgG from a nonimmunized rabbit were purified and administered in human liver–uPA-SCID mice for evaluation of HBV neutralization in vivo. Three days after IgG administration, mice were challenged with HBV and monitored for 8 weeks after inoculation. C, Protective effect against HBV infection at 1, 2, 3, 4, 6, and 8 weeks after first inoculation, as determined by HBV titers at each time point. Each line represents 1 mouse. Two negative control mice (black) received nonimmunized IgG and 3 mice (red) received D4 PreS1 IgG. One mouse of the PreS1-IgG treated group died at week 4, thus week 1, 2, and 3 measurements only are available for this mouse. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus.
Neutralization of HBV Infection In Vivo in Humanized Mice
The ability of D4-induced antibodies to neutralize HBV infection in vivo was determined using the human liver chimeric uPA-SCID mouse model, as described [29, 35]. Total IgG was purified from D4-immunized and nonimmunized rabbits and injected in uPA-SCID mice repopulated with human hepatocytes 3 days prior to HBV challenge. The D4-induced PreS1 IgG antibodies protected, or significantly delayed, peak viremia in all challenged mice (Figure 6). Of 3 challenged mice, 1 was protected (weeks 1 to 3) whereas the other 2 developed serum levels of HBV <104 IU/mL up to 4 weeks postchallenge and remained lower compared to the controls at 8 weeks postchallenge (Figure 6B). The control mice treated with IgG from a naive rabbit all reached serum HBV DNA levels exceeding 108 IU/mL (Figure 6). There were no significant differences between the groups with respect to serum levels of alanine transferase, asparagine transferase, alkaline phosphatase, or bilirubin (Supplementary Figure 4). In conclusion, passive immunization with D4-specific PreS1 IgG antibodies given as a single dose was able to prevent, or significantly delay, HBV infection in vivo in mice repopulated with human hepatocytes. Importantly, the inoculum contained high levels of subviral particle small HBsAg, showing that these antibodies indeed escaped being blocked by small HBsAg. The PreS1 antibodies present at inoculation and during the first weeks clearly block infection, or the first rounds of infection, and limit the number of infected hepatocytes. This limits the viral spread and delays development of peak viremia.
DISCUSSION
The current antiviral treatments for chronic HBV and HDV infections using NAs or other small molecules can effectively suppress the viral load during ongoing therapy and slow down disease progression, but do not fully prevent development of liver disease [4, 9]. None of these compounds effectively induce a functional cure, especially when targeting the immune-tolerant chronic carrier [12, 37]. A functional cure is defined as the stable loss of HBsAg in serum. New therapeutic strategies are needed that overcome, or circumvent, the immune evasion strategies exerted by the virus and stimulate the host to control the infection and induce a functional cure [38].
We therefore designed an immunotherapy targeting an additional step of the viral life cycle to improve off-therapy responses. The therapy was designed with 2 main considerations: (1) to block HBV and HDV infection by stimulating endogenous PreS1 receptor-blocking antibody production; and (2) to avoid using large HBV sequences, as T cells to HBV antigens are highly dysfunctional in chronic HBV. To recruit healthy T cells that do not cross-react with dysfunctional HBV-specific cells in patients with chronic HBV, we used HDAg as a heterologous T-cell carrier. Because >90% of HBV carriers are not coinfected by HDV [5], the HDAg-specific T cells are, in the vast majority of HBV carriers, as naive and healthy as those in as naive and healthy as T cells to any other HBV-unrelated heterologous carrier antigen [30]. However, these HDV-specific T cells can still be relevant for HDV and thus an HBV monoinfected carrier may become immune to HDV superinfection through PreS1-HDAg–based vaccination. Thus, using HDAg as a carrier for PreS1 sequences serves multiple purposes.
Using a genetic vaccine that contains PreS1 and HDAg, we can induce endogenous production of PreS1 antibodies that block the PreS1-mediated entry of HBV using the receptor NTCP [39]. PreS1 is only a minor component on subviral particles that cause the immune dysfunction but it is vital for the infectivity of HBV particles [18, 20, 40]. Therefore, the PreS1-specific antibodies efficiently avoid being blocked by the circulating subviral particles and can neutralize HBV [19]. To test our concept described above, we generated 10 different DNA plasmids as immunogens containing various combinations of PreS1 and/or HDAg sequences. These were tested for induction of specific antibodies and T cells to identify the most immunogenic design. The DNA constructs induced strong PreS1 and HDV T-cell responses. The HDAg-specific T cells were genotype specific in mice of 2 different genetic backgrounds (Figure 2 and data not shown). Thus, to avoid a potential T-cell nonresponder status in humans, we selected constructs carrying 2 strains of HDAg corresponding to the 2 major genotypes of HDV. The constructs combining PreS1 and HDAg sequences effectively induced high levels of PreS1 antibodies in both mice and rabbits. PreS1 antibodies from both species were highly cross-reactive against different HBV types and subtypes. The most immunogenic design, which raised potent HBV/HDV-specific T-cell responses in wild-type and HLA-A2 transgenic mice and high anti-PreS1 titers, comprised HDAg sequences of 2 genotypes coupled to tandem PreS1 sequences. Importantly, we could demonstrate that the antibodies induced by these active immunogens were also the most effective at neutralizing HBV in vitro, using a well-established assay that efficiently supports the full viral life cycle [22, 33]. We were then interested in determining whether these entry-blocking anti-PreS1 antibodies could inhibit HBV infection of human hepatocytes in vivo. From the studies in rabbits, it was evident that antibody levels >103 to PreS1, as determined by ELISA, neutralized HBV in vitro. We therefore purified total IgG from D4 immunized and nonimmunized rabbits and evaluated these antibodies for protection against HBV infection in a mouse model repopulated with human hepatocytes [35]. We found that a single injection of these antibodies indeed protected, or limited, HBV infection in vivo over a 3 to 8-week period. The reduced levels in 2 mice were most likely due to a limited first round infection and neutralization over the first weeks from challenge. Thus this delayed the development of peak HBV viremia. We therefore concluded that a D4 DNA-based immunotherapy protects human hepatocytes against HBV infection both in vitro and in vivo.
There are currently different approaches in development aiming at inhibiting HBV entry and these are mainly based either on PreS1-derived peptides or antibodies targeting the S or PreS1/2 domains of HBsAg. PreS1 antibodies are known to neutralize HBV [41]. The most advanced approach in clinical development so far is a PreS1 peptide-based competitive inhibitor termed Myrcludex B, which has been successful in blocking entry of mainly HDV, but also HBV [42]. This peptide, however, requires daily subcutaneously administration for 12–24 weeks to reach optimal antiviral effects in patients with chronic HDV and/or HBV. Also, although it can successfully prevent HDV/HBV entry by competitively binding to NTCP, alterations in the bile acid transport and metabolite functions of the receptor have been noted [43, 44]. PreS1-peptide–based vaccines have also been shown to be able to break immunotolerance in chronic HBV carrier mice, supporting its important therapeutic role in controlling HBV infection [45]. Other strategies in the pipeline are therapeutic vaccines containing different versions of HBV antigens [46]. Although, it has been shown that vaccines containing only HBV sequences can be safe and occasionally elicit T-cell responses in chronic HBV carriers [23, 24], it still remains unclear if they can overcome, or restore, the T-cell dysfunction of the infected host and, thus, be of clinical benefit [47]. It is likely that additional immunologic triggers are needed to activate the host’s immune responses [48, 49]. We therefore aimed at raising endogenously produced antibodies against the PreS1 antigen using PreS1 sequences linked to HDAg. Here, HDAg acts as a heterologous T-cell epitope carrier that circumvents, or bypasses, the engagement and dependence on the dysfunctional HBV-specific T-cell response present in a chronically infected host. As an added benefit, the immunized chronic carriers monoinfected with HBV can become immune against an HDV superinfection.
In conclusion, we have designed a new immunotherapy approach for HBV and potentially HDV. Our design concept aims at circumventing the major hurdles in the infected host, such as overexpression of antigens that block neutralizing antibodies and the profound T-cell dysfunction/tolerance. Here, HDAg serves as a healthy heterologous T- and B-cell carrier to circumvent the engagement of dysfunctional HBV-specific T cells in the induction of PreS1 antibodies. We show induction of T cells specific for PreS1 and HDAg and antibodies that block HBV entry into hepatocytes in vitro and in vivo. The concept of bypassing T-cell tolerance will be further assessed in the context of a chronic carrier model. This approach, with the host as the producer of the entry inhibitors, may, in combination with existing and coming maturation inhibitors, achieve durable off-therapy responses and a functional cure for chronic HBV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The staff in the animal facility at Karolinska University Hospital are acknowledged for excellent assistance with mouse studies; and Urban Höglund and Adlego are acknowledged for excellent assistance with rabbit studies.
Author contributions. The concept was developed by M. S. and L. F. Experimental design was carried out by M. S., L. F., A. P., G. A., P. M., and M. J. Animal work and immunological experiments were performed by P. M., L. F., N. C. P., G. A., A. P., and N. J. Neutralization assays were performed by Y. N., S. U., G. V., L. V., and Ph. M. Data analysis and writing was performed by all authors. All authors approved the final version of the manuscript.
Financial support. This work was supported by the Swedish Swedish Research Council, The Swedish Cancer Society, Region Stockholm, and Vinnova to M. S.; the Knowledge Foundation to M. J.; and the Research Foundation-Flanders (grant numbers G089515N and G047417N) and Excellence of Science Project (grant number 30981113) to P. M. and Karolinska Institutet (to M. S. and L. F.).
Potential conflicts of interest. M. S. and L. F. are founders and shareholders of Svenska Vaccinfabriken who holds the intellectual property to the immunogens described herein. S. U. is the inventor behind Myrcludex. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Razavi-Shearer D, Gamkrelidze I, Nguyen MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018; 3:383–403. [DOI] [PubMed] [Google Scholar]
- 2. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014; 384:2053–63. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Globalhepatitis report, 2017 Geneva, Switzerland: WHO,2018. [Google Scholar]
- 4. Mitra B, Thapa RJ, Guo H, Block TM. Host functions used by hepatitis B virus to complete its life cycle: implications for developing host-targeting agents to treat chronic hepatitis B. Antiviral Res 2018; 158:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen H-Y, Shen D-T, Ji D-Z, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis [published online ahead of print 18 September 2018]. Gut doi: 10.1136/gutjnl-2018-316601. [DOI] [PubMed] [Google Scholar]
- 6. Liu J, Li T, Zhang L, Xu A. The role of hepatitis B surface antigen in nucleos(t)ide analogues cessation among Asian chronic hepatitis B patients: a systematic review. Hepatology 2019; 70:1045–55. [DOI] [PubMed] [Google Scholar]
- 7. Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015; 64:1972–84. [DOI] [PubMed] [Google Scholar]
- 8. Papatheodoridis GV, Manolakopoulos S, Touloumi G, et al. ; HEPNET Greece Cohort Study Group Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut 2011; 60:1109–16. [DOI] [PubMed] [Google Scholar]
- 9. Zoulim F, Mason WS. Reasons to consider earlier treatment of chronic HBV infections. Gut 2012; 61:333–6. [DOI] [PubMed] [Google Scholar]
- 10. Heidrich B, Yurdaydın C, Kabaçam G, et al. ; HIDIT-1 Study Group Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology 2014; 60:87–97. [DOI] [PubMed] [Google Scholar]
- 11. Wedemeyer H, Yurdaydìn C, Dalekos GN, et al. ; HIDIT Study Group Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011; 364:322–31. [DOI] [PubMed] [Google Scholar]
- 12. Feld JJ, Terrault NA, Lin HS, et al. Entecavir and peginterferon alfa‐2a in adults with hepatitis B e antigen‐positive immune-tolerant chronic hepatitis B virus infection. Hepatology 2019; 69:2338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen M, Sällberg M, Thung SN, Hughes J, Jones J, Milich DR. Nondeletional T-cell receptor transgenic mice: model for the CD4(+) T-cell repertoire in chronic hepatitis B virus infection. J Virol 2000; 74:7587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen M, Sällberg M, Hughes J, et al. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol 2005; 79:3016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen MT, Billaud JN, Sällberg M, et al. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc Natl Acad Sci U S A 2004; 101:14913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mason WS, Gill US, Litwin S, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 2016; 151:986–98.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milich DR. The concept of immune tolerance in chronic hepatitis B virus infection is alive and well. Gastroenterology 2016; 151:801–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Short JM, Chen S, Roseman AM, Butler PJ, Crowther RA. Structure of hepatitis B surface antigen from subviral tubes determined by electron cryomicroscopy. J Mol Biol 2009; 390:135–41. [DOI] [PubMed] [Google Scholar]
- 19. Rydell GE, Prakash K, Norder H, Lindh M. Hepatitis B surface antigen on subviral particles reduces the neutralizing effect of anti-HBs antibodies on hepatitis B viral particles in vitro. Virology 2017; 509:67–70. [DOI] [PubMed] [Google Scholar]
- 20. Dryden KA, Wieland SF, Whitten-Bauer C, Gerin JL, Chisari FV, Yeager M. Native hepatitis B virions and capsids visualized by electron cryomicroscopy. Mol Cell 2006; 22:843–50. [DOI] [PubMed] [Google Scholar]
- 21. Ni Y, Sonnabend J, Seitz S, Urban S. The pre-s2 domain of the hepatitis B virus is dispensable for infectivity but serves a spacer function for L-protein-connected virus assembly. J Virol 2010; 84:3879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ni Y, Lempp FA, Mehrle S, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 2014; 146:1070–83. [DOI] [PubMed] [Google Scholar]
- 23. Mancini-Bourgine M, Fontaine H, Scott-Algara D, Pol S, Bréchot C, Michel ML. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology 2004; 40:874–82. [DOI] [PubMed] [Google Scholar]
- 24. Kosinska AD, Zhang E, Johrden L, et al. Combination of DNA prime-adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog 2013; 9:e1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brass A, Frelin L, Milich DR, Sällberg M, Ahlén G. Functional aspects of intrahepatic hepatitis B virus-specific T cells induced by therapeutic DNA vaccination. Mol Ther 2015; 23:578–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuen MF, Gane EJ, Kim DJ, et al. Antiviral activity, safety, and pharmacokinetics of capsid assembly modulator NVR 3-778 in patients with chronic HBV infection. Gastroenterology 2019; 156:1392–403.e7. [DOI] [PubMed] [Google Scholar]
- 27. Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology 2015; 62:1893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meuleman P, Vanlandschoot P, Leroux-Roels G. A simple and rapid method to determine the zygosity of uPA-transgenic SCID mice. Biochem Biophys Res Commun 2003; 308:375–8. [DOI] [PubMed] [Google Scholar]
- 29. Meuleman P, Libbrecht L, De Vos R, et al. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology 2005; 41:847–56. [DOI] [PubMed] [Google Scholar]
- 30. Chen A, Ahlén G, Brenndörfer ED, et al. Heterologous T cells can help restore function in dysfunctional hepatitis C virus nonstructural 3/4A-specific T cells during therapeutic vaccination. J Immunol 2011; 186:5107–18. [DOI] [PubMed] [Google Scholar]
- 31. Levander S, Holmström F, Frelin L, et al. Immune-mediated effects targeting hepatitis C virus in a syngeneic replicon cell transplantation mouse model. Gut 2018; 67:1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahlén G, Söderholm J, Tjelle T, et al. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol 2007; 179:4741–53. [DOI] [PubMed] [Google Scholar]
- 33. Ni Y, Urban S. Hepatitis B virus infection of HepaRG cells, HepaRG-hNTCP cells, and primary human hepatocytes. Methods Mol Biol 2017; 1540:15–25. [DOI] [PubMed] [Google Scholar]
- 34. Donkers JM, Zehnder B, van Westen GJP, et al. Reduced hepatitis B and D viral entry using clinically applied drugs as novel inhibitors of the bile acid transporter NTCP. Sci Rep 2017; 7:15307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meuleman P, Lerouxroels G. The human liver-uPA-SCID mouse: A model for the evaluation of antiviral compounds against HBV and HCV. Antiviral Res 2008; 80:231–8. [DOI] [PubMed] [Google Scholar]
- 36. Wi J, Jeong MS, Hong HJ. Construction and characterization of an anti-hepatitis B virus preS1 humanized antibody that binds to the essential receptor binding site. J Microbiol Biotechnol 2017; 27:1336–44. [DOI] [PubMed] [Google Scholar]
- 37. Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology 2016; 63:284–306. [DOI] [PubMed] [Google Scholar]
- 38. Revill PA, Chisari FV, Block JM, et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol 2019; 4:545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neurath AR, Kent SB, Strick N, Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell 1986; 46:429–36. [DOI] [PubMed] [Google Scholar]
- 40. Gripon P, Le Seyec J, Rumin S, Guguen-Guillouzo C. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology 1995; 213:292–9. [DOI] [PubMed] [Google Scholar]
- 41. Hong HJ, Ryu CJ, Hur H, et al. In vivo neutralization of hepatitis B virus infection by an anti-preS1 humanized antibody in chimpanzees. Virology 2004; 318:134–41. [DOI] [PubMed] [Google Scholar]
- 42. Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor Myrcludex B: first results of a phase Ib/IIa study. J Hepatol 2016; 65:490–8. [DOI] [PubMed] [Google Scholar]
- 43. Blank A, Eidam A, Haag M, et al. The NTCP-inhibitor Myrcludex B: effects on bile acid disposition and tenofovir pharmacokinetics. Clin Pharmacol Ther 2018; 103:341–8. [DOI] [PubMed] [Google Scholar]
- 44. Passioura T, Watashi K, Fukano K, et al. De novo macrocyclic peptide inhibitors of hepatitis B virus cellular entry. Cell Chem Biol 2018; 25:906–15.e5. [DOI] [PubMed] [Google Scholar]
- 45. Bian Y, Zhang Z, Sun Z, et al. Vaccines targeting PreS1 domain overcome immune tolerance in HBV carrier mice. Hepatology 2017; 66:1067–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen M, Jagya N, Bansal R, Frelin L, Sällberg M. Prospects and progress of DNA vaccines for treating hepatitis B. Expert Rev Vaccines 2016; 15:629–40. [DOI] [PubMed] [Google Scholar]
- 47. Yalcin K, Danis R, Degertekin H, Alp MN, Tekes S, Budak T. The lack of effect of therapeutic vaccination with a pre-S2/S HBV vaccine in the immune tolerant phase of chronic HBV infection. J Clin Gastroenterol 2003; 37:330–5. [DOI] [PubMed] [Google Scholar]
- 48. Zhao HJ, Han QJ, Wang G, et al. Poly I:C-based rHBVvac therapeutic vaccine eliminates HBV via generation of HBV-specific CD8+ effector memory T cells. Gut 2019; 68:2032–43. [DOI] [PubMed] [Google Scholar]
- 49. Suslov A, Boldanova T, Wang X, Wieland S, Heim MH. Hepatitis B virus does not interfere with innate immune responses in the human liver. Gastroenterology 2018; 154:1778–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.