Abstract
Background
Previous studies reported that prostate cancer incidence rates in the United States declined for local-stage disease and increased for regional- and distant-stage disease following the US Preventive Services Task Force recommendations against prostate-specific antigen-based screening for men aged 75 years and older in 2008 and for all men in 2012. It is unknown, however, whether these patterns persisted through 2016.
Methods
Based on the US Cancer Statistics Public Use Research Database, we examined temporal trends in invasive prostate cancer incidence from 2005 to 2016 in men aged 50 years and older stratified by stage (local, regional, and distant), age group (50-74 years and 75 years and older), and race and ethnicity (all races and ethnicities, non-Hispanic Whites, and non-Hispanic Blacks) with joinpoint regression models to estimate annual percent changes. Tests of statistical significance are 2-sided (P < .05).
Results
For all races and ethnicities combined, incidence for local-stage disease declined beginning in 2007 in men aged 50-74 years and 75 years and older, although the decline stabilized during 2013-2016 in men aged 75 years and older. Incidence decreased by 6.4% (95% CI = 4.9%-9% to 7.9%) per year from 2007 to 2016 in men aged 50-74 years and by 10.7% (95% CI = 6.2% to 15.0%) per year from 2007 to 2013 in men aged 75 years and older. In contrast, incidence for regional- and distant-stage disease increased in both age groups during the study period. For example, distant-stage incidence in men aged 75 years and older increased by 5.2% (95% CI = 4.2% to 6.1%) per year from 2010 to 2016.
Conclusions
Regional- and distant-stage prostate cancer incidence continue to increase in the United States in men aged 50 years and older, and future studies are needed to identify reasons for the rising trends.
Prostate cancer is the most commonly diagnosed cancer and the second leading cause of cancer death in men in the United States, with about 192 000 new cases and 33 000 deaths expected to occur in 2020 (1). Based on incidence data through 2015, several previous studies reported that incidence rates declined for local-stage disease, whereas rates increased for distant-stage disease (2-4), following the US Preventive Services Task Force (USPSTF) recommendations against prostate-specific antigen (PSA)–based screening for men aged 75 years and older in 2008 (5) and for all men in 2012 (6). Further, a more recent study, based on incidence data through 2014, documented increased incidence rates for regional-stage disease (7). It is unknown, however, whether these patterns have persisted through 2016 based on nationwide incidence data and whether they are consistent across age groups and by race and ethnicity. Herein, we examined trends in invasive prostate cancer incidence rates from 2005 to 2016 in men stratified by stage, age, and race and ethnicity using a nationwide incidence database.
Methods
Data Source
Invasive prostate cancer cases diagnosed from 2005 to 2016 in men aged 20 years and older were obtained from the US Cancer Statistics 2001-2016 Public Use Research Database, covering 100% of the US population (8). Cases were categorized according to Merged Summary Stage 2000 as local (localized only and regional by direct extension only), regional [regional by lymph node(s) involved only], distant [distant site(s) and node(s) involved], or unknown (not applicable, unknown, unstaged or unspecified, or blanks) (9). Merged Summary Stage 2000 was created by the Centers for Disease Control and Prevention’s National Program of Cancer Registries to harmonize 2 different staging schemes used by the US surveillance community during the 2001-2016 diagnosis years: Surveillance, Epidemiology, and End Results Program (SEER) Summary Stage 2000 for cases diagnosed from 2001 to 2003 and 2016 and Derived SEER Summary Stage 2000 for cases diagnosed from 2004 to 2015 (9).
Statistical Analysis
Incidence rates, age standardized to the 2000 US population, were calculated by age (50 years and older, 50-74 years, and 75 years and older), stage, and race and ethnicity [all races and ethnicities, non-Hispanic Whites (NHWs), and non-Hispanic Blacks (NHBs)] using SEER*Stat software (10). Other major racial and ethnic groups (American Indian and Alaska Natives, Asian and Pacific Islanders, and Hispanics) were not included as separate categories because of sparse data.
Temporal trends in incidence rates, expressed as annual percent change, by race and ethnicity and age groups (50 years and older, 50-74 years, and 75 years and older) were examined by fitting a log-transformed joinpoint regression model, with a maximum of 2 joinpoints (11). The model identifies the time point(s) in which the trend statistically significantly changes. Trends were considered increasing or decreasing when the P value for the corresponding annual percent change was less than .05; otherwise, the trends were considered stable. All statistical tests were 2-sided.
We estimated the excess number of distant-stage prostate cancer cases in men aged 50 years and older because of the increase in distant-stage incidence rates. We first calculated the expected number of cases assuming that the incidence rates remained at their lowest rate, or nadir. This was obtained by applying the 10-year age-specific distant-stage incidence rates (50-59, 60-69, 70-79, and 80 years and older) in the lowest year for age-standardized distant-stage incidence rates in age 50 years and older during the study period to the corresponding age-specific population in the subsequent year through 2016. We then summed the difference between the expected and observed cases over the age groups by calendar year of diagnosis. We similarly calculated the additional number of local-stage prostate cancer cases that would have been diagnosed if incidence rates for local-stage disease in men aged 50 years and older were to remain at their highest rate, or peak.
In supplementary analyses, we similarly examined the incidence patterns for men aged 20-49 years and by narrower age intervals for men aged 50-74 years (50-54, 55-69, and 70-74 years) in view of the 2018 USPSTF recommendation of informed decision making for PSA testing for men aged 55-69 years. We also examined Black-to-White differences in distant-stage incidence rates over time by computing incidence rate ratios (IRRs) and 95% confidence intervals (CIs) using the Tiwari method (12), with 2-year averages. Finally, to explore stage migration, we used the SEER-18 incidence database to examine annual median PSA at regional- or distant-stage diagnosis for men 50 years and older from 2005 to 2016 (13).
Results
From 2005 to 2016, a total of 2 082 874 local-stage (86.8%), 36 568 regional-stage (1.5%), and 121 826 distant-stage (5.1%) prostate cancer cases were diagnosed in men aged 50 years and older in the United States. During the corresponding period, the proportion of local-stage decreased (from 88.1% to 80.5%) but increased for regional-stage (from 1.1% to 2.6%) and distant-stage (from 4.1% to 7.6%) diseases (Table 1; Supplementary Table 1, available online).
Table 1.
Age-standardized prostate cancer number of total cases and incidence rates for all races and ethnicities combined by age group and stage at diagnosis in the United States, 2005 and 2016a
| Stage | 2005 |
2016 |
||
|---|---|---|---|---|
| No. of cases (%) | Rate (95% CI) | No. of cases (%) | Rate (95% CI) | |
| ≥50 years | ||||
| Local | 173 766 (88.1) | 456.4 (454.2 to 458.6) | 150 241 (80.5) | 279.2 (277.7 to 280.6) |
| Regional | 2258 (1.1) | 5.7 (5.4 to 5.9) | 4916 (2.6) | 9.0 (8.8 to 9.3) |
| Distant | 8034 (4.1) | 23.1 (22.6 to 23.6) | 14 162 (7.6) | 29.7 (29.2 to 30.2) |
| Unknown | 13 165 (6.7) | 38.7 (38.1 to 39.4) | 17 252 (9.2) | 34.2 (33.7 to 34.7) |
| All | 197 223 | 523.9 (521.6 to 526.3) | 186 571 | 352.1 (350.4 to 353.7) |
| 50-74 years | ||||
| Local | 134 462 (91.4) | 425.1 (422.8 to 427.4) | 126 532 (83.6) | 278.0 (276.4 to 279.5) |
| Regional | 1959 (1.3) | 6.0 (5.8 to 6.3) | 4174 (2.8) | 9.1(8.8 to 9.4) |
| Distant | 4284 (2.9) | 13.7 (13.3 to 14.2) | 8204 (5.4) | 18.3 (17.9 to 18.7) |
| Unknown | 6418 (4.4) | 21.0 (20.5 to 21.6) | 12 372 (8.2) | 27.5 (27.0 to 28.0) |
| All | 147 123 | 465.9 (463.5 to 468.3) | 151 282 | 332.8 (331.1 to 334.5) |
| ≥75 years | ||||
| Local | 39 304 (78.5) | 568.7 (563.0 to 574.3) | 23 709 (67.1) | 283.4 (279.8 to 287.0) |
| Regional | 299 (0.6) | 4.3 (3.9 to 4.9) | 742 (2.1) | 8.8 (8.2 to 9.5) |
| Distant | 3750 (7.5) | 56.8 (54.9 to 58.6) | 5958 (16.9) | 70.6 (68.8 to 72.4) |
| Unknown | 6747 (13.5) | 102.0 (99.6 to 104.5) | 4880 (13.8) | 58.0 (56.4 to 59.7) |
| All | 50 100 | 731.7 (725.3 to 738.2) | 35 289 | 420.8 (416.5 to 425.3) |
Rates are per 100 000 and age-standardized to the 2000 US standard population. Source: U.S. Cancer Statistics Public Use Research Database, November 2018 submission (2001-2016) (6). CI = confidence interval.
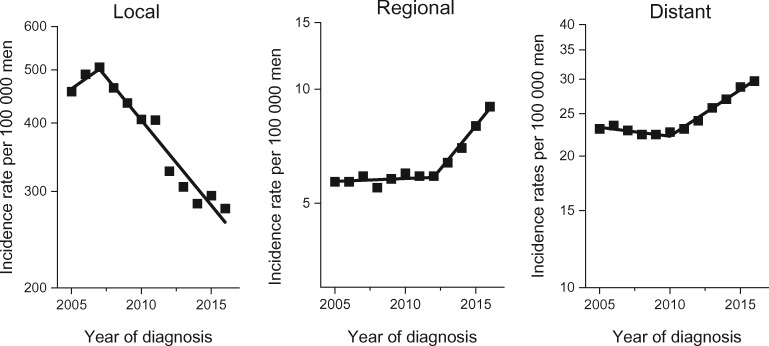
Figure 1 illustrates age-standardized prostate cancer incidence per 100 000 men aged 50 years and older by stage from 2005 through 2016 for all races and ethnicities combined (actual data given in Supplementary Table 1, available online). For local-stage disease, incidence rates per 100 000 men aged 50 years and older increased from 456.4 (95% CI = 454.2 to 458.6) in 2005 to 506.1 (95% CI = 503.9 to 508.3) in 2007 and then decreased in subsequent years to 279.2 (95% CI = 277.7 to 280.6) in 2016. For regional-stage disease, incidence generally increased throughout the study period, from 5.7 (95% CI = 5.4 to 5.9) in 2005 to 9.0 (95% CI = 8.8 to 9.3) in 2016. For distant-stage disease, incidence rates slightly declined from 23.1 (95% CI = 22.6 to 23.6) in 2005 to 22.4 (95% CI = 21.9 to 22.9) in 2008 but then continued to increase to 29.7 (95% CI = 29.2 to 30.2) in 2016.
Figure 1.
Trends in annual age-standardized prostate cancer incidence rates in men aged 50 years and older in the United States by stage, 2005-2016. Solid lines represent joinpoint modeled rates, and symbols represent observed rates. Source: U.S. Cancer Statistics Public Use Research Database, November 2018 submission (2001-2016) (6).
According to joinpoint regression modeling, incidence rates for local-stage disease in men aged 50 years and older statistically significantly decreased by 6.9% (95% CI = 5.3% to 8.5%) per year from 2007 to 2016 (Table 2). In contrast, for regional-stage disease, incidence rates statistically significantly increased by 11.1% (95% CI = 8.9% to 13.3%) per year from 2012 to 2016 after stable rates from 2005 to 2012. Similarly, for distant-stage disease, incidence rates statistically significantly increased from 2010 to 2016 by 5.0% (95% CI = 4.4% to 5.6%) per year after declining by 0.9% (95% CI = 0.1% to 1.8%) per year from 2005 to 2010. Incidence rates for unknown-stage disease statistically significantly decreased by 3.6% (95% CI = 1.6% to 5.6%) per year during 2005-2016.
Table 2.
Trends in annual age-standardized prostate cancer incidence rates by stage at diagnosis, race and ethnicity, and age in the United States, 2005-2016a
| Race and Stage | Age, y | Trends 2005-2016 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st segmenta |
2nd segmenta |
3rd segmenta |
||||||||||
| Years | APC (95% CI) | P b | Years | APC (95% CI) | P b | Years | APC (95% CI) | P b | ||||
| All races/ethnicities | ||||||||||||
| Local | ≥50 | 2005-2007 | 4.2 (−12.6 to 24.3) | .59 | 2007-2016 | −6.9b (−8.5 to −5.3) | <.001 | — | — | — | ||
| 50-74 | 2005-2007 | 6.3 (−11.3 to 27.4) | .45 | 2007-2016 | −6.4b (−7.9 to −4.9) | <.001 | — | — | — | |||
| ≥75 | 2005-2007 | 1.8 (−15.8 to 23.2) | .80 | 2007-2013 | −10.7b (−15.0 to −6.2) | .003 | 2013-2016 | −2.0 (−13.6 to 11.0) | .67 | |||
| Regional | ≥50 | 2005-2012 | 0.3 (−0.8 to 1.4) | .50 | 2012-2016 | 11.1b (8.9 to 13.3) | <.001 | — | — | — | ||
| 50-74 | 2005-2012 | −0.1 (−0.9 to 0.6) | .68 | 2012-2016 | 10.6b (9.0 to 12.3) | .001 | — | — | — | |||
| ≥75 | 2005-2013 | 3.9b (0.9 to 7.0) | .02 | 2013-2016 | 15.9b (6.1 to 26.6) | .006 | — | — | — | |||
| Distant | ≥50 | 2005-2010 | −0.9b (−1.8 to −0.1) | .04 | 2010-2016 | 5.0b (4.4 to 5.6) | <.001 | — | — | — | ||
| 50-74 | 2005-2008 | −1.3 (−3.4 to 0.9) | .18 | 2008-2012 | 2.4b (0.3 to 4.6) | .04 | 2012-2016 | 5.6b (4.4 to 6.8) | <.001 | |||
| ≥75 | 2005-2010 | −1.7b (−3.0 to −0.3) | .02 | 2010-2016 | 5.2b (4.2 to 6.1) | <.001 | — | — | — | |||
| Unknown | ≥50 | 2005-2016 | −3.6b (−5.6 to −1.6) | .003 | — | — | — | — | — | — | ||
| 50-74 | 2005-2016 | −0.3 (−2.9 to 2.5) | .84 | — | — | — | — | — | — | |||
| ≥75 | 2005-2016 | −6.8b (−8.4 to −5.1) | <.001 | — | — | — | — | — | — | |||
| All | ≥50 | 2005-2007 | 4.8 (−13.0 to 26.2) | .53 | 2007-2014 | −7.0b (−10.0 to −4.0) | .003 | 2014-2016 | 1.1 (−16.3 to 22.2) | .88 | ||
| 50-74 | 2005-2007 | 5.4 (−12.3 to 26.7) | .52 | 2007-2016 | −5.6b (−7.1 to −4.0) | <.001 | — | — | — | |||
| ≥75 | 2005-2007 | 0.1 (−13.9 to 16.3) | .99 | 2007-2014 | −8.7b (−11.3 to −5.9) | .001 | 2014-2016 | 4.2 (−13.1 to 24.8) | .57 | |||
| Non-Hispanic White | ||||||||||||
| Local | ≥50 | 2005-2007 | 4.1 (−13.5 to 25.4) | .62 | 2007-2016 | −7.1b (−8.9 to −5.4) | <.001 | — | — | — | ||
| 50-74 | 2005-2007 | 6.3 (−11.7 to 28.1) | .46 | 2007-2016 | −6.7b (−8.3 to −5.0) | <.001 | — | — | — | |||
| ≥75 | 2005-2007 | 1.7 (−16.2 to 23.3) | .82 | 2007-2013 | −10.9b (−15.2 to −6.3) | .003 | 2013-2016 | −1.1 (−13.2 to 12.7) | .83 | |||
| Regional | ≥50 | 2005-2012 | 0.6 (−0.3 to 1.5) | .14 | 2012-2016 | 10.8b (8.6 to 13.1) | <.001 | — | — | — | ||
| 50-74 | 2005-2012 | 0.1 (−0.6 to 0.9) | .76 | 2012-2016 | 10.0b (8.2 to 11.8) | <.001 | — | — | — | |||
| ≥75 | 2005-2013 | 3.6b (0.8 to 6.4) | .02 | 2013-2016 | 17.0b (7.0 to 28.0) | .004 | — | — | — | |||
| Distant | ≥50 | 2005-2010 | −0.7 (−1.6 to 0.2) | .12 | 2010-2016 | 5.8b (5.2 to 6.4) | <.001 | — | — | — | ||
| 50-74 | 2005-2008 | −1.0 (−3.4 to 1.4) | .30 | 2008-2012 | 2.9b (0.6 to 5.3) | .02 | 2012-2016 | 6.4b (5.2 to 7.7) | <.001 | |||
| ≥75 | 2005-2010 | −1.4 (−3.1 to 0.3) | .10 | 2010-2016 | 5.9b (4.7 to 7.1) | <.001 | — | — | — | |||
| Unknown | ≥50 | 2005-2016 | −5.6b (−7.3 to −3.9) | <.001 | — | — | — | — | — | — | ||
| 50-74 | 2005-2014 | −4.0b (−5.8 to −2.2) | .001 | 2014-2016 | 16.9 (−2.0 to 39.5) | .08 | — | — | — | |||
| ≥75 | 2005-2016 | −8.6b (−9.8 to −7.4) | <.001 | — | — | — | — | — | — | |||
| All | ≥50 | 2005-2007 | 4.6 (−13.5 to 26.5) | .55 | 2007-2014 | −7.4b (−10.6 to −4.1) | .004 | 2014-2016 | 1.3 (−17.6 to 24.5) | .87 | ||
| 50-74 | 2005-2007 | 5.4 (−12.5 to 26.8) | .53 | 2007-2016 | −6.0b (−7.6 to −4.3) | <.001 | — | — | — | |||
| ≥75 | 2005-2007 | 0.2 (−14.2 to 17.1) | .97 | 2007-2013 | −9.4b (−13.0 to −5.7) | .002 | 2013-2016 | 0.3 (−9.0 to 10.6) | .93 | |||
| Non-Hispanic Black | ||||||||||||
| Local | ≥50 | 2005-2007 | 4.9 (−8.7 to 20.5) | .44 | 2007-2016 | −5.9b (−7.1 to −4.7) | <.001 | — | — | — | ||
| 50-74 | 2005-2007 | 6.3 (−7.3 to 21.9) | .33 | 2007-2016 | −5.1b (−6.3 to −4.0) | <.001 | — | — | — | |||
| ≥75 | 2005-2007 | 0.8 (−13.4 to 17.4) | .90 | 2007-2016 | −9.0b (−10.4 to −7.6) | <.001 | — | — | — | |||
| Regional | ≥50 | 2005-2012 | 0.7 (−2.5 to 4.1) | .60 | 2012-2016 | 11.7b (4.9 to 19.0) | .004 | — | — | — | ||
| 50-74 | 2005-2013 | 1.3 (−1.2 to 3.9) | .25 | 2013-2016 | 14.3b (4.6 to 24.9) | .009 | — | — | — | |||
| ≥75 | 2005-2016 | 6.2b (2.7 to 9.7) | .002 | — | — | — | — | — | — | |||
| Distant | ≥50 | 2005-2011 | −2.1b (−3.8 to −0.3) | .03 | 2011-2016 | 3.2b (1.2 to 5.3) | .007 | — | — | — | ||
| 50-74 | 2005-2010 | −1.2 (−3.0 to 0.6) | .17 | 2010-2016 | 3.0b (1.8 to 4.3) | .001 | — | — | — | |||
| ≥75 | 2005-2011 | −3.9b (−6.8 to −0.9) | .02 | 2011-2016 | 2.9 (−0.9 to 6.8) | .12 | — | — | — | |||
| Unknown | ≥50 | 2005-2016 | −4.0b (−5.6 to −2.4) | <.001 | — | — | — | — | — | — | ||
| 50-74 | 2005-2016 | −0.5 (−2.6 to 1.6) | .59 | — | — | — | — | — | — | |||
| ≥75 | 2005-2016 | −7.8b (−8.9 to −6.6) | <.001 | — | — | — | — | — | — | |||
| All | ≥50 | 2005-2007 | 3.1 (−10.2 to 18.5) | .61 | 2007-2016 | −5.1b (−6.2 to −3.9) | <.001 | — | — | — | ||
| 50-74 | 2005-2007 | 5.0 (−8.5 to 20.5) | .43 | 2007-2016 | −4.4b (−5.5 to −3.2) | <.001 | — | — | — | |||
| ≥75 | 2005-2007 | 0.1 (−12.4 to 14.4) | .99 | 2007-2014 | −8.4b (−10.7 to −6.2) | .001 | 2014-2016 | −1.2 (−15.5 to 15.4) | .84 | |||
Segments determined by joinpoint model. Source: U.S. Cancer Statistics Public Use Research Database, November 2018 submission (2001-2016) (6). APC = annual percent change; CI = confidence interval.
Monte Carlo Permutation method used to calculate P values; 2-sided test.
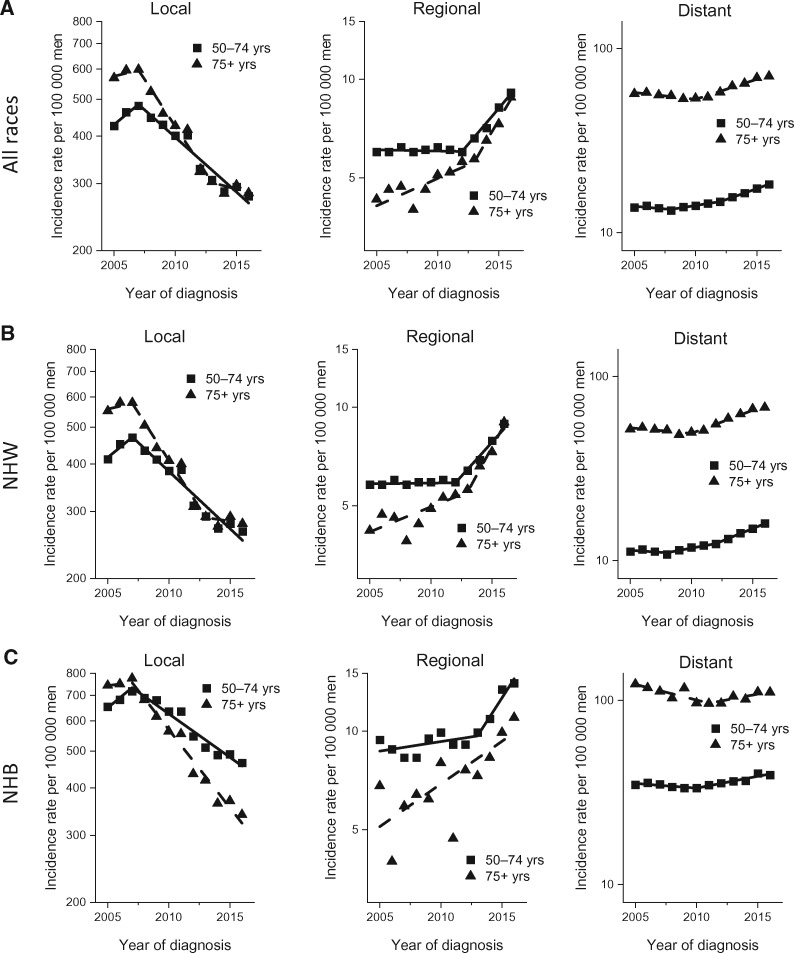
Figure 2 shows stage-specific incidence rates for 2 broad age groups (50-74 years and 75 years and older) by race and ethnicity (all races and ethnicities, NHW, and NHB). For all races and ethnicities combined (Figure 2A), the incidence patterns for age 50–74 years and 75 years and older are generally similar to those of age 50 years and older, with the incidence rates after the late 2000s declining for local-stage disease but increasing for regional- and distant-stage diseases, except local-stage disease stabilized from 2013 to 2016 in men aged 75 years and older. Specifically, incidence for local-stage disease decreased by 6.4% (95% CI = 4.9%-9% to 7.9%) per year from 2007 to 2016 in men aged 50–74 years and by 10.7% (95% CI = 6.2% to 15.0%) per year from 2007 to 2013 in men aged 75 years and older. In contrast, incidence for distant-stage disease increased by 2.4% (95% CI = 0.3% to 4.6%) per year from 2008 to 2012 and by 5.6% (95% CI = 4.4% to 6.8%) per year from 2012 to 2016 in men aged 50–74 years and by 5.2% (95% CI = 4.2% to 6.1%) per year from 2010 to 2016 in men aged 75 years and older. We found generally similar age- and stage-specific incidence patterns in NHW and NHB men, except incidence in NHB men aged 75 years and older during the most recent period continued to decrease for local-stage disease and stabilized for distant-stage disease, and the pace of the increase in distant-stage disease in men aged 50–74 years was slower in NHB men than in NHW men (Figure 2, B and C, and Table 2).
Figure 2.
Trends in annual age-standardized prostate cancer incidence rates by stage at diagnosis, race and ethnicity, and age in the United States, 2005-2016. A) Trends for all races combined by stage at diagnosis for men aged 50-74 years and 75 years and older. Solid lines represent joinpoint modeled rates, and symbols represent observed rates. B) Trends for non-Hispanic Whites (NHW) by stage at diagnosis for men aged 50-74 and 75 years and older. Solid lines represent joinpoint modeled rates, and symbols represent observed rates. C) Trends for non-Hispanic Blacks (NHB) by stage at diagnosis for men aged 50-74 and 75 years and older. Solid lines represent joinpoint modeled rates, and symbols represent observed rates. Source: U.S. Cancer Statistics Public Use Research Database, November 2018 submission (2001-2016) (6).
Supplementary Table 3 (available online) presents the stage-specific incidence trends among men aged 50-74 years by more refined age groups (50-54, 55-69, 70-74 years) and trends in younger men (20-49 years). During the most recent period, incidence rates for men aged 50-54, 55-69, and 70-74 years statistically significantly declined for local-stage disease, whereas incidence statistically significantly increased for regional- and distant-stage diseases. Incidence for men aged 20-49 years decreased for local-stage disease but were stable for regional- and distant-stage diseases.
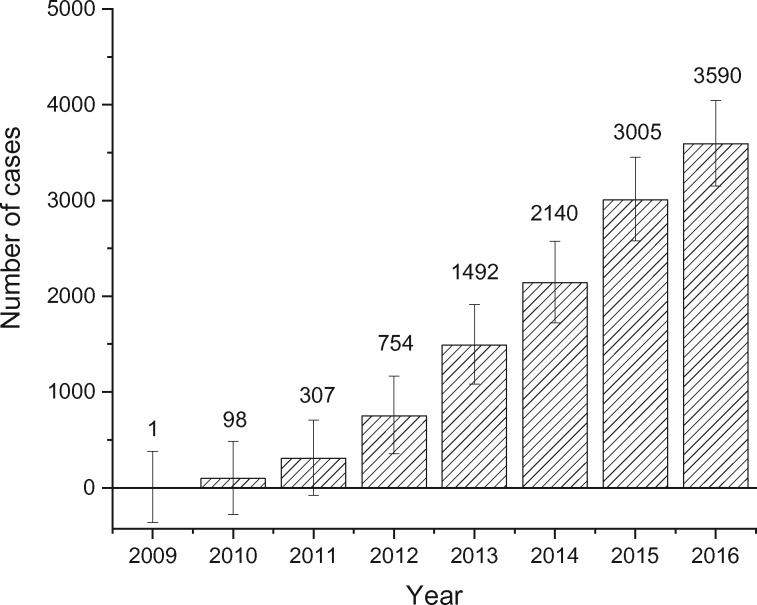
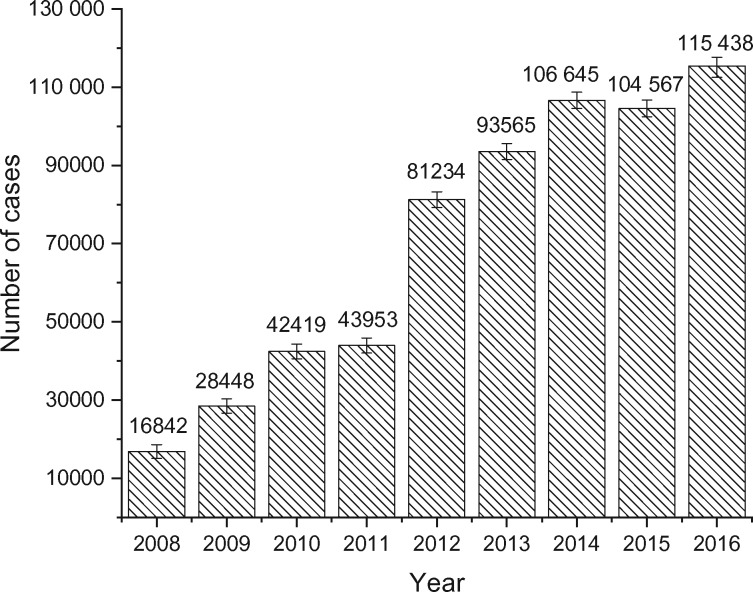
Figure 3 presents the annual excess number of distant-stage disease in men aged 50 years and older had the incidence rates for distant-stage from 2009 through 2016 remained at their nadir, the 2008 rate (Supplementary Table 4, available online). A total of 11 387 more men were diagnosed with distant-stage disease from 2009 to 2016 in the United States than would have been had distant-stage incidence rates remained at the 2008 level, with 3590 cases in 2016 alone. Figure 4 shows the additional number of local-stage disease that would have been diagnosed from 2008 through 2016 had the incidence rates for local-stage disease from 2008 through 2016 remained at the 2007 level, the peak rate. A total of 633 111 additional local-stage diseases would have been diagnosed from 2008 to 2016, with 115 438 of the cases in 2016 alone, had the incidence rate for local-stage disease remained at its peak rate (Supplementary Table 5, available online).
Figure 3.
Annual total number of excess distant-stage cases in men aged 50 years and older in the United States since 2009. Calculated as the differences between observed cases and expected cases if the rate for distant-stage disease had remained at their lowest level (2008 rate). Source: U.S. Cancer Statistics Public Use Research Database, November 2018 submission (2001-2016) (6).
Figure 4.
Annual total number of local-stage cases avoided in men aged 50 years and older in the United States since 2008. Calculated as the differences between observed cases and expected cases if the rate for early stage disease had remained at their peak rate (2007 rate). Source: U.S. Cancer Statistics Public Use Research Database, November 2018 submission (2001-2016) (6).
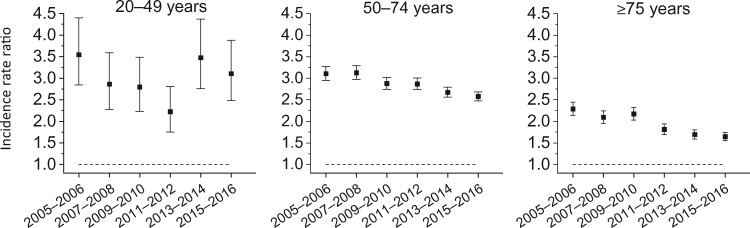
Figure 5 portrays the Black-to-White distant-stage IRRs for men aged 20-49, 50-74, and 75 years and older . Among men aged 20-49 years, the Black-to-White IRRs declined from 3.55 (95% CI = 2.85 to 4.40) during 2005-2006 to 2.22 (95% CI = 1.75 to 2.81) during 2011-2012 and then nonstatistically significantly increased to 3.11 (95% CI = 2.48 to 3.88) during 2015-2016. In contrast, for men aged 50-74 years and 75 years and older, the Black-to-White IRRs for distant-stage disease continued to decrease from 2005-2006 through 2015-2016: from 3.11 (95% CI = 2.95 to 3.27) to 2.58 (95% CI = 2.48 to 2.68) in men aged 50-74 years and from 2.29 (95% CI = 2.14 to 2.45) to 1.65 (95% CI = 1.55 to 1.75) in men aged 75 years and older (Supplementary Table 6, available online).
Figure 5.
Non-Hispanic Black to non-Hispanic White incidence rate ratio for distant-stage prostate cancer by age, 2005-2016. Source: U.S. Cancer Statistics Public Use Research Database, November 2018 submission (2001-2016) (6).
Discussion
Using nationwide population-based incidence data, we report that prostate cancer incidence rates in men aged 50 years and older continued to decline for local-stage disease but increased for regional- and distant-stage disease following the USPSTF recommendations against routine PSA testing for men aged 75 years and older in 2008 (5) and for all men in 2012 (6). According to national self-reported survey data, past-year routine PSA testing rates among men aged 50 and older declined from 40.6% in 2008 to 38.3% in 2010 to 31.5% in 2013 and remained unchanged in 2015 (3). Similar decline in PSA testing was reported based on Medicare claims data (14) and commercial claims data (15).
Reasons for the continued increase in regional- and distant-stage incidence rates are unknown. Family history, an established risk factor for prostate cancer, is unlikely to change during the study period. Cigarette smoking, which increases the risk of fatal prostate cancer (16, 17), is also unlikely to account for observed trends because of the long-term declines in smoking and in tobacco-related cancers (18). Excess body weight, another risk factor associated with fatal prostate cancer, has escalated among men since the 1970s (19), although the extent of its contribution to the rising distant-stage incidence rates is yet to be determined (20). Also unknown is the extent of the contribution of the declining incidence trend in unknown-stage disease (improvements in staging) in men aged 75 years and older to the rising incidence trends for distant-stage disease in this age group. But the increase in regional- and distant-stage incidence rates in men aged 50–74 years is unlikely to be influenced by improvements in staging because the trend for unknown-stage disease remained stable in this age group. Further, stage migration due to more aggressive and sensitive imaging is unlikely to have contributed to the continuously rising regional- and distant-stage incidence rates because median PSA level at diagnosis fluctuated during the study period: from about 55-62 ng/ml in 2005-2007 to 70-75 ng/ml in 2012-2013 and to 57–58 ng/ml in 2015-2016 (Supplementary Table 7, available online).
These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease. Several modeling studies, however, showed that the harms associated with higher PSA screening rates can be mitigated while preserving the benefit of screening (21) through PSA-stratified strategies including a longer screening interval based on baseline PSA (22), higher PSA threshold for biopsy referral in older men (23), and restriction of routine testing to men aged 70 years and younger (24).
Our finding of substantial decline in the racial disparity in the incidence of distant-stage disease, largely confined to men aged 50-74 years, coincided with the steeper increase in distant-stage incidence in NHW men. Nevertheless, distant-stage incidence rates in NHB men remain 2-3 times as high as in NHW men aged 20-74 years and 65% higher in men aged 75 years and older. Reasons for this disparity are not fully understood but, in part, thought to reflect differences in life-style factors, biological susceptibility, and access to care. Obesity (25) and cigarette smoking (26) are more prevalent in NHB than NHW men. Furthermore, the association of obesity with prostate cancer risk is stronger in NHB men (27). Among Black men, recent studies have identified several ancestry-specific risk variants in the 13q34, 22q12 (28), and 8q24 (29) chromosome regions, which are known to harbor multiple prostate cancer susceptibility variants. It is unclear, however, how such genetic variants interact with socioeconomic and environmental exposures to contribute to the higher incidence of distant-stage disease in NHB men. PSA testing in men aged 50 years and older reported to be substantially lower in NHBs than NHWs (3) despite evidence that Black men may have more prevalent preclinical prostate cancer and greater disease progression than White men (30).
One limitation of our study is that the data are not adjusted to account for delay in reporting of cases (31). This may have underestimated the increasing trends for distant-stage disease but overestimated the declining trends for early stage disease. Corrections for delay reporting (1%-4% per year) (32), however, are unlikely to offset the 6%-10% annual decline in incidence for the local-stage disease. In addition, cancer cases in the US Cancer Statistics Public Use Dataset cannot be grouped according to American Joint Committee on Cancer staging because of lack of detailed information on tumor characteristics, including tumor size. However, standardized Merged Summary Stage information in the US Cancer Statistics Public Use Dataset over the study period has allowed us to categorize prostate cancer cases into local-, regional-, and distant-stage diseases (9). Finally, we cannot make causal inference because of the descriptive nature of our study.
Nevertheless, the persistently increasing regional- and distant-stage prostate cancer incidence during the past 5 years has public health implications given the substantial morbidity and premature mortality associated with it and the recent stabilization of prostate cancer death rates after a steady decline since the early 1990s (3). In 2018, the USPSTF updated its recommendations for individualized decision making for PSA-based screening for men aged 55-69 years (33), similar to other public health guidelines. However, only 10% of men without PSA testing report any element of shared decision making in recent years (34). Future studies are needed to elucidate reasons for the rising incidence trends for regional- and distant-stage diseases and for the disproportionately high burden of the disease in Black men.
Funding
This project was supported by the Intramural Research Department of the American Cancer Society.
Notes
Role of the funder: The American Cancer Society had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: The authors are employed by the American Cancer Society, which receives grants from private and corporate foundations, including foundations associated with companies in the health sector for research outside the submitted work. The authors are not funded by any of these grants and their salary is solely funded through American Cancer Society funds.
Disclaimer: The opinions expressed are solely the responsibility of the authors and do not necessarily reflect the official views of the American Cancer Society.
Role of the authors: Ahmedin Jemal: Conceptualization; Writing-original draft; Writing-review & editing. MaryBeth B. Culp: Data curation; Formal analysis; Visualization; Writing—review & editing. Jiemin Ma: Data curation; Writing—review & editing. Farhad Islami: Writing—review & editing. Stacey A. Fedewa: Conceptualization; Writing—original draft; Writing—review & editing.
Acknowledgments: We gratefully acknowledge all cancer registries and their staff for their hard work and diligence in collecting cancer information, without which this research could not have been done.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Ma J, Siegel R, et al. Prostate cancer incidence rates 2 years after the US Preventive Services Task Force Recommendations Against Screening. JAMA Oncol. 2016;2(12):1657–1660. [DOI] [PubMed] [Google Scholar]
- 3. Negoita S, Feuer EJ, Mariotto A, et al. Annual Report to the Nation on the Status of Cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer. 2018;124(13):2801–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu JC, Nguyen P, Mao J, et al. Increase in prostate cancer distant metastases at diagnosis in the United States. JAMA Oncol. 2017;3(5):705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Preventive Services Task Force. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2008;149(3):185–191. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Preventive Services Task Force. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2012;157(2):120–134. [DOI] [PubMed] [Google Scholar]
- 7. Bernstein AN, Shoag JE, Golan R, et al. Contemporary incidence and outcomes of prostate cancer lymph node metastases. J Urol. 2018;199(6):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute Surveillance, Epidemiology and End Results Program SEER, NPCR and SEER Incidence-U.S. Cancer Statistics Public Use Research Database, Nov. 2018 submission (2001–2016). National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. http://www.cdc.gov/cancer/uscs/public-use. Accessed September 2, 2019.
- 9.National Program of Cancer Registries (NPCR), Surveillance Epidemiology & End Results (SEER). NPCR and SEER Incidence-US Cancer Statistics 2001-2016 Public Use Database Data Standards and Data Dictionary2019. https://www.cdc.gov/cancer/uscs/public-use/pdf/npcr-seer-public-use-database-data-dictionary-2001-2016-508.pdf. Accessed September 2, 2019.
- 10.Surveillance Research Program. National Cancer Institute SEER*Stat software (version 8.3.6). seer.cancer.gov/seerstat. Published August 6, 2019. Accessed September 2, 2019.
- 11. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 12. Tiwari RC, Clegg LX, Zou Z.. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute, Surveillance, Epidemiology & End Results SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (1975–2016 varying) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. http://www.seer.cancer.gov. Published April 2019. Accessed September 2, 2019.
- 14. Cooper GS, Kou TD, Schluchter MD, et al. Use of prostate-specific antigen testing in Medicare beneficiaries: association with previous evaluation. Fam Med Commun Health. 2017;5(2):109–118. [Google Scholar]
- 15. Kearns JT, Holt SK, Wright JL, et al. PSA screening, prostate biopsy, and treatment of prostate cancer in the years surrounding the USPSTF recommendation against prostate cancer screening. Cancer. 2018;124(13):2733–2739. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Reports of the Surgeon General. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US; ); 2014. [Google Scholar]
- 17. Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–1034. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics, United States. With Special Feature on Mortality; 2017. http://www.cdc.gov/nchs/hus.htm. [PubMed]
- 19. Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gulati R, Cheng HH, Lange PH, et al. Screening men at increased risk for prostate cancer diagnosis: model estimates of benefits and harms. Cancer Epidemiol Biomarkers Prev. 2017;26(2):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heijnsdijk EAM, Gulati R, Tsodikov A, et al. Lifetime benefits and harms of PSA-based risk screening for prostate cancer. J Natl Cancer Inst. 2020; doi:10.1093/jnci/djaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gulati R, Gore JL, Etzioni R.. Comparative effectiveness of alternative prostate-specific antigen--based prostate cancer screening strategies: model estimates of potential benefits and harms. Ann Intern Med. 2013;158(3):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gulati R, Tsodikov A, Etzioni R, et al. Expected population impacts of discontinued prostate-specific antigen screening. Cancer. 2014;120(22):3519–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hales CM, Fryar CD, Carroll MD, et al. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. JAMA. 2018;319(23):2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. Health, United States, 2018. Hyattsville, MD; 2019. https://www.cdc.gov/nchs/data/hus/hus18.pdf. Accessed October 5, 2019. [PubMed]
- 27. Barrington WE, Schenk JM, Etzioni R, et al. Difference in association of obesity with prostate cancer risk between us African American and non-Hispanic White men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol. 2015;1(3):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conti DV, Wang K, Sheng X, et al. Two novel susceptibility loci for prostate cancer in men of African ancestry. J Natl Cancer Inst. 2017;109(8):djx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han Y, Rand KA, Hazelett DJ, et al. Prostate cancer susceptibility in men of African ancestry at 8q24. J Natl Cancer Inst. 2016;108(7):djv431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsodikov A, Gulati R, de Carvalho TM, et al. Is prostate cancer different in Black men? Answers from 3 natural history models. Cancer. 2017;123(12):2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clegg LX, Feuer EJ, Midthune DN, et al. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–1545. [DOI] [PubMed] [Google Scholar]
- 32. Midthune DN, Fay MP, Clegg LX, Feuer EJ.. Modeling reporting delays and reporting corrections in cancer registry data. J Am Stat Assoc. 2005;100(469):61–70. [Google Scholar]
- 33. Grossman DC, Curry SJ, Owens DK, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901–1913. [DOI] [PubMed] [Google Scholar]
- 34. Fedewa SA, Gansler T, Smith R, et al. Recent patterns in shared decision making for prostate-specific antigen testing in the United States. Ann Fam Med. 2018;16(2):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.